Color Doppler Ultrasound Improves Machine Learning Diagnosis of Breast Cancer
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient and Image Acquisition
2.2. Feature Extraction
2.2.1. BI-RADSUS
2.2.2. Age
2.2.3. Grayscale Features
2.2.4. Color Doppler Features
2.3. Machine Learning
2.4. Class Imbalance Correction Using SMOTE
2.5. Pruning by Drop Rate
3. Results
3.1. Patient and Image Characteristics
3.2. Feature Statistics and Effect Size
3.3. Performance of Each Diagnostic Model
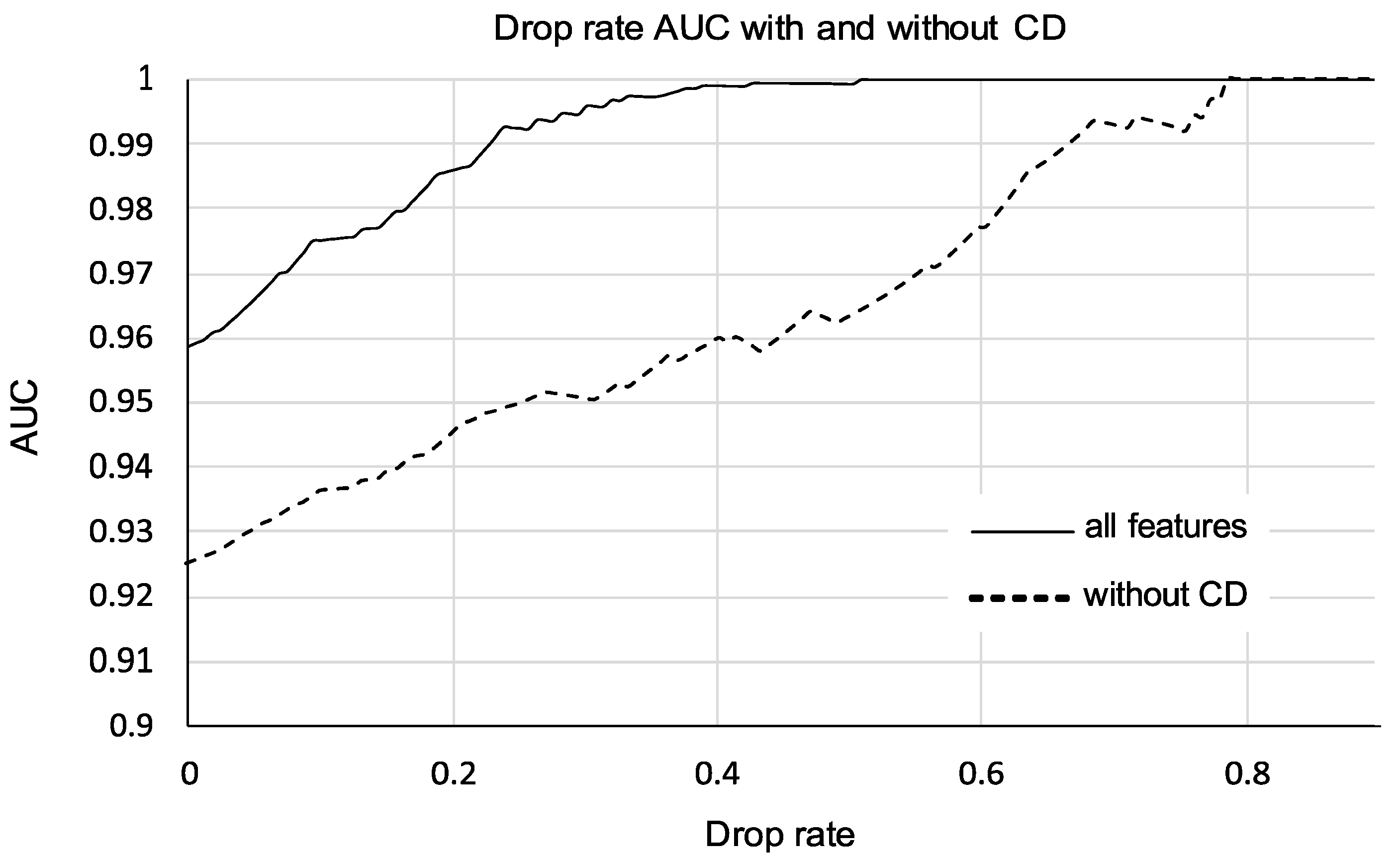
3.4. Performance of Models Pruned by Drop Rate
4. Discussion
Author Contributions
Funding
Conflicts of Interest
Appendix A
| Feature | Formulas |
|---|---|
| Angular variation margin (AVM): Brightness dispersion in sections around the margin using coefficient of variation (CV). AVM quantifies the inhomogeneity of margin brightness with angle. | where for all pixels x,y in sector i |
| Angular variation interior (AVI): Brightness dispersion in sectors around margin through center of mass using coefficient of variation (CV). AVI quantifies angular inhomogeneity. | |
| Brightness difference (BD): Mean intensity (I) difference between the lesion’s interior (int) near the margin and the lesion’s exterior (ext). | |
| Margin sharpness (MS): Diffuseness of the mass margin. Calculated as the fraction of sectors with significant differences in grayscale between interior and exterior. | N = total number of sectors; Nsig counts sectors (p < 0.05) with significant difference: |
| Tortuosity (T): The perimeter of the lesion divided by the circumference of its best-fit ellipse (elliptically normalized circumference). | |
| Depth-to- width ratio (DWR): Ratio of the ROI’s depth to its width on the sonogram. | |
| Axis ratio (AR): Ratio of the major to minor axis of a best-fit ellipse to the ROI. | |
| Radius variation (RV): Coefficient of variation in the radius of the lesion, from center of gravity to each pixel in the lesion’s border. | |
| Ellipse-normalized skeleton (ENS): Number of pixels (Nskeleton) in medial-axis discrete skeleton set S of the shape divided by the circumference of its best-fit ellipse (Cellipse). | |
| Vascular fractional area (VFA): Percentage area of lesion occupied by blood vessels. | |
| Blood flow velocity index (VI): Mean local blood velocity, mapped from color bar to pixels. | |
| Formulas were compiled from the literature [5,9]. | |
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2019. Cancer J. Clin. 2019, 69, 7–34. [Google Scholar] [CrossRef] [PubMed]
- Breast Cancer Surveillance Consortium. Clin. Gov 2019. Available online: https://www.bcsc-research.org (accessed on 26 July 2019).
- Flobbe, K.; Kessels, A.G.H.; Nelemans, P.J.; van Engelshoven, J.M.A.; Bosch, A.M.; Beets, G.; von Meyenfeldt, M. The additional diagnostic value of ultrasonography in the diagnosis of breast cancer. Arch. Intern. Med. 2003, 163, 1194–1199. [Google Scholar] [CrossRef] [PubMed]
- Gartlehner, G.; Thaler, K.; Chapman, A.; Kaminski-Hartenthaler, A.; Berzaczy, D.; Van Noord, M.G.; Helbich, T.H. Mammography in combination with breast ultrasonography versus mammography for breast cancer screening in women at average risk. Cochrane Database Syst. Rev. 2013. [Google Scholar] [CrossRef] [PubMed]
- Sehgal, C.M.; Arger, P.H.; Rowling, S.E.; Conant, E.F.; Reynolds, C.; Patton, J.A. Quantitative vascularity of breast masses by Doppler imaging: Regional variations and diagnostic implications. J. Ultrasound Med. 2000, 19, 427–440. [Google Scholar] [CrossRef]
- Song, S.E.; Seo, B.K.; Cho, K.R.; Woo, O.H.; Son, G.S.; Kim, C.; Cho, S.B.; Kwon, S.S. Computer-aided detection (CAD) system for breast MRI in assessment of local tumor extent, nodal status, and multifocality of invasive breast cancers: Preliminary study. Cancer Imaging 2015, 15, 1–9. [Google Scholar] [CrossRef][Green Version]
- Saarenmaa, I.; Salminen, T.; Geiger, U.; Heikkinen, P.; Rinen, S.; Isola, J.; Kataja, V.; Kokko, M.L.; Kumpulainen, E.; Kärkkäinen, A.; et al. The effect of age and density of the breast on the sensitivity of breast cancer diagnostic by mammography and ultasonography. Breast Cancer Res. Treat. 2001, 67, 117–123. [Google Scholar] [CrossRef]
- Kolb, T.M.; Lichy, J.; Newhouse, J.H. Comparison of the Performance of Screening Mammography, Physical Examination, and Breast US and Evaluation of Factors that Influence Them: An Analysis of 27,825 Patient Evaluations. Radiology 2002, 225, 165–175. [Google Scholar] [CrossRef]
- Sehgal, C.M.; Cary, T.W.; Kangas, S.A.; Weinstein, S.P.; Schultz, S.M.; Arger, P.H.; Conant, E.F. Computer-based margin analysis of breast sonography for differentiating malignant and benign masses. J. Ultrasound Med. 2004, 23, 1201–1209. [Google Scholar] [CrossRef]
- Venkatesh, S.S.; Levenback, B.J.; Sultan, L.R.; Bouzghar, G.; Sehgal, C.M. Going beyond a First Reader: A Machine Learning Methodology for Optimizing Cost and Performance in Breast Ultrasound Diagnosis. Ultrasound Med. Biol. 2015, 41, 3148–3162. [Google Scholar] [CrossRef]
- Niu, J.; Ma, J.; Guan, X.; Zhao, X.; Li, P.; Zhang, M. Correlation Between Doppler Ultrasound Blood Flow Parameters and Angiogenesis and Proliferation Activity in Breast Cancer. Med. Sci. Monit. 2019, 25, 7035–7041. [Google Scholar] [CrossRef]
- Yang, W.; Dempsey, P.J. Diagnostic Breast Ultrasound: Current Status and Future Directions. Ultrasound Clin. 2009, 45, 845–861. [Google Scholar] [CrossRef]
- He, T.; Qi, F.; Jia, L.; Wang, S.; Wang, C.; Song, N.; Fu, Y.; Li, L.; Luo, Y. Tumor cell-secreted angiogenin induces angiogenic activity of endothelial cells by suppressing miR-542-3p. Cancer Lett. 2015, 368, 115–125. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Sultan, L.R.; Tian, J.; Cary, T.W.; Sehgal, C.M. Machine learning for diagnostic ultrasound of triple-negative breast cancer. Breast Cancer Res. Treat. 2019, 173, 365–373. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, T.; Kaoku, S.; Yamaguchi, T.; Izumori, A.; Konno, S.; Okuno, T.; Tsunoda, H.; Ban, K.; Hirokaga, K.; Sawada, T.; et al. Multicenter Prospective Study of Color Doppler Ultrasound for Breast Masses: Utility of Our Color Doppler Method. Ultrasound Med. Biol. 2019, 45, 1367–1379. [Google Scholar] [CrossRef]
- Cho, N.; Jang, M.; Lyou, C.Y.; Park, J.S.; Choi, H.Y.; Moon, W.K. Distinguishing benign from malignant masses at breast US: Combined US elastography and color Doppler US-Influence on radiologist accuracy. Radiology 2012, 262, 80–90. [Google Scholar] [CrossRef]
- Li, L.; Zhou, X.; Zhao, X.; Hao, S.; Yao, J.; Zhong, W.; Zhi, H. B-Mode Ultrasound Combined with Color Doppler and Strain Elastography in the Diagnosis of Non-mass Breast Lesions: A Prospective Study. Ultrasound Med. Biol. 2017, 43, 2582–2590. [Google Scholar] [CrossRef]
- Svensson, W.E.; Pandian, A.J.; Hashimoto, H. The use of breast ultrasound color Doppler vascular pattern morphology improves diagnostic sensitivity with minimal change in specificity. Ultraschall Med. 2010, 31, 466–474. [Google Scholar] [CrossRef]
- Sehgal, C.M.; Weinstein, S.P.; Arger, P.H.; Conant, E.F. A review of breast ultrasound. J. Mammary Gland. Biol. Neoplasia 2006, 11, 113–123. [Google Scholar] [CrossRef]
- Song, J.H.; Venkatesh, S.S.; Conant, E.A.; Arger, P.H.; Sehgal, C.M. Comparative analysis of logistic regression and artificial neural network for computer-aided diagnosis of breast masses. Acad. Radiol. 2005, 12, 487–495. [Google Scholar] [CrossRef]
- Cary, T.W.; Cwanger, A.; Venkatesh, S.S.; Conant, E.F.; Sehgal, C.M. Comparison of Naïve Bayes and logistic regression for computer-aided diagnosis of breast masses using ultrasound imaging. In Proceedings of the SPIE Medical Imaging 2012: Ultrasonic Imaging, Tomography, and Therapy, San Diego, CA, USA, 4–9 February 2012. [Google Scholar] [CrossRef]
- Freund, Y.; Schapire, R.E. A Decision-Theoretic Generalization of On-Line Learning and an Application to Boosting. J. Comput. Syst. Sci. 1997, 55, 119–139. [Google Scholar] [CrossRef]
- Sehgal, C.M.; Cary, T.W.; Cwanger, A.; Levenback, B.J.; Venkatesh, S.S. Combined Naïve Bayes and logistic regression for quantitative breast sonography. In Proceedings of the 2012 IEEE International Ultrasonics Symposium, Dresden, Germany, 7–10 October 2012; pp. 1686–1689. [Google Scholar] [CrossRef]
- Sedgwick, E. The Breast Ultrasound Lexicon: Breast Imaging Reporting and Data System (BI-RADS). Semin. Roentgenol. 2011, 46, 245–251. [Google Scholar] [CrossRef] [PubMed]
- Chawla, N.V.; Bowyer, K.W.; Hall, L.O.; Kegelmeyer, W.P. SMOTE: Synthetic minority over-sampling technique. J. Artif. Intell. Res. 2002, 16, 321–357. [Google Scholar] [CrossRef]
- Stavros, A.T.; Thickman, D.; Rapp, C.L.; Dennis, M.A.; Parker, S.H.; Sisney, G.A. Solid breast nodules: Use of sonography to distinguish between benign and malignant lesions. Radiology 1995, 196, 123–134. [Google Scholar] [CrossRef]
- D’Souza, J.C.; Sultan, L.R.; Hunt, S.J.; Gade, T.P.; Karmacharya, M.B.; Schultz, S.M.; Brice, A.K.; Wood, A.; Sehgal, C.M. Microbubble-enhanced ultrasound for the antivascular treatment and monitoring of hepatocellular carcinoma. Nanotheranostics 2019, 3, 331–341. [Google Scholar] [CrossRef] [PubMed]
- Frank, E.; Hall, M.A.; Witten, I. H; The WEKA Workbench. Data Mining: Practical Machine Learning Tools and Techniques, 4th ed.; Morgan Kaufmann: Burlington, MA, USA, 2016; Volume 31, pp. 76–77. [Google Scholar] [CrossRef]
- Hall, M.; Frank, E.; Holmes, G.; Pfahringer, B.; Reutemann, P.; Witten, I.H. The WEKA data mining software: An update. ACM SIGKDD Explor. Newsl. 2009, 11, 10–18. [Google Scholar] [CrossRef]
- Bantis, L.E.; Nakas, C.T.; Reiser, B. Construction of confidence intervals for the maximum of the Youden index and the corresponding cutoff point of a continuous biomarker. Biom. J. 2019, 61, 138. [Google Scholar] [CrossRef]
- Perneger, T.V. What’s wrong with Bonferroni adjustments. Br. Med. J. 1998, 316, 1236–1238. [Google Scholar] [CrossRef]
- Patyk, M.; Silicki, J.; Mazur, R.; Kręcichwost, R.; Sokołowska-Dąbek, D.; Zaleska-Dorobisz, U. Radiomics—The value of the numbers in present and future radiology. Pol. J. Radiol. 2018. [Google Scholar] [CrossRef]
- Valdora, F.; Houssami, N.; Rossi, F.; Calabrese, M.; Tagliafico, A.S. Rapid review: Radiomics and breast cancer. Breast Cancer Res. Treat. 2018, 83, e171. [Google Scholar] [CrossRef]
- Losurdo, L.; Fanizzi, A.; Basile, T.M.A.; Bellotti, R.; Bottigli, U.; Dentamaro, R.; Didonna, V.; Lorusso, V.; Massafra, R.; Tamborra, P.; et al. Radiomics analysis on contrast-enhanced spectral mammography images for breast cancer diagnosis: A pilot study. Entropy 2019, 21, 1110. [Google Scholar] [CrossRef]
- Tagliafico, A.S.; Mariscotti, G.; Valdora, F.; Durando, M.; Nori, J.; La Forgia, D.; Rosenberg, I.; Caumo, F.; Gandolfo, N.; Sormani, M.P.; et al. A prospective comparative trial of adjunct screening with tomosynthesis or ultrasound in women with mammography-negative dense breasts (ASTOUND-2). Eur. J. Cancer 2018, 104, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Tagliafico, A.S.; Valdora, F.; Mariscotti, G.; Durando, M.; Nori, J.; La Forgia, D.; Rosenberg, I.; Caumo, F.; Gandolfo, N.; Houssami, N.; et al. An exploratory radiomics analysis on digital breast tomosynthesis in women with mammographically negative dense breasts. Breast 2018, 40, 92–96. [Google Scholar] [CrossRef] [PubMed]
- Dellaportas, D.; Koureas, A.; Contis, J.; Lykoudis, P.M.; Vraka, I.; Psychogios, D.; Kondi-Pafiti, A.; Voros, D.K. Contrast-Enhanced Color Doppler Ultrasonography for Preoperative Evaluation of Sentinel Lymph Node in Breast Cancer Patients. Breast Care 2015, 10, 331–335. [Google Scholar] [CrossRef] [PubMed]
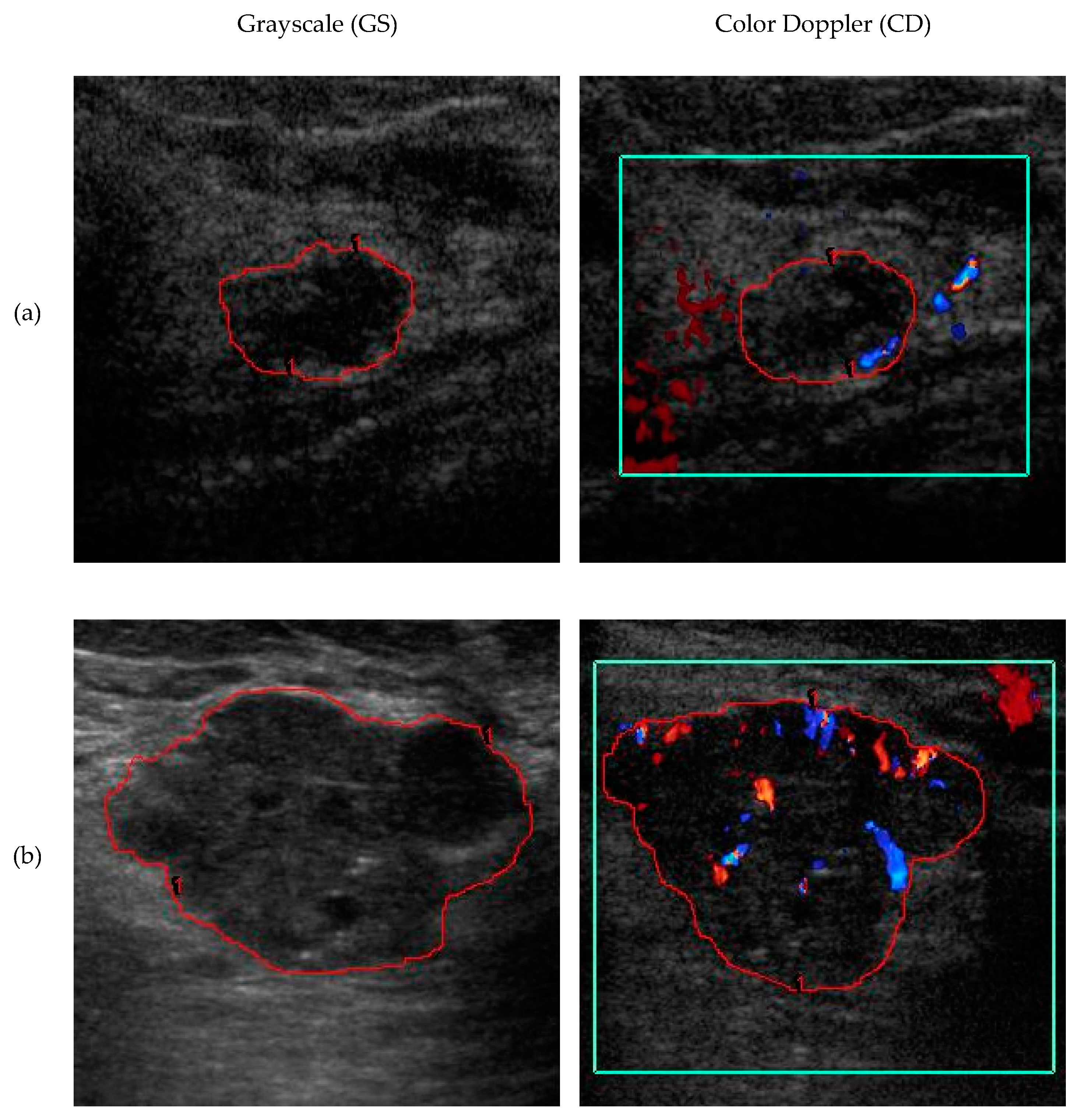

| Feature | Benign | Malignant | U-Test p Value | Effect Size |
|---|---|---|---|---|
| Age | 46.2 ± 13.3 | 57.1 ± 11.5 | <0.0001 | 5.78+ |
| Angular interior (AVI) | 4.18 ± 1.66 | 4.24 ± 1.84 | 0.4441 | 2.97 |
| Angular margin (AVM) | 3.35 ± 1.13 | 3.60 ± 1.46 | 0.2261 | 10.3+ |
| Bright difference (BD) | 17.2 ± 7.2 | 11.0 ± 6.2 | <0.0001 | 12.00 |
| Margin sharpness (MS) | 82.8 ± 8.1 | 74.0 ± 10.2 | <0.0001 | 1.97 |
| Axis ratio (AR) | 1.74 ± 0.46 | 1.60 ± 0.37 | 0.0308 | 5.89+ |
| Depth–width ratio (DWR) | 0.71 ± 0.19 | 0.86 ± 0.27 | 0.0002 | 2.38+ |
| Radius variation (RV) | 0.21 ± 0.07 | 0.19 ± 0.07 | 0.1261 | 2.36 |
| Skeleton (ENS) | 0.14 ± 0.02 | 0.15 ± 0.03 | 0.0113 | 1.16+ |
| Tortuosity (T) | 1.16 ± 0.07 | 1.19 ± 0.12 | 0.0318 | 1.54+ |
| Vascular velocity (VI) | 0.42 ± 0.58 | 0.85 ± 0.64 | <0.0001 | 2.63+ |
| Vascular area (VFA) | 0.82 ± 1.51 | 2.59 ± 3.40 | <0.0001 | 2.12+ |
| Feature Set | AUC ± sErr | YI | Se at YI | Sp at YI | Sp at Se98 | Sp at Se95 |
|---|---|---|---|---|---|---|
| BI-RADS w/SMOTE | 0.664 ± 0.052 0.770 ± 0.051 | 0.515 0.526 | 54.7 54.7 | 96.8 97.9 | 0.0 0.0 | 0.0 0.0 |
| BI-RADS, Age w/SMOTE | 0.864 ± 0.030 0.865 ± 0.031 | 0.608 0.607 | 76.6 85.9 | 84.2 74.7 | 27.5 9.5 | 42.1 43.2 |
| BI-RADS, Age, CD w/SMOTE | 0.891 ± 0.026 0.901 ± 0.025 | 0.661 0.661 | 73.4 73.4 | 92.6 92.6 | 45.1 26.3 | 47.4 60.0 |
| BI-RADS, Age, GSz w/SMOTE | 0.900 ± 0.024 0.925 ± 0.022 | 0.665 0.754 | 81.2 85.9 | 85.2 89.5 | 48.4 32.6 | 59.0 67.4 |
| BI-RADS, Age, CD, GS w/SMOTE | 0.934 ± 0.018 0.958 ± 0.013 | 0.780 0.811 | 93.8 96.9 | 84.2 84.2 | 44.2 76.8 | 82.1 85.3 |
| Feature Set 1 | Feature Set 2 | p Value |
|---|---|---|
| BI-RADSUS, Age | BI-RADSUS, Age, GS | 0.0086 (SMOTE) 0.0116 |
| BI-RADSUS, Age | BI-RADSUS, Age, CD | 0.0320 (SMOTE) 0.1355 |
| BI-RADSUS, Age | BI-RADSUS, Age, GS, CD | 0.0003 (SMOTE) 0.0006 |
| BI-RADSUS, Age, GS | BI-RADSUS, Age, CD | 0.7595 (SMOTE) 0.3746 |
| BI-RADSUS, Age, CD | BI-RADSUS, Age, GS, CD | 0.0104 (SMOTE) 0.0050 |
| BI-RADSUS, Age, GS | BI-RADSUS, Age, GS, CD | 0.0161 (SMOTE) 0.0352 |
| Feature Set | AUC ± sErr | 95% CI | YI | Se at YI | Sp at YI |
|---|---|---|---|---|---|
| CD, 20% | 0.986 ± 0.007 | 0.947–0.999 | 0.913 | 100 | 91.3 |
| no CD, 20% | 0.944 ± 0.022 | 0.889–0.977 | 0.817 | 0091.7 | 90.0 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moustafa, A.F.; Cary, T.W.; Sultan, L.R.; Schultz, S.M.; Conant, E.F.; Venkatesh, S.S.; Sehgal, C.M. Color Doppler Ultrasound Improves Machine Learning Diagnosis of Breast Cancer. Diagnostics 2020, 10, 631. https://doi.org/10.3390/diagnostics10090631
Moustafa AF, Cary TW, Sultan LR, Schultz SM, Conant EF, Venkatesh SS, Sehgal CM. Color Doppler Ultrasound Improves Machine Learning Diagnosis of Breast Cancer. Diagnostics. 2020; 10(9):631. https://doi.org/10.3390/diagnostics10090631
Chicago/Turabian StyleMoustafa, Afaf F., Theodore W. Cary, Laith R. Sultan, Susan M. Schultz, Emily F. Conant, Santosh S. Venkatesh, and Chandra M. Sehgal. 2020. "Color Doppler Ultrasound Improves Machine Learning Diagnosis of Breast Cancer" Diagnostics 10, no. 9: 631. https://doi.org/10.3390/diagnostics10090631
APA StyleMoustafa, A. F., Cary, T. W., Sultan, L. R., Schultz, S. M., Conant, E. F., Venkatesh, S. S., & Sehgal, C. M. (2020). Color Doppler Ultrasound Improves Machine Learning Diagnosis of Breast Cancer. Diagnostics, 10(9), 631. https://doi.org/10.3390/diagnostics10090631