Hyperpolarized 129Xe Time-of-Flight MR Imaging of Perfusion and Brain Function
Abstract
1. Introduction
2. Materials and Methods
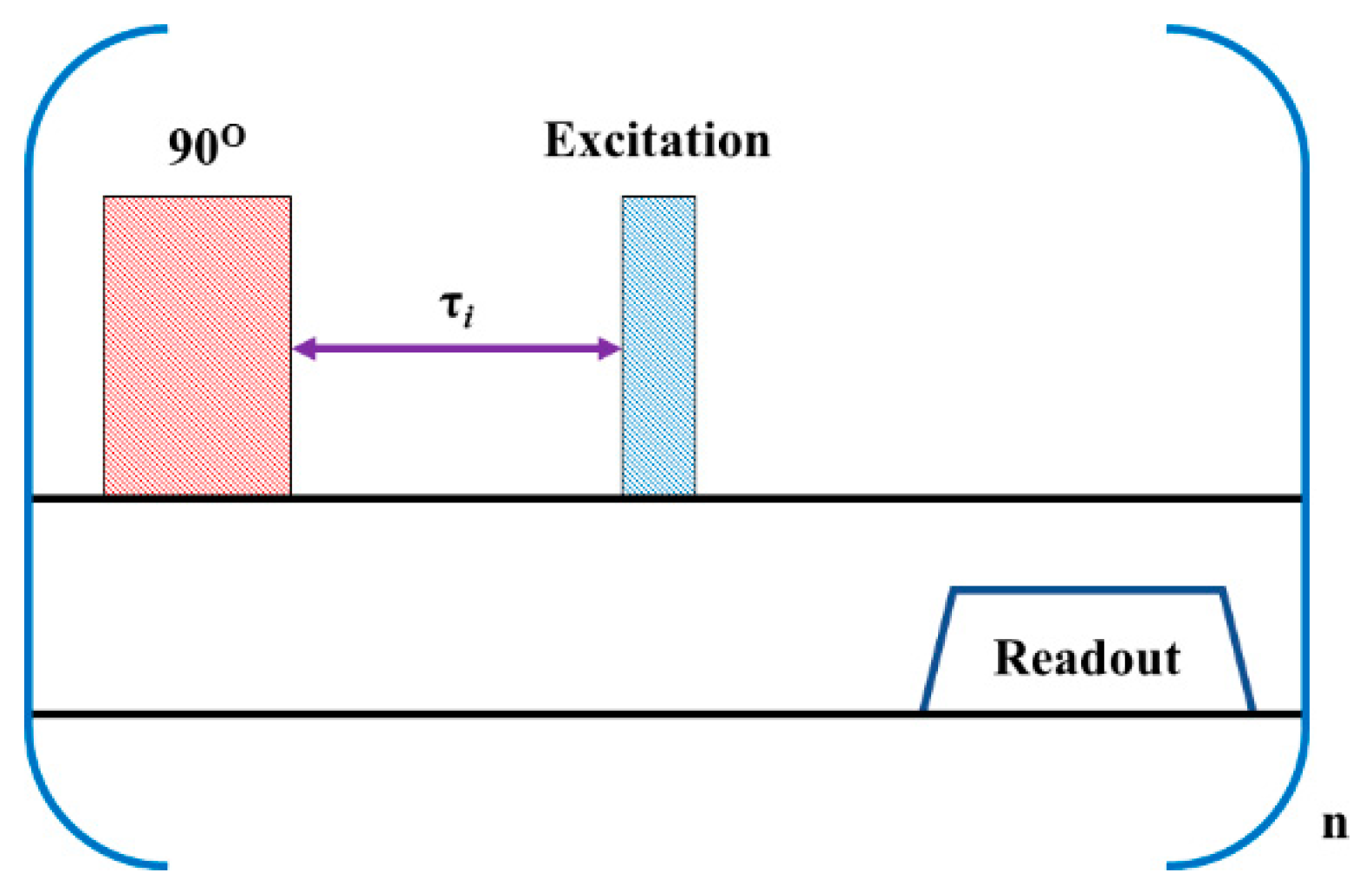
2.1. Pulse Sequence Design
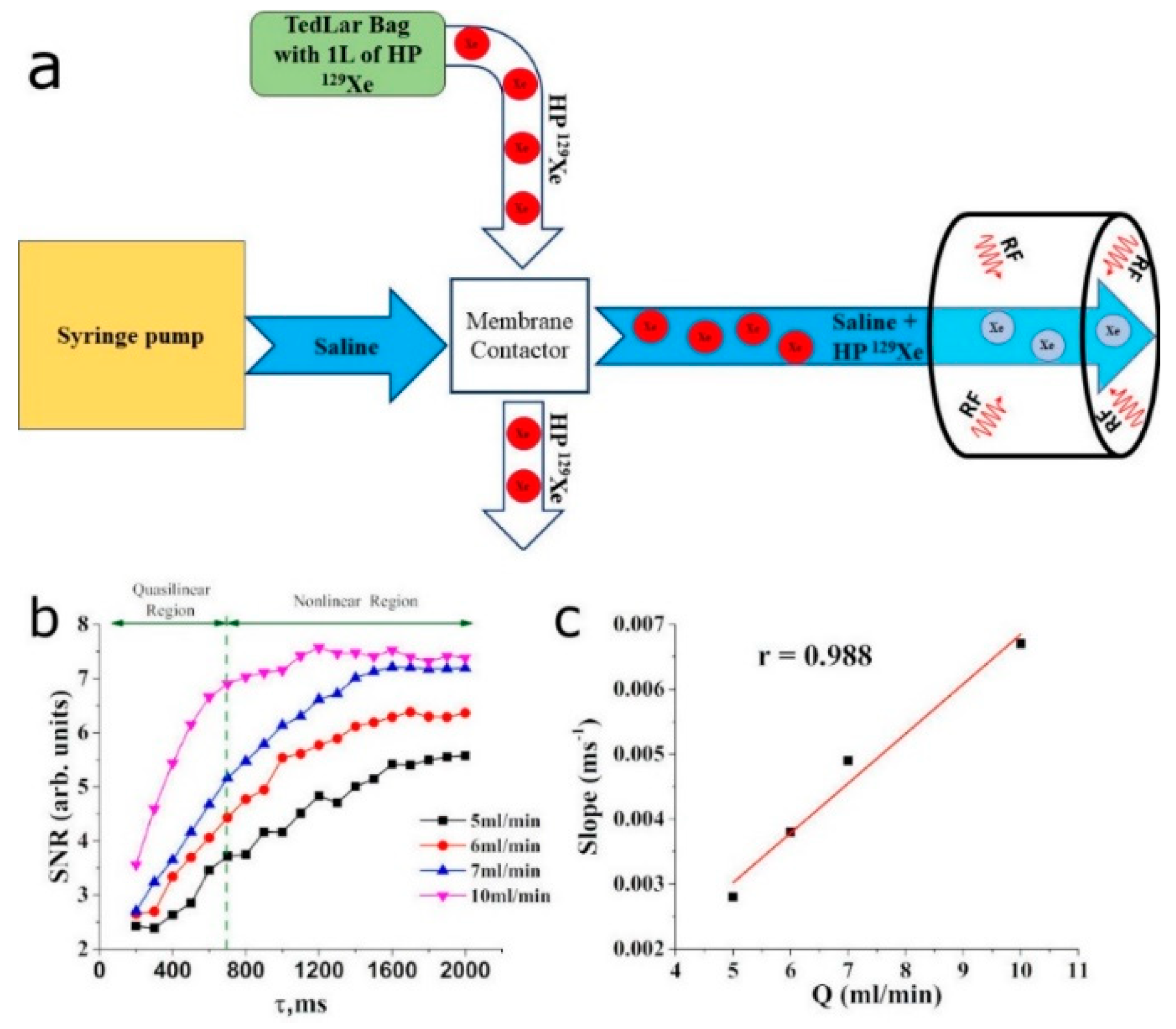
2.2. In Vitro Flow Measurements
2.3. In Vivo Cerebral Perfusion Measurements in Healthy Volunteers
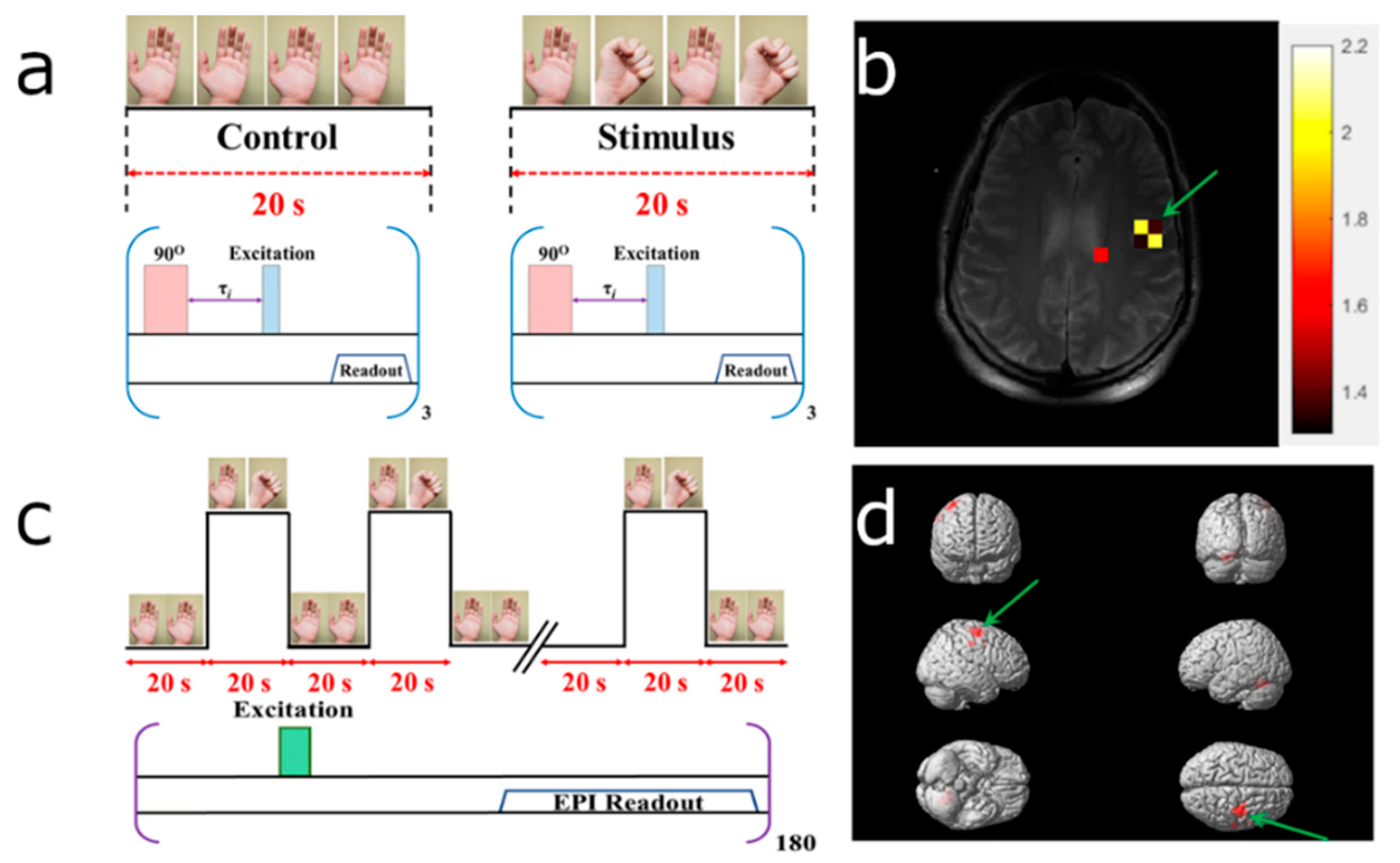
2.3.1. 1H Magnetic Resonance Imaging
2.3.2. 129Xe Perfusion Mapping Using Time of Flight (TOF)
2.3.3. 129Xe Perfusion Mapping Image Processing
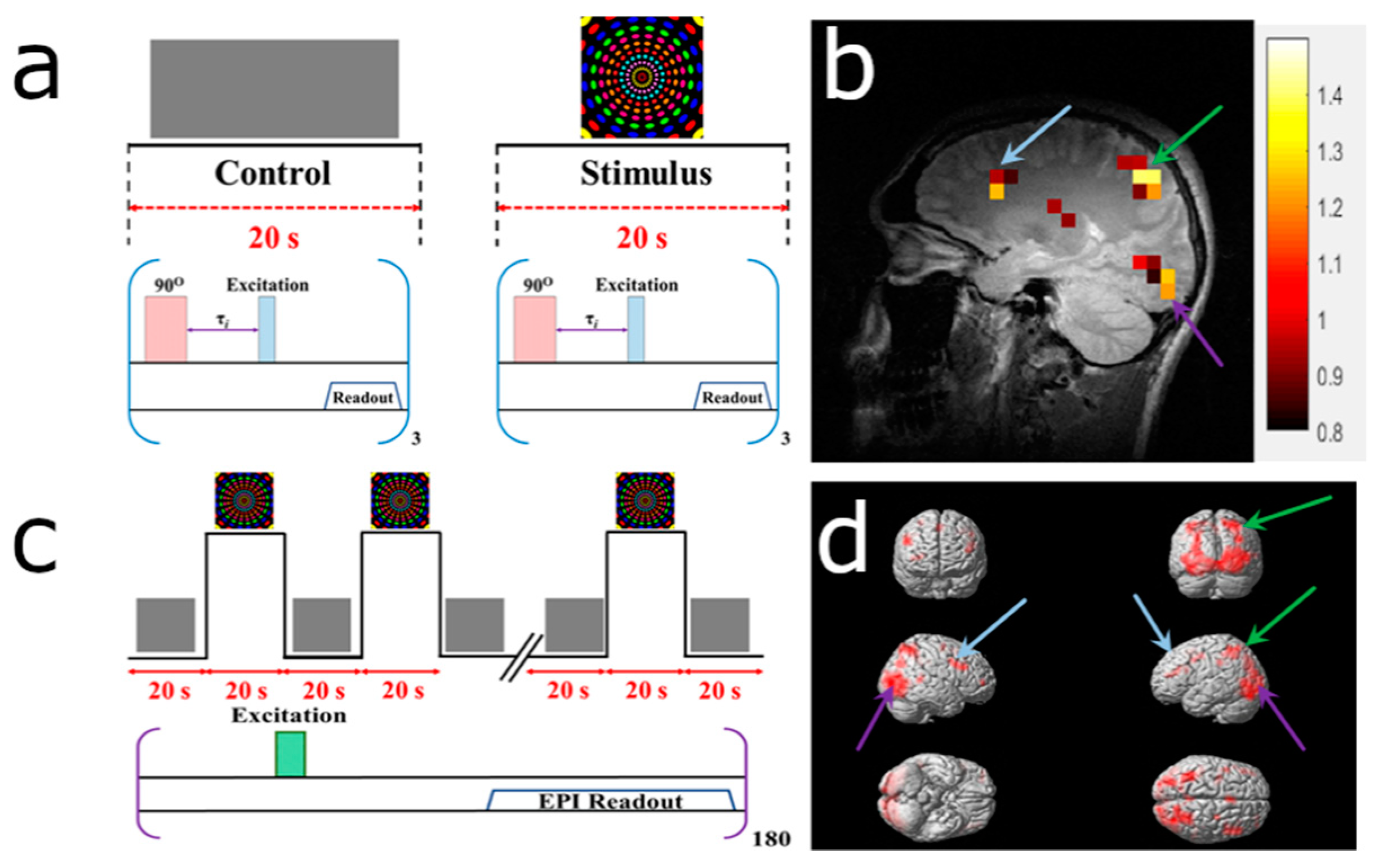
2.3.4. BOLD fMRI Image Acquisition
2.3.5. BOLD fMRI Image Processing
3. Results
3.1. In Vitro Evaluation of HP 129Xe TOF Pulse Sequence
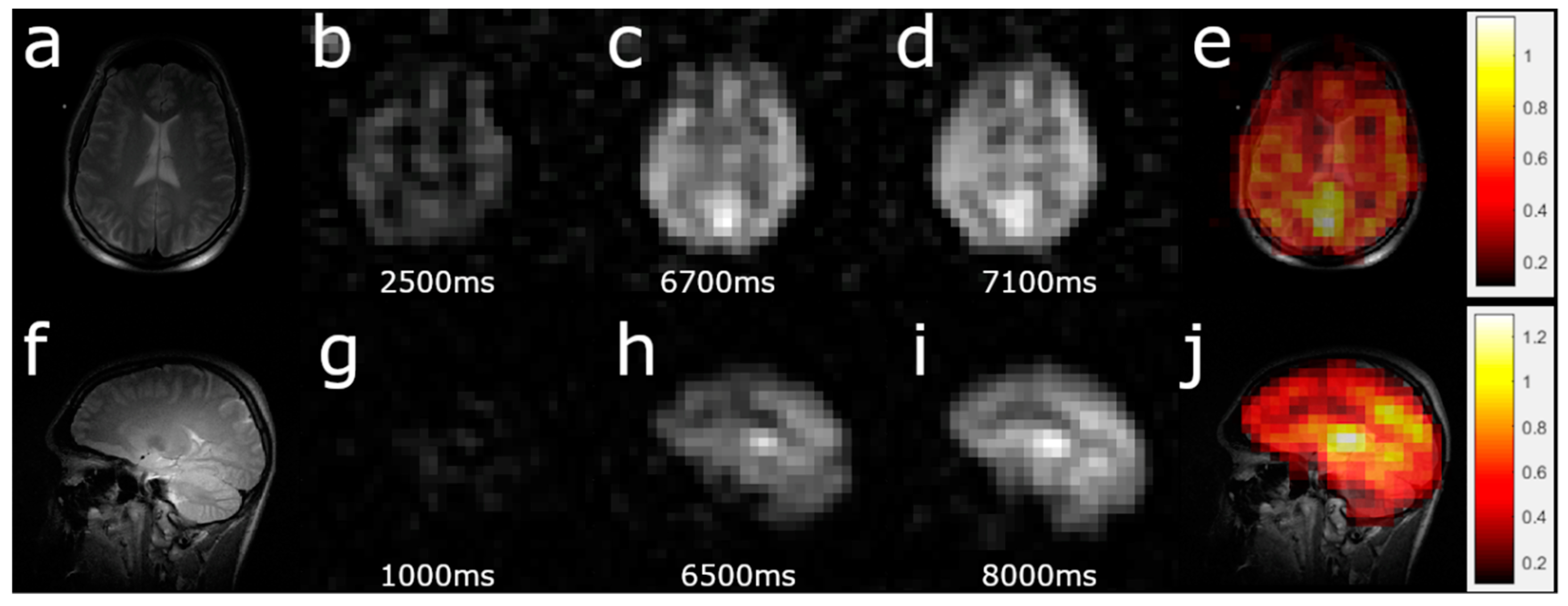
3.2. HP 129Xe TOF Cerebral Perfusion Imaging
3.3. HP 129Xe Hemodynamic Response Detection
4. Discussion
5. Patents
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Petersen, E.T.; Zimine, I.; Ho, Y.C.L.; Golay, X. Non-invasive measurement of perfusion: A critical review of arterial spin labelling techniques. Br. J. Radiol. 2006, 79, 688–701. [Google Scholar] [CrossRef] [PubMed]
- Lubbers, M.; Coenen, A.; Kofflard, M.; Bruning, T.; Kietselaer, B.; Galema, T.; Kock, M.; Niezen, A.; Das, M.; van Gent, M.; et al. Comprehensive Cardiac CT With Myocardial Perfusion Imaging Versus Functional Testing in Suspected Coronary Artery Disease: The Multicenter, Randomized CRESCENT-II Trial. JACC Cardiovasc. Imaging 2018, 11, 1625–1636. [Google Scholar] [CrossRef] [PubMed]
- Mori, H.; Isobe, S.; Suzuki, S.; Unno, K.; Morimoto, R.; Kano, N.; Okumura, T.; Yasuda, Y.; Kato, K.; Murohara, T. Prognostic value of left ventricular dyssynchrony evaluated by gated myocardial perfusion imaging in patients with chronic kidney disease and normal perfusion defect scores. J. Nucl. Cardiol. 2019, 26, 288–297. [Google Scholar] [CrossRef] [PubMed]
- Gillis, K.A.; McComb, C.; Patel, R.K.; Stevens, K.K.; Schneider, M.P.; Radjenovic, A.; Morris, S.T.W.; Roditi, G.H.; Delles, C.; Mark, P.B. Non-Contrast Renal Magnetic Resonance Imaging to Assess Perfusion and Corticomedullary Differentiation in Health and Chronic Kidney Disease. Nephron 2016, 133, 183–192. [Google Scholar] [CrossRef] [PubMed]
- Greenberg, D.A.; Jin, K. From angiogenesis to neuropathology. Nature 2005, 438, 954–959. [Google Scholar] [CrossRef] [PubMed]
- Rao, M.R.; Norquay, G.; Stewart, N.J.; Hoggard, N.; Griffiths, P.D.; Wild, J.M. Assessment of brain perfusion using hyperpolarized 129Xe MRI in a subject with established stroke. J. Magn. Reson. Imaging 2019, 50, 1002–1004. [Google Scholar] [CrossRef]
- Hu, W.T.; Wang, Z.; Lee, M.-Y.; Trojanowski, J.Q.; Detre, J.A.; Grossman, M. Distinct cerebral perfusion patterns in FTLD and AD. Neurology 2010, 75, 881–888. [Google Scholar] [CrossRef]
- Ito, H.; Inoue, K.; Goto, R.; Kinomura, S.; Taki, Y.; Okada, K.; Sato, K.; Sato, T.; Kanno, I.; Fukuda, H. Database of normal human cerebral blood flow measured by SPECT: I. Comparison between 1-123-IMP, Tc-99m-HMPAO, and Tc-99m-ECD as referred with O-15 labeled water PET and voxel-based morphometry. Ann. Nucl. Med. 2006, 20, 131–138. [Google Scholar] [CrossRef]
- Okazawa, H.; Higashino, Y.; Tsujikawa, T.; Arishima, H.; Mori, T.; Kiyono, Y.; Kimura, H.; Kikuta, K. ichiro Noninvasive method for measurement of cerebral blood flow using O-15 water PET/MRI with ASL correlation. Eur. J. Radiol. 2018, 105, 102–109. [Google Scholar] [CrossRef]
- Kamath, A.; Smith, W.S.; Powers, W.J.; Cianfoni, A.; Chien, J.D.; Videen, T.; Lawton, M.T.; Finley, B.; Dillon, W.P.; Wintermark, M. Perfusion CT compared to H215O/O15O PET in patients with chronic cervical carotid artery occlusion. Neuroradiology 2008, 50, 745–751. [Google Scholar] [CrossRef]
- Yonas, H.; Darby, J.M.; Marks, E.C.; Durham, S.R.; Maxwell, C. CBF Measured by Xe-CT: Approach to Analysis and Normal Values. J. Cereb. Blood Flow Metab. 1991, 11, 716–725. [Google Scholar] [CrossRef] [PubMed]
- Johnson, U.; Engquist, H.; Howells, T.; Nilsson, P.; Ronne-Engström, E.; Lewén, A.; Rostami, E.; Enblad, P. Bedside Xenon-CT Shows Lower CBF in SAH Patients with Impaired CBF Pressure Autoregulation as Defined by Pressure Reactivity Index (PRx). Neurocrit. Care 2016, 25, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, T.; Ogasawara, K.; Kobayashi, T.; Komoribayashi, N.; Endo, H.; Inoue, T.; Kuzu, Y.; Nishimoto, H.; Terasaki, K.; Ogawa, A. Measurement of cerebral hemodynamics with perfusion-weighted MR imaging: Comparison with pre- and post-acetazolamide 133Xe-SPECT in occlusive carotid disease. AJNR Am. J. Neuroradiol. 2001, 22, 248–254. [Google Scholar]
- Golay, X.; Petersen, E.T. Arterial Spin Labeling: Benefits and Pitfalls of High Magnetic Field. Neuroimaging Clin. N. Am. 2006, 16, 259–268. [Google Scholar] [CrossRef] [PubMed]
- Nery, F.; Gordon, I.; Thomas, D. Non-Invasive Renal Perfusion Imaging Using Arterial Spin Labeling MRI: Challenges and Opportunities. Diagnostics 2018, 8, 2. [Google Scholar] [CrossRef] [PubMed]
- Vidorreta, M.; Wang, Z.; Chang, Y.V.; Wolk, D.A.; Fernández-Seara, M.A.; Detre, J.A. Whole-brain background-suppressed pCASL MRI with 1D-accelerated 3D RARE Stack-Of-Spirals readout. PLoS ONE 2017, 12, e0183762. [Google Scholar] [CrossRef]
- Kilroy, E.; Apostolova, L.; Liu, C.; Yan, L.; Ringman, J.; Wang, D.J.J. Reliability of two-dimensional and three-dimensional pseudo-continuous arterial spin labeling perfusion MRI in elderly populations: Comparison with 15o-water positron emission tomography. J. Magn. Reson. Imaging 2014, 39, 931–939. [Google Scholar] [CrossRef]
- Harteveld, A.A.; de Boer, A.; Franklin, S.L.; Leiner, T.; van Stralen, M.; Bos, C. Comparison of multi-delay FAIR and pCASL labeling approaches for renal perfusion quantification at 3T MRI. Magn. Reson. Mater. Phys. Biol. Med. 2020, 33, 81–94. [Google Scholar] [CrossRef]
- Ni, L.; Li, J.; Li, W.; Zhou, F.; Wang, F.; Schwarz, C.G.; Liu, R.; Zhao, H.; Wu, W.; Zhang, X.; et al. The value of resting-state functional MRI in subacute ischemic stroke: Comparison with dynamic susceptibility contrast-enhanced perfusion MRI. Sci. Rep. 2017, 7, 1–8. [Google Scholar] [CrossRef]
- Hashido, T.; Saito, S.; Ishida, T. A radiomics-based comparative study on arterial spin labeling and dynamic susceptibility contrast perfusion-weighted imaging in gliomas. Sci. Rep. 2020, 10, 6121. [Google Scholar] [CrossRef]
- Sourbron, S.; Ingrisch, M.; Siefert, A.; Reiser, M.; Herrmann, K. Quantification of cerebral blood flow, cerebral blood volume, and blood-brain-barrier leakage with DCE-MRI. Magn. Reson. Med. 2009, 62, 205–217. [Google Scholar] [CrossRef] [PubMed]
- Yerram, P.; Saab, G.; Karuparthi, P.R.; Hayden, M.R.; Khanna, R. Nephrogenic systemic fibrosis: A mysterious disease in patients with renal failure—Rrole of gadolinium-based contrast media in causation and the beneficial effect of intravenous sodium thiosulfate. Clin. J. Am. Soc. Nephrol. 2007, 2, 258–263. [Google Scholar] [CrossRef] [PubMed]
- Perazella, M.A. Nephrogenic systemic fibrosis, kidney disease, and gadolinium: Is there a link? Clin. J. Am. Soc. Nephrol. 2007, 2, 200–202. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Madhankumar, A.B.; Miller, P.A.; Duck, K.A.; Hafenstein, S.; Rizk, E.; Slagle-Webb, B.; Sheehan, J.M.; Connor, J.R.; Yang, Q.X. MRI contrast agent for targeting glioma: Interleukin-13 labeled liposome encapsulating gadolinium-DTPA. Neuro. Oncol. 2016, 18, 691–699. [Google Scholar] [CrossRef] [PubMed]
- Le Mignon, M.M.; Chambon, C.; Warrington, S.; Davies, R.; Bonnemain, B. Gd-DOTA. Pharmacokinetics and tolerability after intravenous injection into healthy volunteers. Investig. Radiol. 1990, 25, 933–937. [Google Scholar] [CrossRef]
- Runge, V.M.; Clanton, J.A.; Price, A.C.; Wehr, C.J.; Herzer, W.A.; Leon Partain, C.; James, E. The Use of Gd dtpa as a Perfusion Agent and Marker of Blood-Brain Barrier Disruption. Mqnnrrrc Krsonance Imaging 1985, 3, 43–55. [Google Scholar] [CrossRef]
- Albert, M.S.; Cates, G.D.; Driehuys, B.; Happer, W.; Saam, B.; Springer, C.S.; Wishnia, A. Biological Magnetic Resonance Imaging Using Laser-Polarized 129Xe. Nature 1994, 370, 199–201. [Google Scholar] [CrossRef]
- Kruger, S.J.; Nagle, S.K.; Couch, M.J.; Ohno, Y.; Albert, M.; Fain, S.B. Functional imaging of the lungs with gas agents. J. Magn. Reson. Imaging 2016, 43, 295–315. [Google Scholar] [CrossRef]
- Norquay, G.; Collier, G.J.; Rao, M.; Stewart, N.J.; Wild, J.M. Xe 129 -Rb Spin-Exchange Optical Pumping with High Photon Efficiency. Phys. Rev. Lett. 2018, 121, 153201. [Google Scholar] [CrossRef]
- Skinner, J.G.; Ranta, K.; Whiting, N.; Coffey, A.M.; Nikolaou, P.; Rosen, M.S.; Chekmenev, E.Y.; Morris, P.G.; Barlow, M.J.; Goodson, B.M. High Xe density, high photon flux, stopped-flow spin-exchange optical pumping: Simulations versus experiments. J. Magn. Reson. 2020, 312, 106686. [Google Scholar] [CrossRef]
- Ruset, I.C.; Ketel, S.; Hersman, F.W. Optical pumping system design for large production of hyperpolarized 129Xe. Phys. Rev. Lett. 2006, 96, 053002. [Google Scholar] [CrossRef] [PubMed]
- Rao, M.; Stewart, N.J.; Norquay, G.; Griffiths, P.D.; Wild, J.M. High Resolution Spectroscopy and Chemical Shift Imaging of Hyperpolarized 129Xe Dissolved in the Human Brain In Vivo at 1.5 Tesla. Magn. Reson. Med. 2016, 75, 2227–2234. [Google Scholar] [CrossRef] [PubMed]
- Rao, M.R.; Stewart, N.J.; Griffiths, P.D.; Norquay, G.; Wild, J.M. Imaging Human Brain Perfusion with Inhaled Hyperpolarized 129Xe MR Imaging. Radiology 2017, 286, 659–665. [Google Scholar] [CrossRef] [PubMed]
- Hane, F.T.; Li, T.; Plata, J.-A.; Hassan, A.; Granberg, K.; Albert, M.S. Inhaled Xenon Washout as a Biomarker of Alzheimer’s Disease. Diagnostics 2018, 8, 41. [Google Scholar] [CrossRef]
- Chacon-Caldera, J.; Maunder, A.; Rao, M.; Norquay, G.; Rodgers, O.I.; Clemence, M.; Puddu, C.; Schad, L.R.; Wild, J.M. Dissolved hyperpolarized xenon-129 MRI in human kidneys. Magn. Reson. Med. 2019. [Google Scholar] [CrossRef]
- Chang, Y.V. MOXE: A model of gas exchange for hyperpolarized 129Xe magnetic resonance of the lung. Magn. Reson. Med. 2013, 69, 884–890. [Google Scholar] [CrossRef]
- Fox, M.S.; Ouriadov, A.; Thind, K.; Hegarty, E.; Wong, E.; Hope, A.; Santyr, G.E. Detection of radiation induced lung injury in rats using dynamic hyperpolarized 129Xe magnetic resonance spectroscopy. Med. Phys. 2014, 41, 72302. [Google Scholar] [CrossRef]
- Driehuys, B.; Cofer, G.P.; Pollaro, J.; Mackel, J.B.; Hedlund, L.W.; Johnson, G.A. Imaging alveolar-capillary gas transfer using hyperpolarized 129Xe MRI. Proc. Natl. Acad. Sci. USA 2006, 103, 18278–18283. [Google Scholar] [CrossRef]
- Hare, H.V.; Blockley, N.P.; Gardener, A.G.; Clare, S.; Bulte, D.P. Investigating the field-dependence of the Davis model: Calibrated fMRI at 1.5, 3 and 7T. Neuroimage 2015, 112, 189–196. [Google Scholar] [CrossRef]
- Ashburner, J.; Barnes, G.; Chen, C.C.; Daunizeau, J.; Flandin, G.; Friston, K.; Jafarian, A.; Kiebel, S.; Litvak, V.; Moran, R.; et al. SPM (Statistical Parametric Mapping); Wellcome Trust Centre for Neuroimaging: London, UK, 2014. [Google Scholar]
- Kilian, W.; Seifert, F.; Rinneberg, H. Dynamic NMR Spectroscopy of Hyperpolarized 129Xe in Human Brain Analyzed by an Uptake Model. Magn. Reson. Med. 2004, 51, 843–847. [Google Scholar] [CrossRef]
- Zhou, X.; Mazzanti, M.L.; Chen, J.J.; Tzeng, Y.S.; Mansour, J.K.; Gereige, J.D.; Venkatesh, A.K.; Sun, Y.; Mulkern, R.V.; Albert, M.S. Reinvestigating hyperpolarized 129Xe longitudinal relaxation time in the rat brain with noise considerations. NMR Biomed. 2008, 21, 217–225. [Google Scholar] [CrossRef] [PubMed]
- Wolber, J.; Cherubini, A.; Leach, M.O.; Bifone, A. On the oxygenation-dependent 129 Xe T 1 in blood. NMR Biomed. 2000, 13, 234–237. [Google Scholar] [CrossRef]
- Albert, M.S.; Kacher, D.F.; Balamore, D.; Venkatesh, A.K.; Jolesz, F.A. T 1 of 129 Xe in Blood and the Role of Oxygenation. J. Magn. Reson. 1999, 140, 264–273. [Google Scholar] [CrossRef] [PubMed]
- Norquay, G.; Leung, G.; Stewart, N.J.; Tozer, G.M.; Wolber, J.; Wild, J.M. Relaxation and exchange dynamics of hyperpolarized 129Xe in human blood. Magn. Reson. Med. 2015, 74, 303–311. [Google Scholar] [CrossRef]
- Peled, S.; Jolesz, F.A.; Tseng, C.-H.; Nascimben, L.; Albert, M.S.; Walsworth, R.L. Determinants of tissue delivery for 129Xe magnetic resonance in humans. Magn. Reson. Med. 1996, 36, 340–344. [Google Scholar] [CrossRef] [PubMed]
- Neale, C.; Johnston, P.; Hughes, M.; Scholey, A. Functional Activation during the Rapid Visual Information Processing Task in a Middle Aged Cohort: An fMRI Study. PLoS ONE 2015, 10, e0138994. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Liu, Y.; Shen, H.; Zhou, Z.; Hu, D. Sustained Negative BOLD Response in Human fMRI Finger Tapping Task. PLoS ONE 2011, 6, e23839. [Google Scholar] [CrossRef]
- Turesky, T.K.; Olulade, O.A.; Luetje, M.M.; Eden, G.F. An fMRI study of finger tapping in children and adults. Hum. Brain Mapp. 2018, 39, 3203–3215. [Google Scholar] [CrossRef]
- Gountouna, V.E.; Job, D.E.; McIntosh, A.M.; Moorhead, T.W.J.; Lymer, G.K.L.; Whalley, H.C.; Hall, J.; Waiter, G.D.; Brennan, D.; McGonigle, D.J.; et al. Functional Magnetic Resonance Imaging (fMRI) reproducibility and variance components across visits and scanning sites with a finger tapping task. Neuroimage 2010, 49, 552–560. [Google Scholar] [CrossRef]
- Zhao, L.; Fielden, S.W.; Feng, X.; Wintermark, M.; Mugler, J.P.; Meyer, C.H. Rapid 3D dynamic arterial spin labeling with a sparse model-based image reconstruction. Neuroimage 2015, 121, 205–216. [Google Scholar] [CrossRef]
- Cui, Y.; Liang, X.; Gu, H.; Hu, Y.; Zhao, Z.; Yang, X.Y.; Qian, C.; Yang, Y.; Teng, G.J. Cerebral perfusion alterations in type 2 diabetes and its relation to insulin resistance and cognitive dysfunction. Brain Imaging Behav. 2017, 11, 1248–1257. [Google Scholar] [CrossRef] [PubMed]
- Dai, W.; Garcia, D.; De Bazelaire, C.; Alsop, D.C. Continuous flow-driven inversion for arterial spin labeling using pulsed radio frequency and gradient fields. Magn. Reson. Med. 2008, 60, 1488–1497. [Google Scholar] [CrossRef] [PubMed]
- Luttropp, H.H.; Thomasson, R.; Dahm, S.; Persson, J. Clinical experience with minimal flow xenon anesthesia. Acta Anaesihessiol. Scand. 1994, 38, 121–125. [Google Scholar] [CrossRef] [PubMed]
- Latchaw, R.E.; Yonas, H.; Pentheny, S.; Gur, D. Adverse Reactions to Xenon-enhanced CT Cerebral Blood Flow Determination. Radiology 1987, 163, 251–254. [Google Scholar] [CrossRef]
- Imai, H.; Matsumoto, H.; Miyakoshi, E.; Okumura, S.; Fujiwara, H.; Kimura, A. Regional fractional ventilation mapping in spontaneously breathing mice using hyperpolarized 129Xe MRI. NMR Biomed. 2015, 28, 24–29. [Google Scholar] [CrossRef]
- Loza, L.A.; Kadlecek, S.J.; Pourfathi, M.; Hamedani, H.; Duncan, I.F.; Ruppert, K.; Rizi, R.R. Quantification of Ventilation and Gas Uptake in Free-Breathing Mice With Hyperpolarized 129Xe MRI. IEEE Trans. Med. Imaging 2019, 38, 2081–2091. [Google Scholar] [CrossRef]
- Homi, H.M.; Yokoo, N.; Ma, D.; Warner, D.S.; Franks, N.P.; Maze, M.; Ch, M.B.B.; Grocott, H.P. The Neuroprotective Effect of Xenon Administration during Transient Middle Cerebral Artery Occlusion in Mice. Anesthesiology 2003, 99, 876–881. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shepelytskyi, Y.; Hane, F.T.; Grynko, V.; Li, T.; Hassan, A.; Albert, M.S. Hyperpolarized 129Xe Time-of-Flight MR Imaging of Perfusion and Brain Function. Diagnostics 2020, 10, 630. https://doi.org/10.3390/diagnostics10090630
Shepelytskyi Y, Hane FT, Grynko V, Li T, Hassan A, Albert MS. Hyperpolarized 129Xe Time-of-Flight MR Imaging of Perfusion and Brain Function. Diagnostics. 2020; 10(9):630. https://doi.org/10.3390/diagnostics10090630
Chicago/Turabian StyleShepelytskyi, Yurii, Francis T. Hane, Vira Grynko, Tao Li, Ayman Hassan, and Mitchell S. Albert. 2020. "Hyperpolarized 129Xe Time-of-Flight MR Imaging of Perfusion and Brain Function" Diagnostics 10, no. 9: 630. https://doi.org/10.3390/diagnostics10090630
APA StyleShepelytskyi, Y., Hane, F. T., Grynko, V., Li, T., Hassan, A., & Albert, M. S. (2020). Hyperpolarized 129Xe Time-of-Flight MR Imaging of Perfusion and Brain Function. Diagnostics, 10(9), 630. https://doi.org/10.3390/diagnostics10090630





