Deep Learning Versus Iterative Reconstruction for CT Pulmonary Angiography in the Emergency Setting: Improved Image Quality and Reduced Radiation Dose
Abstract
1. Introduction
2. Materials and Methods
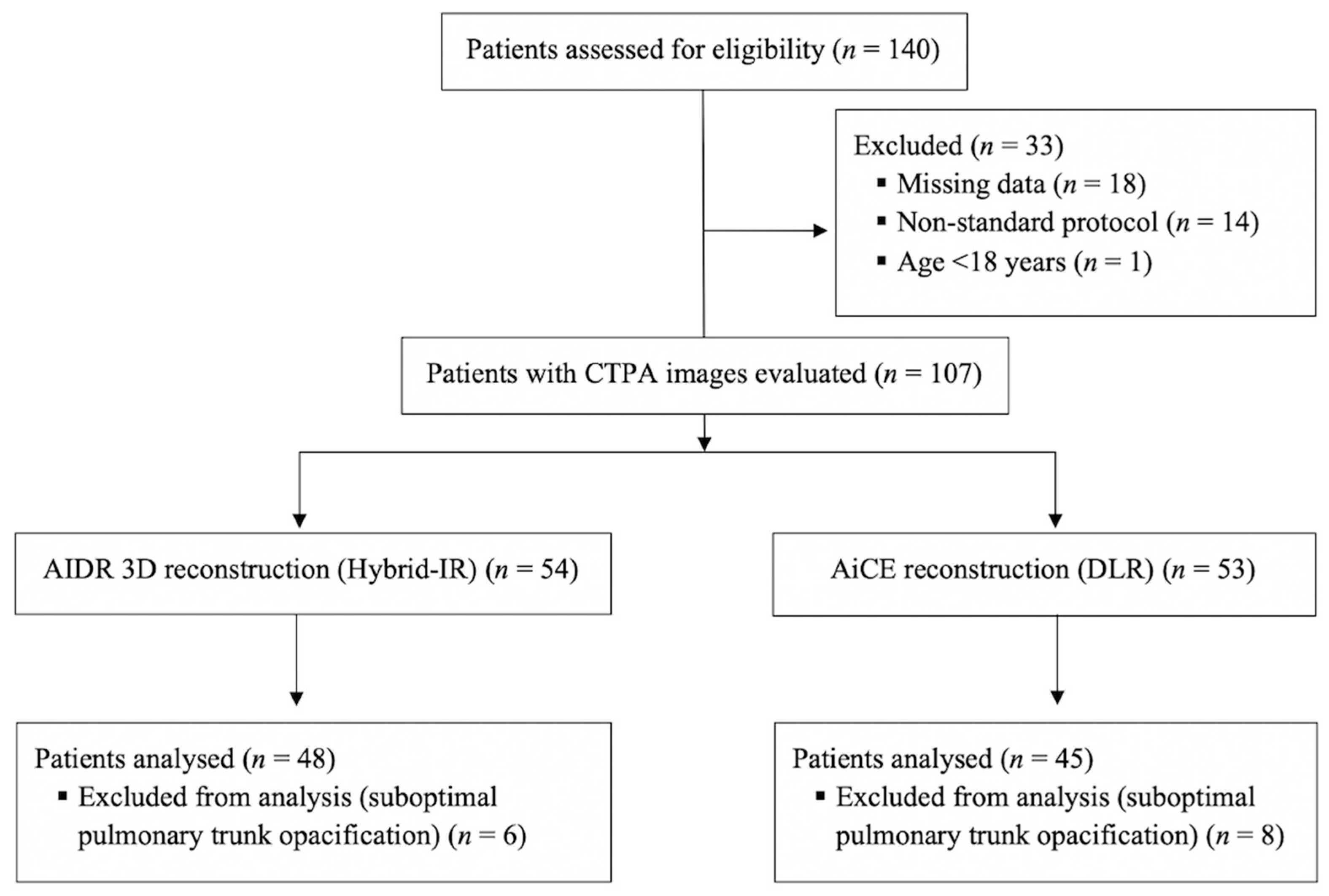
2.1. Study Population
2.2. CT Scanning Protocol
2.3. Image Quality Assessment
2.3.1. Objective Quantitative Evaluation of Image Quality
2.3.2. Subjective Qualitative Evaluation of Image Quality
2.4. Radiation Dose Measurements
2.5. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. CT Image Quality
3.2.1. Quantitative Image Analysis
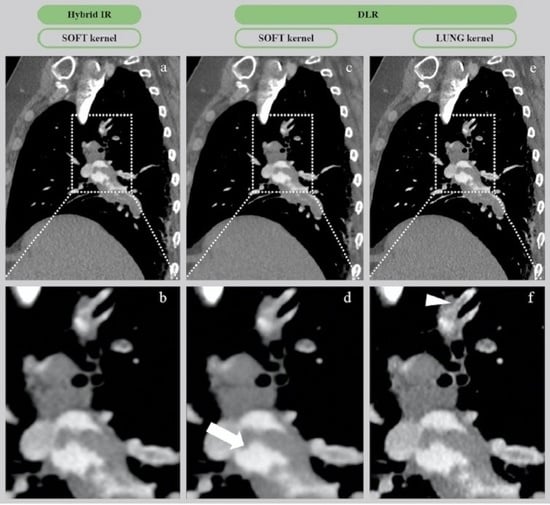
3.2.2. Qualitative Image Analysis
3.3. Radiation Exposure
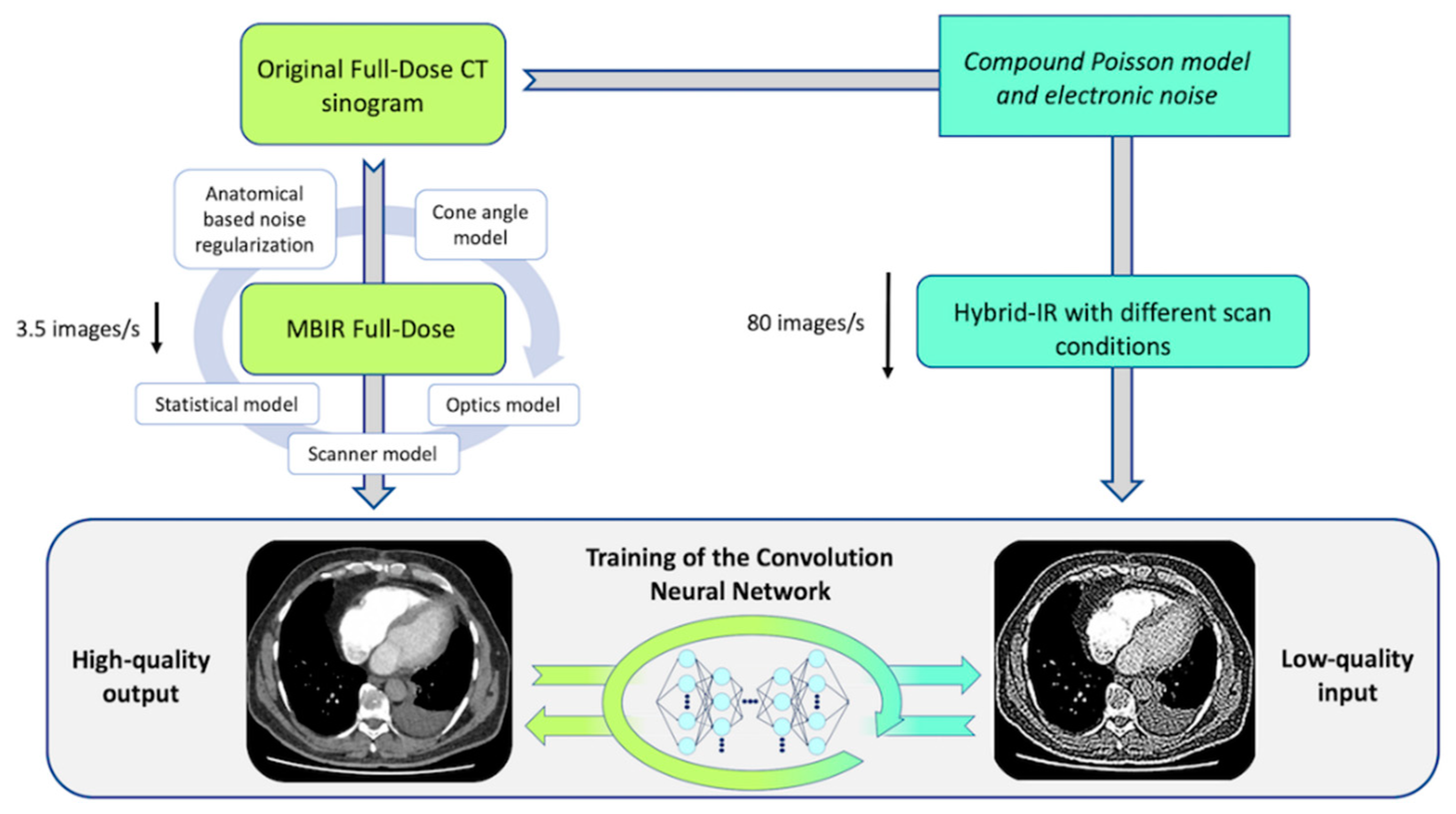
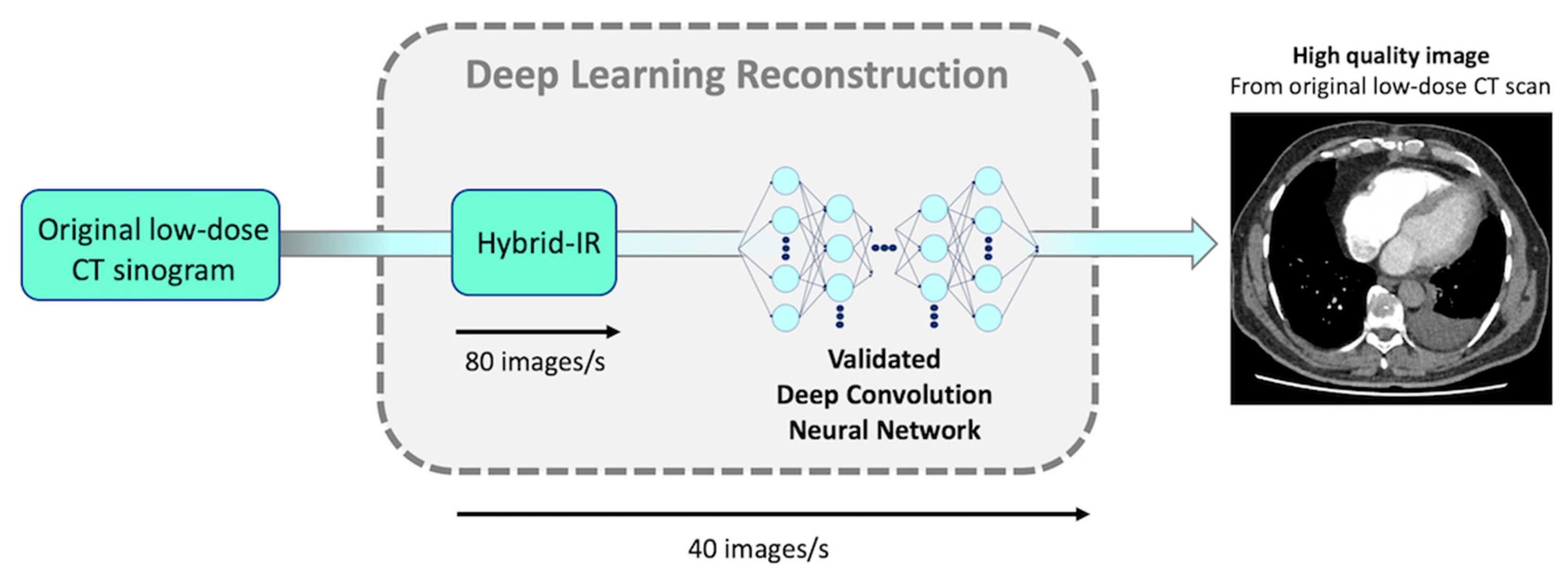
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Giuntini, C.; Di Ricco, G.; Marini, C.; Melillo, E.; Palla, A. Epidemiology. Chest 1995, 107, 3S–9S. [Google Scholar] [CrossRef] [PubMed]
- Doğan, H.; de Roos, A.; Geleijins, J.; Huisman, M.V.; Kroft, L.J.M. The role of computed tomography in the diagnosis of acute and chronic pulmonary embolism. Diagn. Interv. Radiol. 2015, 21, 307–316. [Google Scholar] [CrossRef] [PubMed]
- Van Rossum, A.B.; Pattynama, P.M.; Ton, E.R.; Treurniet, F.E.; Arndt, J.W.; van Eck, B.; Kieft, G.J. Pulmonary embolism: Validation of spiral CT angiography in 149 patients. Radiology 1996, 201, 467–470. [Google Scholar] [CrossRef] [PubMed]
- Moore, A.J.E.; Wachsmann, J.; Chamarthy, M.R.; Panjikaran, L.; Tanabe, Y.; Rajiah, P. Imaging of acute pulmonary embolism: An update. Cardiovasc. Diagn. Ther. 2018, 8, 225–243. [Google Scholar] [CrossRef] [PubMed]
- Kabrhel, C.; Matts, C.; McNamara, M.; Katz, J.; Ptak, T. A highly sensitive ELISA D-dimer increases testing but not diagnosis of pulmonary embolism. Acad. Emerg. Med. 2006, 13, 519–524. [Google Scholar] [CrossRef] [PubMed]
- Brenner, D.J.; Hall, E.J. Computed tomography - An increasing source of radiation exposure. N. Engl. J. Med. 2007, 357, 2277–2284. [Google Scholar] [CrossRef]
- Einstein, A.J.; Henzlova, M.J.; Rajagopalan, S. Estimating risk of cancer associated with radiation exposure from 64-slice computed tomography coronary angiography. JAMA 2007, 298, 317. [Google Scholar] [CrossRef]
- Hall, E.J.; Brenner, D.J. Cancer risks from diagnostic radiology. Br. J. Radiol. 2008, 81, 362–378. [Google Scholar] [CrossRef]
- Berrington de González, A.; Mahesh, M.; Kim, K.P.; Bhargavan, M.; Lewis, R.; Mettler, F.; Land, C. Projected cancer risks from computed tomographic scans performed in the United States in 2007. Arch. Intern. Med. 2009, 169, 2071. [Google Scholar] [CrossRef]
- Sulagaesuan, C.; Saksobhavivat, N.; Asavaphatiboon, S.; Kaewlai, R. Reducing emergency CT radiation doses with simple techniques: A quality initiative project. J. Med. Imaging Radiat. Oncol. 2016, 60, 23–34. [Google Scholar] [CrossRef]
- Grupp, U.; Schäfer, M.L.; Meyer, H.; Lembcke, A.; Pöllinger, A.; Wieners, G.; Renz, D.; Schwabe, P.; Streitparth, F. Reducing radiation dose in emergency CT scans while maintaining equal image quality: Just a promise or reality for severely injured patients? Emerg. Med. Int. 2013, 2013, 984645. [Google Scholar] [CrossRef] [PubMed]
- McCollough, C.H.; Primak, A.N.; Braun, N.; Kofler, J.; Yu, L.; Christner, J. Strategies for reducing radiation dose in CT. Radiol. Clin. N. Am. 2009, 47, 27–40. [Google Scholar] [CrossRef] [PubMed]
- Fleischmann, D.; Boas, F.E. Computed tomography-old ideas and new technology. Eur. Radiol. 2011, 21, 510–517. [Google Scholar] [CrossRef] [PubMed]
- Willemink, M.J.; de Jong, P.A.; Leiner, T.; de Heer, L.M.; Nievelstein, R.A.J.; Budde, R.P.J.; Schilham, A.M.R. Iterative reconstruction techniques for computed tomography Part 1: Technical principles. Eur. Radiol. 2013, 23, 1623–1631. [Google Scholar] [CrossRef]
- Stiller, W. Basics of iterative reconstruction methods in computed tomography: A vendor-independent overview. Eur. J. Radiol. 2018, 109, 147–154. [Google Scholar] [CrossRef]
- Beister, M.; Kolditz, D.; Kalender, W.A. Iterative reconstruction methods in X-ray CT. Phys. Med. 2012, 28, 94–108. [Google Scholar] [CrossRef]
- Löve, A.; Olsson, M.L.; Siemund, R.; Stålhammar, F.; Björkman-Burtscher, I.M.; Söderberg, M. Six iterative reconstruction algorithms in brain CT: A phantom study on image quality at different radiation dose levels. Br. J. Radiol. 2013, 86, 20130388. [Google Scholar] [CrossRef]
- Nichols, J.A.; Herbert Chan, H.W.; Baker, M.A.B. Machine learning: Applications of artificial intelligence to imaging and diagnosis. Biophys. Rev. 2019, 11, 111–118. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, J.; Xie, L.; Hu, Y.; Shu, H.; Luo, L.; Zhang, L.; Gui, Z.; Coatrieux, G. Discriminative prior - prior image constrained compressed sensing reconstruction for low-dose CT imaging. Sci. Rep. 2017, 7, 13868. [Google Scholar] [CrossRef]
- Wu, D.; Kim, K.; El Fakhri, G.; Li, Q. Iterative low-dose CT reconstruction with priors trained by artificial neural network. IEEE Trans. Med. Imaging 2017, 36, 2479–2486. [Google Scholar] [CrossRef]
- Higaki, T.; Nakamura, Y.; Zhou, J.; Yu, Z.; Nemoto, T.; Tatsugami, F.; Awai, K. Deep learning reconstruction at CT: Phantom study of the image characteristics. Acad. Radiol. 2020, 27, 82–87. [Google Scholar] [CrossRef] [PubMed]
- Tatsugami, F.; Higaki, T.; Nakamura, Y.; Yu, Z.; Zhou, J.; Lu, Y.; Fujioka, C.; Kitagawa, T.; Kihara, Y.; Lida, M.; et al. Deep learning-based image restoration algorithm for coronary CT angiography. Eur. Radiol. 2019, 29, 5322–5329. [Google Scholar] [CrossRef] [PubMed]
- Akagi, M.; Nakamura, Y.; Higaki, T.; Narita, K.; Honda, Y.; Zhou, J.; Awai, K. Deep learning reconstruction improves image quality of abdominal ultra-high-resolution CT. Eur. Radiol. 2019, 29, 6163–7171. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.I.; Kim, K.J.; Ahn, M.I.; Kim, H.R.; Park, H.J.; Jung, S.; Lim, H.W.; Park, S.H. Detection of pulmonary embolism using 64-slice multidetector-row computed tomography: Accuracy and reproducibility on different image reconstruction parameters. Acta Radiol. 2011, 52, 417–421. [Google Scholar] [CrossRef] [PubMed]
- Manson, E.N.; Fletcher, J.J.; Atuwo-Ampoh, V.D.; Addison, E.K.; Schandorf, C.; Bambara, L. Assessment of some image quality tests on a 128-slice computed tomography scanner using a Catphan700 phantom. J. Med. Phys. 2016, 41, 153–156. [Google Scholar] [CrossRef] [PubMed]
- Szucs-Farkas, Z.; Kurmann, L.; Strautz, T.; Patak, M.A.; Vock, P.; Schindera, S.T. Patient exposure and image quality of low-dose pulmonary computed tomography angiography. Investig. Radiol. 2008, 43, 871–876. [Google Scholar] [CrossRef]
- Yuan, R.; Shuman, W.P.; Earls, J.P.; Hague, C.J.; Mumtaz, H.A.; Scott-Moncrieff, A.; Ellis, J.D.; Mayo, J.R.; Leipsic, J.A. Reduced iodine load at CT pulmonary angiography with dual-energy monochromatic imaging: Comparison with standard CT pulmonary angiography-A prospective randomized trial. Radiology 2012, 262, 290–297. [Google Scholar] [CrossRef]
- Pontana, F.; Pagniez, J.; Duhamel, A.; Flohr, T.; Faivre, J.B.; Murphy, C.; Remy, J.; Remy-Jardin, M. Reduced-dose low-voltage chest CT angiography with sinogram-affirmed iterative reconstruction versus standard-dose filtered back projection. Radiology 2013, 267, 609–618. [Google Scholar] [CrossRef]
- Pontana, F.; Moureau, D.; Schmidt, B.; Duhamel, A.; Faivre, J.B.; Yasunaga, K.; Remy, J.; Remy-Jardin, M. CT pulmonary angiogram with 60% dose reduction: Influence of iterative reconstructions on image quality. Diagn. Interv. Imaging 2015, 96, 487–493. [Google Scholar] [CrossRef]
- Gill, M.K.; Vijayananthan, A.; Kumar, G.; Jayarani, K.; Ng, K.H.; Sun, Z. Use of 100 kV versus 120 kV in computed tomography pulmonary angiography in the detection of pulmonary embolism: Effect on radiation dose and image quality. Quant. Imaging Med. Surg. 2015, 5, 524–533. [Google Scholar] [CrossRef]
- Narita, K.; Nakamura, Y.; Higaki, T.; Akagi, M.; Honda, Y.; Awai, K. Deep learning reconstruction of drip-infusion cholangiography acquired with ultra-high-resolution computed tomography. Abdom. Radiol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Higaki, T.; Nakamura, Y.; Fukumoto, W.; Honda, Y.; Tatsugami, F.; Awai, K. Clinical application of radiation dose reduction at abdominal CT. Eur. J. Radiol. 2019, 111, 68–75. [Google Scholar] [CrossRef] [PubMed]
- McNitt-Gray, M.F. AAPM/RSNA physics tutorial for residents: Topics in CT. Radiation dose in CT. Radiographics 2002, 22, 1541–1553. [Google Scholar] [CrossRef] [PubMed]
- Modica, M.J.; Kanal, K.M.; Gunn, M.L. The obese emergency patient: Imaging challenges and solutions. Radiographics 2011, 31, 811–823. [Google Scholar] [CrossRef]
| Parameters | AIDR 3D | AiCE |
|---|---|---|
| Reconstruction technique | Iterative | Deep learning |
| Acquisition mode | Helical | Helical |
| Tube voltage (kVp) | 100 | 100 |
| Tube current (mA) | 150–500 | 140–600 |
| Collimation (mm) | 0.5 × 80 row | 0.5 × 80 row |
| Rotation time (s) | 0.275 | 0.275 |
| Field of view (mm) | 400 | 400 |
| Slice thickness (mm) | 1.0 | 0.5 |
| Interval (mm) | 0.8 | 0.3 |
| Pitch | 0.813 | 0.813 |
| Noise index | 8.5 | 10 |
| Parameters | AIDR | AiCE | % Change | p-Value | ||
|---|---|---|---|---|---|---|
| All patients, n | 45 | 48 | - | - | ||
| Image noise (HU) | ||||||
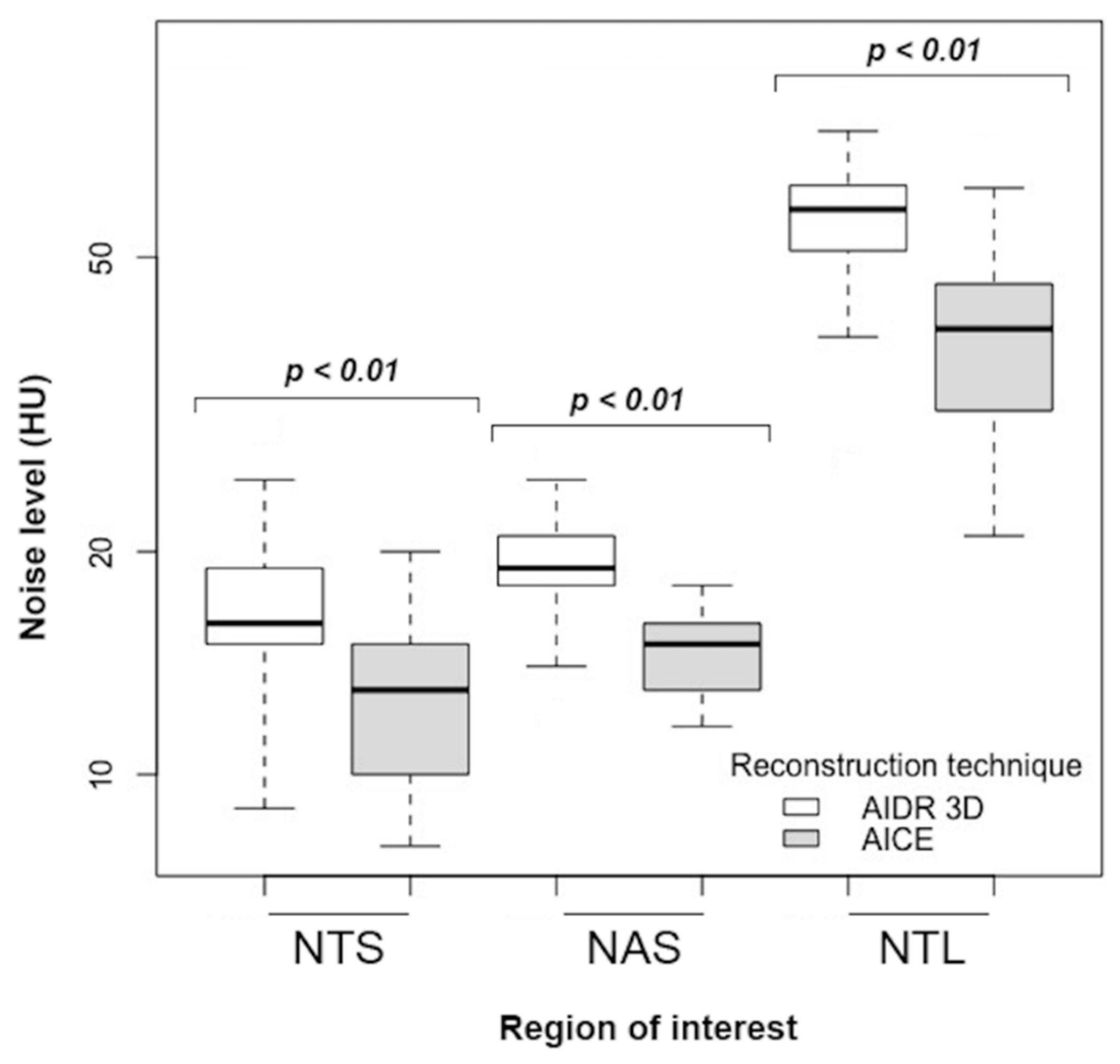
| NAS | 19.2 ± 3.0 | 14.0 ± 2.0 | −27% | <0.01 | ||
| NTS | 16.7 ± 3.7 | 11.9 ± 2.3 | −29% | <0.01 | ||
| NTL | 56.9 ± 10.8 | 38.7 ± 11.3 | −32% | <0.01 | ||
| SNR | 20.7 ± 6.1 | 24.4 ± 5.9 | +18% | <0.01 | ||
| CNR | 18.6 ± 6.0 | 21.8 ± 5.8 | +17% | <0.01 | ||
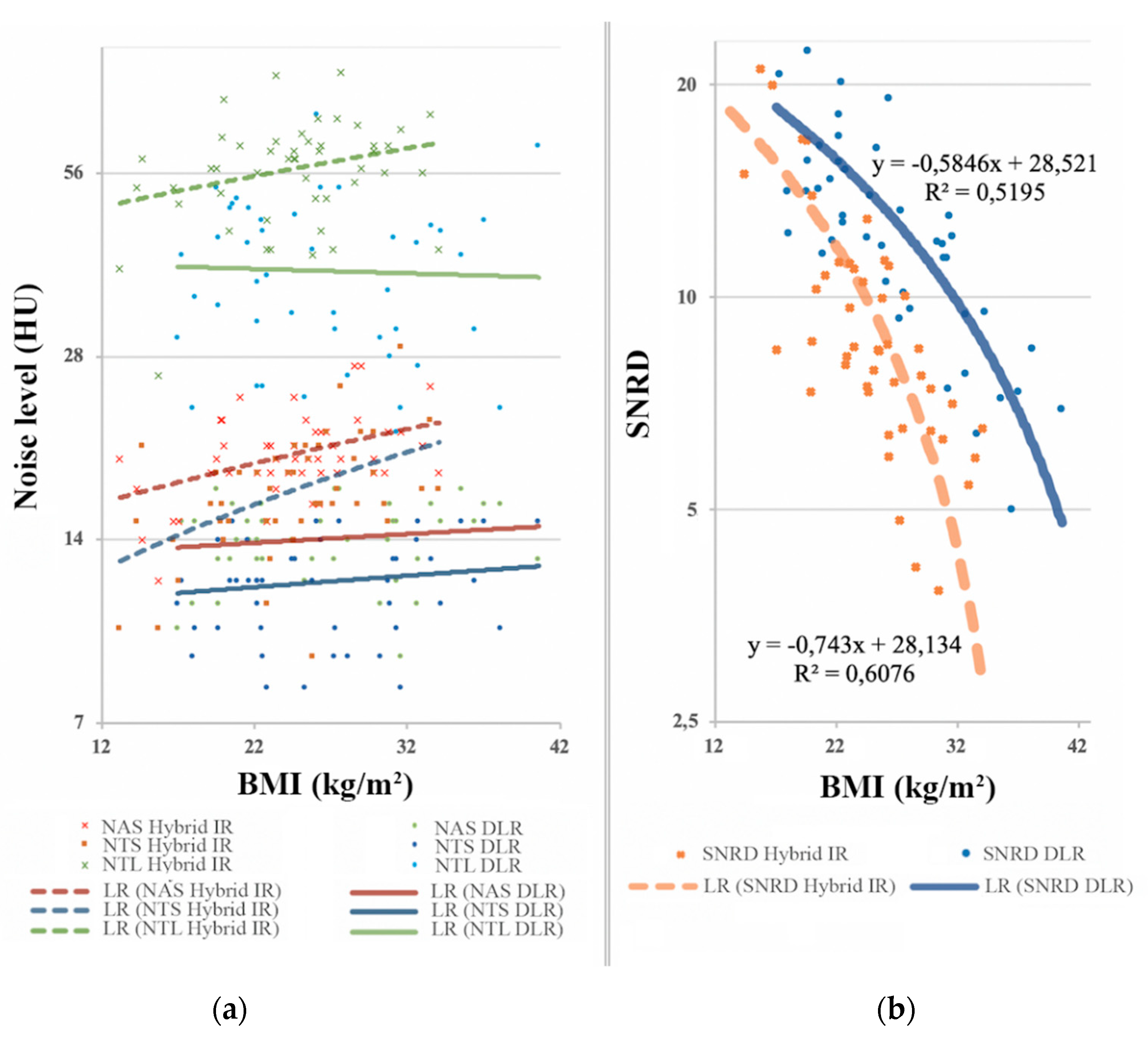
| SNRD | 10.3 ± 4.8 | 13.0 ± 5.0 | +26% | <0.01 | ||
| CNRD | 9.3 ± 4.6 | 11.7 ± 4.8 | +26% | <0.01 | ||
| BMI < 25, n | 24 | 21 | - | - | ||
| Image noise (HU) | ||||||
| NAS | 18.5 ± 2.9 | 13.9 ± 1.7 | −25% | <0.01 | ||
| NTS | 15.5 ± 2.9 | 11.9 ± 2.3 | −23% | <0.01 | ||
| NTL | 55.0 ± 11.5 | 39.6 ± 9.3 | −28% | <0.01 | ||
| SNR | 23.4 ± 6.2 | 27.1 ± 5.3 | +16% | 0.02 | ||
| CNR | 21.3 ± 6.0 | 24.5 ± 5.4 | +15% | 0.05 | ||
| SNRD | 12.7 ± 5.4 | 16.2 ± 5.0 | +28% | <0.01 | ||
| CNRD | 11.5 ± 5.1 | 14.6 ± 4.8 | +27% | <0.01 | ||
| BMI 25–30, n | 18 | 8 | - | - | ||
| Image noise (HU) | ||||||
| NAS | 20.1 ± 3.1 | 14.6 ± 1.9 | −27% | <0.01 | ||
| NTS | 17.8 ± 3.4 | 11.5 ± 2.9 | −35% | <0.01 | ||
| NTL | 58.7 ± 10.4 | 41.5 ± 16.1 | −29% | 0.02 | ||
| SNR | 18.1 ± 4.3 | 24.6 ± 5.2 | +36% | <0.01 | ||
| CNR | 16.1 ± 4.5 | 21.9 ± 4.9 | +36% | <0.01 | ||
| SNRD | 7.8 ± 2.0 | 12.5 ± 3.5 | +60% | <0.01 | ||
| CNRD | 7.0 ± 2.0 | 11.1 ± 3.4 | +59% | <0.01 | ||
| BMI > 30, n | 6 | 16 | - | - | ||
| Image noise (HU) | ||||||
| NAS | 20.5 ± 2.6 | 14.0 ± 2.3 | −32% | <0.01 | ||
| NTS | 19.3 ± 5.3 | 12.3 ± 2.3 | −36% | <0.01 | ||
| NTL | 58.7 ± 9.9 | 36.1 ± 11.3 | −39% | <0.01 | ||
| SNR | 14.3 ± 2.0 | 20.8 ± 5.2 | +45% | <0.01 | ||
| CNR | 12.4 ± 1.8 | 18.2 ± 4.9 | +47% | <0.01 | ||
| SNRD | 5.8 ± 1.1 | 9.2 ± 2.5 | +59% | <0.01 | ||
| CNRD | 5.1 ± 1.0 | 8.1 ± 2.3 | +59% | <0.01 | ||
| Parameters | AIDR | AiCE | p-Value | ||
|---|---|---|---|---|---|
| All patients, n | 45 | 48 | - | ||
| Overall image quality | |||||
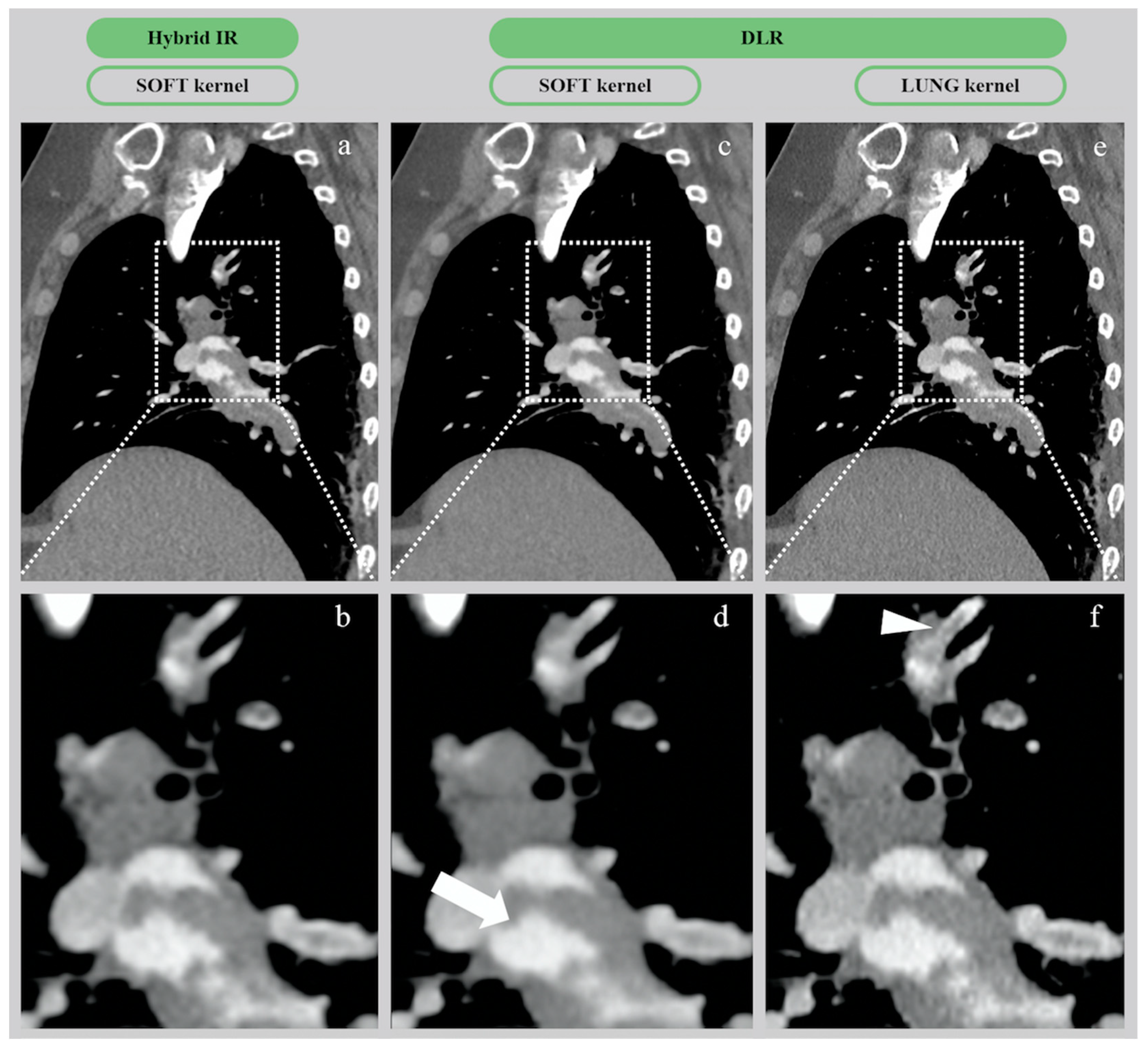
| Soft | 3.8 ± 0.8 | 4.4 ± 0.7 | <0.01 | ||
| Lung | 3.6 ± 0.9 | 4.1 ± 0.7 | <0.01 | ||
| Diagnostic confidence level * | |||||
| Proximal | 3.0 ± 0.0 | 3.0 ± 0.0 | - | ||
| Segmental | 2.9 ± 0.3 | 2.9 ± 0.3 | 0.93 | ||
| Subsegmental | 2.6 ± 0.6 | 2.6 ± 0.7 | 0.90 | ||
| BMI < 25, n | 24 | 21 | - | ||
| Overall image quality | |||||
| Soft | 3.7 ± 0.9 | 4.4 ± 0.7 | <0.01 | ||
| Lung | 3.7 ± 0.9 | 4.2 ± 0.6 | 0.03 | ||
| Diagnostic confidence level * | |||||
| Proximal | 3.0 ± 0.0 | 3.0 ± 0.0 | - | ||
| Segmental | 2.9 ± 0.3 | 3.0 ± 0.0 | 0.63 | ||
| Subsegmental | 2.7 ± 0.5 | 2.8 ± 0.5 | 0.85 | ||
| BMI 25–30, n | 18 | 8 | - | ||
| Overall image quality | |||||
| Soft | 3.9 ± 0.6 | 4.6 ± 0.7 | 0.01 | ||
| Lung | 3.5 ± 0.8 | 4.4 ± 0.5 | 0.01 | ||
| Diagnostic confidence level * | |||||
| Proximal | 3.0 ± 0.0 | 3.0 ± 0.0 | - | ||
| Segmental | 2.9 ± 0.2 | 3.0 | 0.57 | ||
| Subsegmental | 2.5 ± 0.7 | 2.9 ± 0.4 | 0.14 | ||
| BMI > 30, n | 5 | 16 | - | ||
| Overall image quality | |||||
| Soft | 4.0 ± 0.9 | 4.3 ± 0.9 | 0.6 | ||
| Lung | 3.7 ± 1.0 | 3.8 ± 0.8 | 1 | ||
| Diagnostic confidence level * | |||||
| Proximal | 3.0 ± 0.0 | 3.0 ± 0.0 | - | ||
| Segmental | 2.8 ± 0.4 | 2.8 ± 0.4 | 0.96 | ||
| Subsegmental | 2.5 ± 0.8 | 2.3 ± 0.9 | 0.54 | ||
| Parameters | AIDR | AiCE | % Change | p-Value | |
|---|---|---|---|---|---|
| All patients, n | 45 | 48 | - | - | |
| CTDIvol | 4.8 ± 1.4 | 4.0 ± 1.2 | −17% | <0.01 | |
| DLP | 157.9 ± 44.9 | 130.8 ± 41.2 | −17% | <0.01 | |
| BMI < 25, n | 24 | 21 | - | - | |
| CTDIvol | 3.9 ± 1.2 | 3.0 ± 0.8 | −23% | 0.02 | |
| DLP | 130.8 ± 37.9 | 100.7 ± 30.0 | −23% | <0.001 | |
| BMI 25–30, n | 18 | 8 | - | - | |
| CTDIvol | 5.4 ± 0.6 | 4.1 ± 0.8 | −24% | <0.01 | |
| DLP | 179.6 ± 28.0 | 139.0 ± 34.4 | −23% | <0.01 | |
| BMI > 30, n | 5 | 16 | - | - | |
| CTDIvol | 6.2 ± 1.4 | 5.2 ± 0.6 | −16% | 0.04 | |
| DLP | 200.8 ± 47.2 | 166.3 ± 23.9 | −17% | 0.08 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lenfant, M.; Chevallier, O.; Comby, P.-O.; Secco, G.; Haioun, K.; Ricolfi, F.; Lemogne, B.; Loffroy, R. Deep Learning Versus Iterative Reconstruction for CT Pulmonary Angiography in the Emergency Setting: Improved Image Quality and Reduced Radiation Dose. Diagnostics 2020, 10, 558. https://doi.org/10.3390/diagnostics10080558
Lenfant M, Chevallier O, Comby P-O, Secco G, Haioun K, Ricolfi F, Lemogne B, Loffroy R. Deep Learning Versus Iterative Reconstruction for CT Pulmonary Angiography in the Emergency Setting: Improved Image Quality and Reduced Radiation Dose. Diagnostics. 2020; 10(8):558. https://doi.org/10.3390/diagnostics10080558
Chicago/Turabian StyleLenfant, Marc, Olivier Chevallier, Pierre-Olivier Comby, Grégory Secco, Karim Haioun, Frédéric Ricolfi, Brivaël Lemogne, and Romaric Loffroy. 2020. "Deep Learning Versus Iterative Reconstruction for CT Pulmonary Angiography in the Emergency Setting: Improved Image Quality and Reduced Radiation Dose" Diagnostics 10, no. 8: 558. https://doi.org/10.3390/diagnostics10080558
APA StyleLenfant, M., Chevallier, O., Comby, P.-O., Secco, G., Haioun, K., Ricolfi, F., Lemogne, B., & Loffroy, R. (2020). Deep Learning Versus Iterative Reconstruction for CT Pulmonary Angiography in the Emergency Setting: Improved Image Quality and Reduced Radiation Dose. Diagnostics, 10(8), 558. https://doi.org/10.3390/diagnostics10080558