Updating the Risk Stratification for Sudden Cardiac Death in Cardiomyopathies: The Evolving Role of Cardiac Magnetic Resonance Imaging. An Approach for the Electrophysiologist
Abstract
1. Introduction
2. CMR Basics
3. CMR Imaging Patterns Reveal the Arrhythmogenic Burden in Cardiomyopathies
3.1. Nonischemic Dilated Cardiomyopathy
3.2. Ischemic Cardiomyopathy
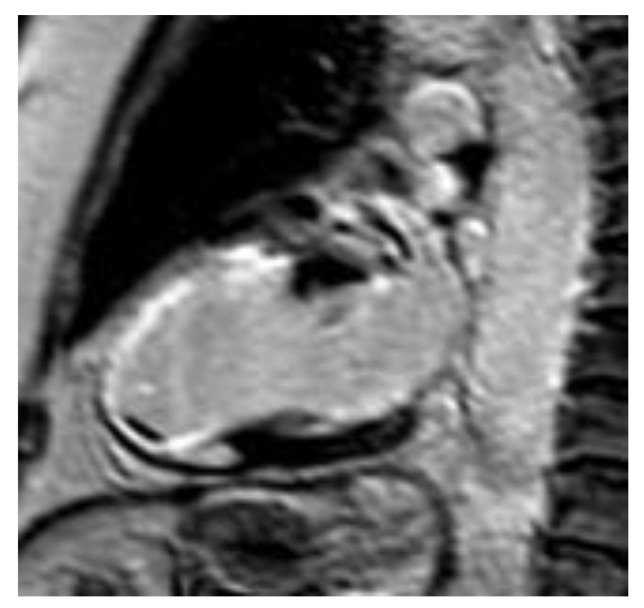
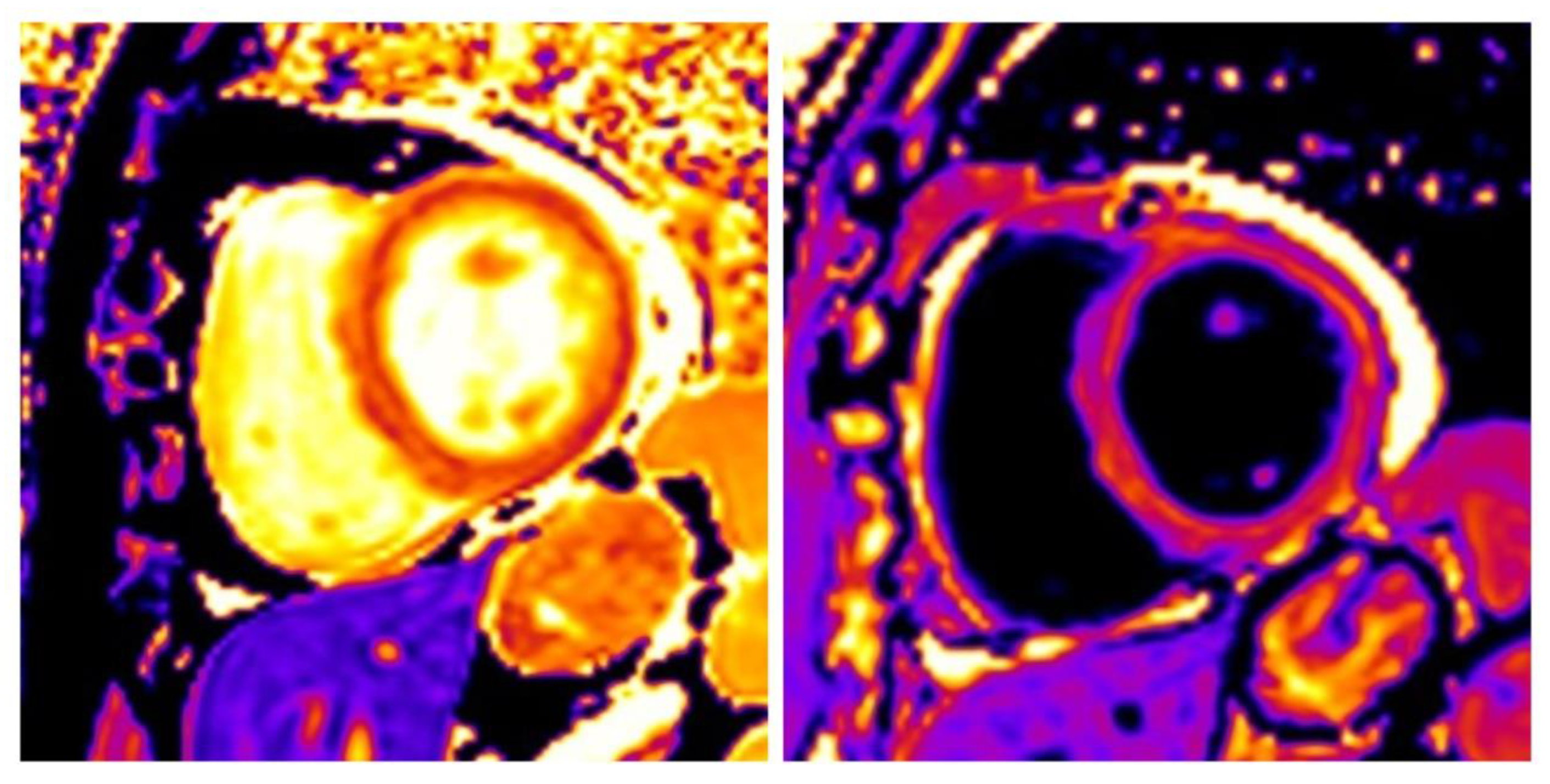
3.3. Hypertrophic Cardiomyopathy
3.4. Inflammatory Cardiomyopathy
4. The Complementary Role of CMR and Electroanatomical Mapping in Ventricular Tachycardia Ablation
- Generate anatomic images during the procedure. Consequently, any registration mismatch errors are avoided. The operator uses the CMR scanner virtually in the same manner as a fluoroscope, with an attached pedalfor image acquisition whenever e.g., tissue characterization is needed [117].
- Produce substrate maps to be compared with those derived from the “classical” EAM module and improve area of interest targeting.
- Evaluate the formation of permanent, irreversible lesions [124,125,126] prior to catheter withdrawal. Regarding real-time lesion formation assessment, acute LGE grossly overestimates the effect (ECV expansion), while thenoncontrast method, using fast T1- and T2-weighted imaging [127,128,129,130] has only been able to acutely detect a minority (~30%) of lesions. CMR thermography is a newly introduced parameter with promising results [42,131,132].
5. Future Perspectives in the Arrhythmic Risk Stratification in Cardiomyopathies
6. Conclusions
Funding
Conflicts of Interest
References
- Pitoulis, F.G.; Terracciano, C.M. Heart plasticity in response to pressure- and volume-overload: A review of findings in compensated and decompensated phenotypes. Front. Physiol. 2020, 11, 92. [Google Scholar] [CrossRef] [PubMed]
- Elliott, P.; Andersson, B.; Arbustini, E.; Bilinska, Z.; Cecchi, F.; Charron, P.; Dubourg, O.; Kühl, U.; Maisch, B.; McKenna, W.J.; et al. Classification of the cardiomyopathies: A position statement from the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur. Heart. J. 2008, 29, 270–276. [Google Scholar] [CrossRef] [PubMed]
- McKenna, W.J.; Maron, B.J.; Thiene, G. Classification, epidemiology, and global burden of cardiomyopathies. Circ. Res. 2017, 121, 722–730. [Google Scholar] [CrossRef] [PubMed]
- Buja, L.M.; Ottaviani, G.; Mitchell, R.N. Pathobiology of cardiovascular diseases: An update. Cardiovasc. Pathol. 2019, 42, 44–53. [Google Scholar] [CrossRef]
- Ottaviani, G.; Buja, L.M. Anatomopathological changes of the cardiac conduction system in sudden cardiac death, particularly in infants: Advances over the last 25 years. Cardiovasc.Pathol. 2016, 25, 489–499. [Google Scholar] [CrossRef]
- Srinivasan, N.T.; Schilling, R.J. Sudden cardiac death and arrhythmias. Arrhythm. Electrophysiol. Rev. 2018, 7, 111–117. [Google Scholar] [CrossRef]
- Sen-Chowdhry, S.; McKenna, W.J. Sudden death from genetic and acquired cardiomyopathies. Circulation 2012, 125, 1563–1576. [Google Scholar] [CrossRef]
- Gatzoulis, K.A.; Sideris, A.; Kanoupakis, E.; Sideris, S.; Nikolaou, N.; Antoniou, C.K.; Kolettis, T.M. Arrhythmic risk stratification in heart failure: Time for the next step? Ann. Noninvasive. Electrocardiol. 2017, 22, e12430. [Google Scholar] [CrossRef]
- Moss, A.J.; Zareba, W.; Hall, W.J.; Klein, H.; Wilber, D.J.; Cannom, D.S.; Daubert, J.P.; Higgins, S.L.; Brown, M.W.; Andrews, M.L.; et al. Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. N. Engl. J. Med. 2002, 346, 877–883. [Google Scholar] [CrossRef]
- Bänsch, D.; Antz, M.; Boczor, S.; Volkmer, M.; Tebbenjohanns, J.; Seidl, K.; Block, M.; Gietzen, F.; Berger, J.; Kuck, K.H. Primary prevention of sudden cardiac death in idiopathic dilated cardiomyopathy: The Cardiomyopathy Trial (CAT). Circulation 2002, 105, 1453–1458. [Google Scholar] [CrossRef]
- Bardy, G.H.; Lee, K.L.; Mark, D.B.; Poole, J.E.; Packer, D.L.; Boineau, R.; Domanski, M.; Troutman, C.; Anderson, J.; Johnson, G.; et al. Amiodarone or an implantable cardioverter-defibrillator for congestive heart failure. N. Engl. J. Med. 2005, 352, 225–237. [Google Scholar] [CrossRef] [PubMed]
- Bristow, M.R.; Saxon, L.A.; Boehmer, J.; Krueger, S.; Kass, D.A.; De Marco, T.; Carson, P.; DiCarlo, L.; DeMets, D.; White, B.G.; et al. Cardiac-resynchronization therapy with or without an implantable defibrillator in advanced chronic heart failure. N. Engl. J. Med. 2004, 350, 2140–2150. [Google Scholar] [CrossRef] [PubMed]
- Desai, A.S.; Fang, J.C.; Maisel, W.H.; Baughman, K.L. Implantable defibrillators for the prevention of mortality in patients with nonischemic cardiomyopathy: A meta-analysis of randomized controlled trials. JAMA 2004, 292, 2874–2879. [Google Scholar] [CrossRef] [PubMed]
- Kadish, A.; Dyer, A.; Daubert, J.P.; Quigg, R.; Estes, N.A.; Anderson, K.P.; Calkins, H.; Hoch, D.; Goldberger, J.; Shalaby, A.; et al. Prophylactic defibrillator implantation in patients with nonischemic dilated cardiomyopathy. N. Engl. J. Med. 2004, 350, 2151–2158. [Google Scholar] [CrossRef]
- Køber, L.; Thune, J.J.; Nielsen, J.C.; Haarbo, J.; Videbæk, L.; Korup, E.; Jensen, G.; Hildebrandt, P.; Steffensen, F.H.; Bruun, N.E.; et al. Defibrillator implantation in patients with nonischemic systolic heart failure. N. Engl. J. Med. 2016, 375, 1221–1230. [Google Scholar] [CrossRef]
- Strickberger, S.A.; Hummel, J.D.; Bartlett, T.G.; Frumin, H.I.; Schuger, C.D.; Beau, S.L.; Bitar, C.; Morady, F.; AMIOVIRT Investigators. Amiodarone versus implantable cardioverter-defibrillator: Randomized trial in patients with nonischemic dilated cardiomyopathy and asymptomatic nonsustained ventricular tachycardia—AMIOVIRT. J. Am. Coll. Cardiol. 2003, 41, 1707–1712. [Google Scholar] [CrossRef]
- Marwick, T.H. Ejection fraction pros and cons: JACC state-of-the-art review. J. Am. Coll. Cardiol. 2018, 72, 2360–2379. [Google Scholar] [CrossRef]
- Al-Khatib, S.M.; Stevenson, W.G.; Ackerman, M.J.; Bryant, W.J.; Callans, D.J.; Curtis, A.B.; Deal, B.J.; Dickfeld, T.; Field, M.E.; Fonarow, G.C.; et al. 2017 AHA/ACC/HRS guideline for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: Executive summary: A report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines and the Heart Rhythm Society. J. Am. Coll. Cardiol. 2018, 72, 1677–1749. [Google Scholar] [CrossRef]
- Priori, S.G.; Blomström-Lundqvist, C.; Mazzanti, A.; Blom, N.; Borggrefe, M.; Camm, J.; Elliott, P.M.; Fitzsimons, D.; Hatala, R.; Hindricks, G.; et al. 2015 ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: The task force for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death of the European Society of Cardiology (ESC). Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC). Eur. Heart J. 2015, 36, 2793–2867. [Google Scholar] [CrossRef]
- Gorgels, A.P.; Gijsbers, C.; de Vreede-Swagemakers, J.; Lousberg, A.; Wellens, H.J. Out-of-hospital cardiac arrest—The relevance of heart failure. The Maastricht Circulatory Arrest Registry. Eur. Heart, J. 2003, 24, 1204–1209. [Google Scholar] [CrossRef]
- Stecker, E.C.; Vickers, C.; Waltz, J.; Socoteanu, C.; John, B.T.; Mariani, R.; McAnulty, J.H.; Gunson, K.; Jui, J.; Chugh, S.S. Population-based analysis of sudden cardiac death with and without left ventricular systolic dysfunction: Two-year findings from the Oregon sudden unexpected death study. J. Am. Coll. Cardiol. 2006, 47, 1161–1166. [Google Scholar] [CrossRef]
- Sabbag, A.; Suleiman, M.; Laish-Farkash, A.; Samania, N.; Kazatsker, M.; Goldenberg, I.; Glikson, M.; Beinart, R.; Israeli Working Group of Pacing and Electrophysiology. Contemporary rates of appropriate shock therapy in patients who receive implantable device therapy in a real-world setting: From the Israeli ICD Registry. Heart Rhythm 2015, 12, 2426–2433. [Google Scholar] [CrossRef] [PubMed]
- Pattanayak, P.; Bleumke, D.A. Tissue characterization of the myocardium: State of the art characterization by magnetic resonance and computed tomography imaging. Radiol. Clin. 2015, 53, 413–423. [Google Scholar] [CrossRef] [PubMed]
- Assomull, R.G.; Prasad, S.K.; Lyne, J.; Smith, G.; Burman, E.D.; Khan, M.; Sheppard, M.N.; Poole-Wilson, P.A.; Pennell, D.J. Cardiovascular magnetic resonance, fibrosis, and prognosis in dilated cardiomyopathy. J. Am. Coll. Cardiol. 2006, 48, 1977–1985. [Google Scholar] [CrossRef]
- Wu, K.C.; Weiss, R.G.; Thiemann, D.R.; Kitagawa, K.; Schmidt, A.; Dalal, D.; Lai, S.; Bluemke, D.A.; Gerstenblith, G.; Marbán, E.; et al. Late gadolinium enhancement by cardiovascular magnetic resonance heralds an adverse prognosis in nonischemic cardiomyopathy. J. Am. Coll. Cardiol. 2008, 51, 2414–2421. [Google Scholar] [CrossRef] [PubMed]
- Iles, L.; Pfluger, H.; Lefkovits, L.; Butler, M.J.; Kistler, P.M.; Kaye, D.M.; Taylor, A.J. Myocardial fibrosis predicts appropriate device therapy in patients with implantable cardioverter-defibrillators for primary prevention of sudden cardiac death. J. Am. Coll. Cardiol. 2011, 57, 821–828. [Google Scholar] [CrossRef]
- PerazzoloMarra, M.; De Lazzari, M.; Zorzi, A.; Migliore, F.; Zilio, F.; Calore, C.; Vettor, G.; Tona, F.; Tarantini, G.; Cacciavillani, L.; et al. Impact of the presence and amount of myocardial fibrosis by cardiac magnetic resonance on arrhythmic outcome and sudden cardiac death in nonischemic dilated cardiomyopathy. Heart Rhythm 2014, 11, 856–863. [Google Scholar] [CrossRef] [PubMed]
- Masci, P.G.; Doulaptsis, C.; Bertella, E.; Del Torto, A.; Symons, R.; Pontone, G.; Barison, A.; Droogné, W.; Andreini, D.; Lorenzoni, V.; et al. Incremental prognostic value of myocardial fibrosis in patients with non-ischemic cardiomyopathy without congestive heart failure. Circ. Heart Fail. 2014, 7, 448–456. [Google Scholar] [CrossRef]
- Piers, S.R.; Everaerts, K.; van der Geest, R.J.; Hazebroek, M.R.; Siebelink, H.M.; Pison, L.A.; Schalij, M.J.; Bekkers, S.C.; Heymans, S.; Zeppenfeld, K. Myocardial scar predicts monomorphic ventricular tachycardia but not polymorphic ventricular tachycardia or ventricular fibrillation in nonischemic dilated cardiomyopathy. Heart Rhythm 2015, 12, 2106–2114. [Google Scholar] [CrossRef]
- Shin, D.G.; Lee, H.J.; Park, J.; Uhm, J.S.; Pak, H.N.; Lee, M.H.; Kim, Y.J.; Joung, B. Pattern of late gadolinium enhancement predicts arrhythmic events in patients with non-ischemic cardiomyopathy. Int. J. Cardiol. 2016, 222, 9–15. [Google Scholar] [CrossRef]
- Lehrke, S.; Lossnitzer, D.; Schöb, M.; Steen, H.; Merten, C.; Kemmling, H.; Pribe, R.; Ehlermann, P.; Zugck, C.; Korosoglou, G.; et al. Use of cardiovascular magnetic resonance for risk stratification in chronic heart failure: Prognostic value of late gadolinium enhancement in patients with non-ischaemic dilated cardiomyopathy. Heart 2011, 97, 727–732. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Guallar, E.; Weiss, R.G.; Stillabower, M.; Gerstenblith, G.; Tomaselli, G.F.; Wu, K.C. Associations between scar characteristics by cardiac magnetic resonance and changes in left ventricular ejection fraction in primary prevention defibrillator recipients. Heart Rhythm 2016, 13, 1661–1666. [Google Scholar] [CrossRef]
- Scott, P.A.; Rosengarten, J.A.; Curzen, N.P.; Morgan, J.M. Late gadolinium enhancement cardiac magnetic resonance imaging for the prediction of ventricular tachyarrhythmic events: A meta-analysis. Eur. J. Heart Fail. 2013, 15, 1019–1027. [Google Scholar] [CrossRef] [PubMed]
- Van der Bijl, P.; Delgado, V.; Bax, J.J. Noninvasive imaging markers associated with sudden cardiac death. Trends Cardiovasc. Med. 2016, 26, 348–360. [Google Scholar] [CrossRef]
- Pohost, G.M.; Nayak, K.S. Handbook of Cardiovascular Magnetic Resonance Imaging, 1st ed.; CRC Press: New York, NY, USA, 2006; pp. 1–30. [Google Scholar]
- Kwong, R.Y. Cardiovascular Magnetic Resonance Imaging; Humana Press Inc.: Totowa, NJ, USA, 2008. [Google Scholar]
- Kim, P.K.; Hong, Y.J.; Im, D.J.; Suh, Y.J.; Park, C.H.; Kim, J.Y.; Chang, S.; Lee, H.J.; Hur, J.; Kim, Y.J.; et al. Myocardial T1 and T2 mapping: Techniques and clinical applications. Korean J. Radiol. 2017, 18, 113–131. [Google Scholar] [CrossRef] [PubMed]
- Mavrogeni, S.; Apostolou, D.; Argyriou, P.; Velitsista, S.; Papa, L.; Efentakis, S.; Vernardos, E.; Kanoupaki, M.; Kanoupakis, G.; Manginas, A. T1 and T2 mapping in cardiology: “Mapping the obscure object of desire”. Cardiology 2017, 138, 207–217. [Google Scholar] [CrossRef]
- Moon, J.C.; Messroghli, D.R.; Kellman, P.; Piechnik, S.K.; Robson, M.D.; Ugander, M.; Gatehouse, P.D.; Arai, A.E.; Friedrich, M.G.; Neubauer, S.; et al. Myocardial T1 mapping and extracellular volume quantification: A Society for Cardiovascular Magnetic Resonance (SCMR) and CMR Working Group of the European Society of Cardiology consensus statement. J. Cardiovasc. Magn. Reson. 2013, 15, 92. [Google Scholar] [CrossRef]
- Xiao, Y.D.; Paudel, R.; Liu, J.; Ma, C.; Zhang, Z.S.; Zhou, S.K. MRI contrast agents: Classification and application (Review). Int. J. Mol. Med. 2016, 38, 1319–1326. [Google Scholar] [CrossRef]
- Ranga, A.; Agarwal, Y.; Garg, K.J. Gadolinium based contrast agents in current practice: Risks of accumulation and toxicity in patients with normal renal function. Indian, J. Radiol. Imaging 2017, 27, 141–147. [Google Scholar] [CrossRef]
- Choi, J.W.; Moon, W.J. Gadolinium deposition in the brain: Current updates. Korean, J. Radiol. 2019, 20, 134–147. [Google Scholar] [CrossRef]
- Gulani, V.; Calamante, F.; Shellock, F.G.; Kanal, E.; Reeder, S.B.; International Society for Magnetic Resonance in Medicine. Gadolinium deposition in the brain: Summary of evidence and recommendations. Lancet Neurol. 2017, 16, 564–570. [Google Scholar] [CrossRef]
- Mewton, N.; Liu, C.Y.; Croisille, P.; Bluemke, D.; Lima, J.A. Assessment of myocardial fibrosis with cardiovascular magnetic resonance. J. Am. Coll. Cardiol. 2011, 57, 891–903. [Google Scholar] [CrossRef] [PubMed]
- Haaf, P.; Garg, P.; Messroghli, D.R.; Broadbent, D.A.; Greenwood, J.P.; Plein, S. Cardiac T1 mapping and Extracellular Volume (ECV) in clinical practice: A comprehensive review. J. Cardiovasc. Magn. Reson. 2016, 18, 89. [Google Scholar] [CrossRef] [PubMed]
- Barison, A.; Del Torto, A.; Chiappino, S.; Aquaro, G.D.; Todiere, G.; Vergaro, G.; Passino, C.; Lombardi, M.; Emdin, M.; Masci, P.G. Prognostic significance of myocardial extracellular volume fraction in nonischaemic dilated cardiomyopathy. J. Cardiovasc. Med. 2015, 16, 681–687. [Google Scholar] [CrossRef] [PubMed]
- Aus dem Siepen, F.; Buss, S.J.; Messroghli, D.; Andre, F.; Lossnitzer, D.; Seitz, S.; Keller, M.; Schnabel, P.A.; Giannitsis, E.; Korosoglou, G.; et al. T1 mapping in dilated cardiomyopathy with cardiac magnetic resonance: Quantification of diffuse myocardial fibrosis and comparison with endomyocardial biopsy. Eur. Heart J. Cardiovasc. Imaging 2015, 16, 210–216. [Google Scholar] [CrossRef]
- Kammerlander, A.A.; Marzluf, B.A.; Zotter-Tufaro, C.; Aschauer, S.; Duca, F.; Bachmann, A.; Knechtelsdorfer, K.; Wiesinger, M.; Pfaffenberger, S.; Greiser, A.; et al. T1 mapping by CMR imaging: From histological validation to clinical implication. JACC Cardiovasc. Imaging 2016, 9, 14–23. [Google Scholar] [CrossRef]
- Taylor, A.J.; Salerno, M.; Dharmakumar, R.; Jerosch-Herold, M. T1 mapping: Basic techniques and clinical applications. JACC Cardiovasc. Imaging 2016, 9, 67–81. [Google Scholar] [CrossRef]
- Nazarian, S.; Bluemke, D.A.; Lardo, A.C.; Zviman, M.M.; Watkins, S.P.; Dickfeld, T.L.; Meininger, G.R.; Roguin, A.; Calkins, H.; Tomaselli, G.F.; et al. Magnetic resonance assessment of the substrate for inducible ventricular tachycardia in nonischemic cardiomyopathy. Circulation 2005, 112, 2821–2825. [Google Scholar] [CrossRef]
- Dawson, D.K.; Hawlisch, K.; Prescott, G.; Roussin, I.; Di Pietro, E.; Deac, M.; Wong, J.; Frenneaux, M.P.; Pennell, D.J.; Prasad, S.K. Prognostic role of CMR in patients presenting with ventricular arrhythmias. JACC Cardiovasc. Imaging 2013, 6, 335–344. [Google Scholar] [CrossRef]
- Gulati, A.; Jabbour, A.; Ismail, T.F.; Guha, K.; Khwaja, J.; Raza, S.; Morarji, K.; Brown, T.D.; Ismail, N.A.; Dweck, M.R.; et al. Association of fibrosis with mortality and sudden cardiac death in patients with nonischemic dilated cardiomyopathy. JAMA 2013, 309, 896–908. [Google Scholar] [CrossRef]
- Demirel, F.; Adiyaman, A.; Timmer, J.R.; Dambrink, J.H.; Kok, M.; Boeve, W.J.; Elvan, A. Myocardial scar characteristics based on cardiac magnetic resonance imaging is associated with ventricular tachyarrhythmia in patients with ischemic cardiomyopathy. Int. J. Cardiol. 2014, 177, 392–399. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Desjardins, B.; Baman, T.; Ilg, K.; Good, E.; Crawford, T.; Oral, H.; Pelosi, F.; Chugh, A.; Morady, F.; et al. Delayed-enhanced MR scar imaging and intraprocedural registration into an electroanatomical mapping system in post-infarction patients. JACC Cardiovasc. Imaging 2012, 5, 207–210. [Google Scholar] [CrossRef] [PubMed]
- Andreu, D.; Berruezo, A.; Ortiz-Pérez, J.T.; Silva, E.; Mont, L.; Borràs, R.; de Caralt, T.M.; Perea, R.J.; Fernández-Armenta, J.; Zeljko, H.; et al. Integration of 3D electroanatomic maps and magnetic resonance scar characterization into the navigation system to guide ventricular tachycardia ablation. Circ. Arrhythmia Electrophysiol. 2011, 4, 674–683. [Google Scholar] [CrossRef]
- Halliday, B.P.; Gulati, A.; Ali, A.; Guha, K.; Newsome, S.; Arzanauskaite, M.; Vassiliou, V.S.; Lota, A.; Izgi, C.; Tayal, U.; et al. Association between midwall late gadolinium enhancement and sudden cardiac death in patients with dilated cardiomyopathy and mild and moderate left ventricular systolic dysfunction. Circulation 2017, 135, 2106–2115. [Google Scholar] [CrossRef] [PubMed]
- Becker, M.A.J.; Cornel, J.H.; van de Ven, P.M.; van Rossum, A.C.; Allaart, C.P.; Germans, T. The prognostic value of late gadolinium-enhanced cardiac magnetic resonance imaging in nonischemic dilated cardiomyopathy: A review and meta-analysis. JACC Cardiovasc. Imaging 2018, 11, 1274–1284. [Google Scholar] [CrossRef] [PubMed]
- Di Marco, A.; Anguera, I.; Schmitt, M.; Klem, I.; Neilan, T.G.; White, J.A.; Sramko, M.; Masci, P.G.; Barison, A.; Mckenna, P.; et al. Late gadolinium enhancement and the risk for ventricular arrhythmias or sudden death in dilated cardiomyopathy: Systematic review and meta-analysis. JACC Heart Fail. 2017, 5, 28–38. [Google Scholar] [CrossRef]
- Halliday, B.P.; Baksi, A.J.; Gulati, A.; Ali, A.; Newsome, S.; Izgi, C.; Arzanauskaite, M.; Lota, A.; Tayal, U.; Vassiliou, V.S.; et al. Outcome in dilated cardiomyopathy related to the extent, location, and pattern of late gadolinium enhancement. JACC Cardiovasc. Imaging 2019, (Pt 2), 1645–1655. [Google Scholar] [CrossRef]
- Mueller, K.A.; Heck, C.; Heinzmann, D.; Schwille, J.; Klingel, K.; Kandolf, R.; Kramer, U.; Gramlich, M.; Geisler, T.; Gawaz, M.P.; et al. Comparison of ventricular inducibility with late gadolinium enhancement and myocardial inflammation in endomyocardial biopsy in patients with dilated cardiomyopathy. PLoS ONE 2016, 11, e0167616. [Google Scholar] [CrossRef] [PubMed]
- Tachi, M.; Amano, Y.; Inui, K.; Takeda, M.; Yamada, F.; Asai, K.; Kumita, S. Relationship of postcontrast myocardial T1 value and delayed enhancement to reduced cardiac function and serious arrhythmia in dilated cardiomyopathy with left ventricular ejection fraction less than 35. Acta Radiol. 2016, 57, 430–436. [Google Scholar] [CrossRef] [PubMed]
- Puntmann, V.O.; Carr-White, G.; Jabbour, A.; Yu, C.Y.; Gebker, R.; Kelle, S.; Hinojar, R.; Doltra, A.; Varma, N.; Child, N.; et al. T1-Mapping and Outcome in Nonischemic Cardiomyopathy: All-Cause Mortality and Heart Failure. JACC Cardiovasc. Imaging 2016, 9, 40–50. [Google Scholar] [CrossRef]
- Hong, Y.J.; Park, C.H.; Kim, Y.J.; Hur, J.; Lee, H.J.; Hong, S.R.; Suh, Y.J.; Greiser, A.; Paek, M.Y.; Choi, B.W.; et al. Extracellular volume fraction in dilated cardiomyopathy patients without obvious late gadolinium enhancement: Comparison with healthy control subjects. Int. J. Cardiovasc. Imaging 2015, 31 (Suppl. 1), 115–122. [Google Scholar] [CrossRef] [PubMed]
- Nakamori, S.; Dohi, K.; Ishida, M.; Goto, Y.; Imanaka-Yoshida, K.; Omori, T.; Goto, I.; Kumagai, N.; Fujimoto, N.; Ichikawa, Y.; et al. Native T1 mapping and extracellular volume mapping for the assessment of diffuse myocardial fibrosis in dilated cardiomyopathy. JACC Cardiovasc. Imaging 2018, 11, 48–59. [Google Scholar] [CrossRef] [PubMed]
- Buss, S.J.; Breuninger, K.; Lehrke, S.; Voss, A.; Galuschky, C.; Lossnitzer, D.; Andre, F.; Ehlermann, P.; Franke, J.; Taeger, T.; et al. Assessment of myocardial deformation with cardiac magnetic resonance strain imaging improves risk stratification in patients with dilated cardiomyopathy. Eur. Heart J. Cardiovasc. Imaging 2015, 16, 307–315. [Google Scholar] [CrossRef]
- Riffel, J.H.; Keller, M.G.; Rost, F.; Arenja, N.; Andre, F.; Aus dem Siepen, F.; Fritz, T.; Ehlermann, P.; Taeger, T.; Frankenstein, L.; et al. Left ventricular long axis strain: A new prognosticator in non-ischemic dilated cardiomyopathy? J. Cardiovasc. Magn. Reson. 2016, 18, 36. [Google Scholar] [CrossRef] [PubMed]
- Pi, S.H.; Kim, S.M.; Choi, J.O.; Kim, E.K.; Chang, S.A.; Choe, Y.H.; Lee, S.C.; Jeon, E.S. Prognostic value of myocardial strain and late gadolinium enhancement on cardiovascular magnetic resonance imaging in patients with idiopathic dilated cardiomyopathy with moderate to severely reduced ejection fraction. J. Cardiovasc. Magn. Reson. 2018, 20, 36. [Google Scholar] [CrossRef] [PubMed]
- Kron, I.L.; DiMarco, J.P.; Lerman, B.B.; Nolan, S.P. Resection of scarred papillary muscles improves outcome after surgery for ventricular tachycardia. Ann. Surg. 1986, 203, 685–690. [Google Scholar] [CrossRef]
- Josephson, M.E.; Harken, A.H.; Horowitz, L.N. Endocardial excision: A new surgical technique for the treatment of recurrent ventricular tachycardia. Circulation 1979, 60, 1430–1439. [Google Scholar] [CrossRef]
- Moran, J.M.; Kehoe, R.F.; Loeb, J.M.; Lichtenthal, P.R.; Sanders, J.H., Jr.; Michaelis, L.L. Extended endocardial resection for the treatment of ventricular tachycardia and ventricular fibrillation. Ann. Thorac. Surg. 1982, 34, 538–552. [Google Scholar] [CrossRef]
- Kalarus, Z.; Svendsen, J.H.; Capodanno, D.; Dan, G.A.; De Maria, E.; Gorenek, B.; Jędrzejczyk-Patej, E.; Mazurek, M.; Podolecki, T.; Sticherling, C.; et al. Cardiac arrhythmias in the emergency settings of acute coronary syndrome and revascularization: An European Heart Rhythm Association (EHRA) consensus document, endorsed by the European Association of Percutaneous Cardiovascular Interventions (EAPCI), and European Acute Cardiovascular Care Association (ACCA). Europace 2019, 21, 1603–1604. [Google Scholar] [CrossRef]
- Mavrogeni, S.; Petrou, E.; Kolovou, G.; Theodorakis, G.; Iliodromitis, E. Prediction of ventricular arrhythmias using cardiovascular magnetic resonance. Eur. Heart, J. Cardiovasc. Imaging 2013, 14, 518–525. [Google Scholar] [CrossRef]
- Stevenson, W.G. Ventricular scars and ventricular tachycardia. Trans. Am. Clin. Climatol. Assoc. 2009, 120, 403–412. [Google Scholar] [PubMed]
- Martin, R.; Maury, P.; Bisceglia, C.; Wong, T.; Estner, H.; Meyer, C.; Dallet, C.; Martin, C.A.; Shi, R.; Takigawa, M.; et al. Characteristics of scar-related ventricular tachycardia circuits using ultra-high-density mapping: A multi-center study. Circ. Arrhythmia Electrophysiol. 2018, 11, e006569. [Google Scholar] [CrossRef]
- Fenoglio, J.J., Jr.; Pham, T.D.; Harken, A.H.; Horowitz, L.N.; Josephson, M.E.; Wit, A.L. Recurrent sustained ventricular tachycardia: Structure and ultrastructure of subendocardial regions in which tachycardia originates. Circulation 1983, 68, 518–533. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, A.; Azevedo, C.F.; Cheng, A.; Gupta, S.N.; Bluemke, D.A.; Foo, T.K.; Gerstenblith, G.; Weiss, R.G.; Marbán, E.; Tomaselli, G.F.; et al. Infarct tissue heterogeneity by magnetic resonance imaging identifies enhanced cardiac arrhythmia susceptibility in patients with left ventricular dysfunction. Circulation 2007, 115, 2006–2014. [Google Scholar] [CrossRef] [PubMed]
- Jablonowski, R.; Chaudhry, U.; van der Pals, J.; Engblom, H.; Arheden, H.; Heiberg, E.; Wu, K.C.; Borgquist, R.; Carlsson, M. Cardiovascular magnetic resonance to predict appropriate implantable cardioverter defibrillator therapy in ischemic and nonischemic cardiomyopathy patients using late gadolinium enhancement border zone: Comparison of four analysis methods. Circ. Cardiovasc. Imaging 2017, 10, e006105. [Google Scholar] [CrossRef]
- Roes, S.D.; Borleffs, C.J.; van der Geest, R.J.; Westenberg, J.J.; Marsan, N.A.; Kaandorp, T.A.; Reiber, J.H.; Zeppenfeld, K.; Lamb, H.J.; de Roos, A.; et al. Infarct tissue heterogeneity assessed with contrast-enhanced MRI predicts spontaneous ventricular arrhythmia in patients with ischemic cardiomyopathy and implantable cardioverter-defibrillator. Circ. Cardiovasc. Imaging 2009, 2, 183–190. [Google Scholar] [CrossRef]
- Olausson, E.; Fröjdh, F.; Maanja, M.; Niklasson, L.; Fridman, Y.; Bering, P.; Wertz, J.; Wong, T.; Kellman, P.; Miller, C.; et al. Diffuse myocardial fibrosis measured by extracellular volume associates with incident ventricular arrhythmia in implantable cardioverter defibrillator recipients more than focal fibrosis. J. Am. Coll. Cardiol. 2018, 71, A1454. [Google Scholar] [CrossRef]
- Chen, Z.; Sohal, M.; Voigt, T.; Sammut, E.; Tobon-Gomez, C.; Child, N.; Jackson, T.; Shetty, A.; Bostock, J.; Cooklin, M.; et al. Myocardial tissue characterization by cardiac magnetic resonance imaging using T1 mapping predicts ventricular arrhythmia in ischemic and non-ischemic cardiomyopathy patients with implantable cardioverter-defibrillators. Heart Rhythm 2015, 12, 792–801. [Google Scholar] [CrossRef]
- Gatzoulis, K.A.; Tsiachris, D.; Arsenos, P.; Antoniou, C.K.; Dilaveris, P.; Sideris, S.; Kanoupakis, E.; Simantirakis, E.; Korantzopoulos, P.; Goudevenos, I.; et al. Arrhythmic risk stratification in post-myocardial infarction patients with preserved ejection fraction: The PRESERVE EF study. Eur. Heart, J. 2019, 40, 2940–2949. [Google Scholar] [CrossRef]
- Gatzoulis, K.A.; Antoniou, C.K.; Dilaveris, P.; Chrysohoou, C.; Arsenos, P.; Trachanas, K.; Tousoulis, D. Ventricular arrhythmogenic potential assessment in an asymptomatic ischemic cardiomyopathy patient with a normal ejection fraction. Hell. J. Cardiol. 2017, 58, 443–445. [Google Scholar] [CrossRef]
- Velasquez, A.; Goldberger, J.J. Risk stratification for sudden cardiac death: Show me the money! Eur. Heart, J. 2019, 40, 2950–2952. [Google Scholar] [CrossRef]
- Elliott, P.M.; Anastasakis, A.; Borger, M.A.; Borggrefe, M.; Cecchi, F.; Charron, P.; Hagege, A.A.; Lafont, A.; Limongelli, G.; Mahrholdt, H.; et al. 2014 ESC Guidelines on diagnosis and management of hypertrophic cardiomyopathy: The task force for the diagnosis and management of hypertrophic cardiomyopathy of the European Society of Cardiology (ESC). Eur. Heart J. 2014, 35, 2733–2779. [Google Scholar] [CrossRef]
- Gersh, B.J.; Maron, B.J.; Bonow, R.O.; Dearani, J.A.; Fifer, M.A.; Link, M.S.; Naidu, S.S.; Nishimura, R.A.; Ommen, S.R.; Rakowski, H.; et al. 2011 ACCF/AHA guideline for the diagnosis and treatment of hypertrophic cardiomyopathy: A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation 2011, 124, 2761–2796. [Google Scholar] [CrossRef] [PubMed]
- Maron, B.J.; Casey, S.A.; Chan, R.H.; Garberich, R.F.; Rowin, E.J.; Maron, M.S. Independent assessment of the european society of cardiology sudden death risk model for hypertrophic cardiomyopathy. Am. J. Cardiol. 2015, 116, 757–764. [Google Scholar] [CrossRef] [PubMed]
- Vriesendorp, P.A.; Schinkel, A.F.; Liebregts, M.; Theuns, D.A.; van Cleemput, J.; Ten Cate, F.J.; Willems, R.; Michels, M. Validation of the 2014 European Society of Cardiology guidelines risk prediction model for the primary prevention of sudden cardiac death in hypertrophic cardiomyopathy. Circ. Arrhythmia Electrophysiol. 2015, 8, 829–835. [Google Scholar] [CrossRef] [PubMed]
- O’Mahony, C.; Jichi, F.; Pavlou, M.; Monserrat, L.; Anastasakis, A.; Rapezzi, C.; Biagini, E.; Gimeno, J.R.; Limongelli, G.; McKenna, W.J.; et al. A novel clinical risk prediction model for sudden cardiac death in hypertrophic cardiomyopathy (HCM risk-SCD). Eur. Heart J. 2014, 35, 2010–2020. [Google Scholar] [CrossRef]
- Gatzoulis, K.A.; Georgopoulos, S.; Antoniou, C.K.; Anastasakis, A.; Dilaveris, P.; Arsenos, P.; Sideris, S.; Tsiachris, D.; Archontakis, S.; Sotiropoulos, E.; et al. Programmed ventricular stimulation predicts arrhythmic events and survival in hypertrophic cardiomyopathy. Int. J. Cardiol. 2018, 254, 175–181. [Google Scholar] [CrossRef]
- Maron, B.J.; Rowin, E.J.; Casey, S.A.; Link, M.S.; Lesser, J.R.; Chan, R.H.; Garberich, R.F.; Udelson, J.E.; Maron, M.S. Hypertrophic cardiomyopathy in adulthood associated with low cardiovascular mortality with contemporary management strategies. J. Am. Coll. Cardiol. 2015, 65, 1915–1928. [Google Scholar] [CrossRef] [PubMed]
- Lorenzini, M.; Anastasiou, Z.; O’Mahony, C.; Guttman, O.P.; Gimeno, J.R.; Monserrat, L.; Anastasakis, A.; Rapezzi, C.; Biagini, E.; Garcia-Pavia, P.; et al. Mortality among referral patients with hypertrophic cardiomyopathy vs the general European population. JAMA Cardiol. 2019, 27, e194534. [Google Scholar] [CrossRef]
- Maron, M.S.; Rowin, E.J.; Wessler, B.S.; Mooney, P.J.; Fatima, A.; Patel, P.; Koethe, B.C.; Romashko, M.; Link, M.S.; Maron, B.J. Enhanced American College of Cardiology/American Heart Association strategy for prevention of sudden cardiac death in high-risk patients with hypertrophic cardiomyopathy. JAMA Cardiol. 2019, 4, 644–657. [Google Scholar] [CrossRef]
- Schinkel, A.F.; Vriesendorp, P.A.; Sijbrands, E.J.; Jordaens, L.J.; ten Cate, F.J.; Michels, M. Outcome and complications after implantable cardioverter defibrillator therapy in hypertrophic cardiomyopathy: Systematic review and meta-analysis. Circ. Heart Fail. 2012, 5, 552–559. [Google Scholar] [CrossRef] [PubMed]
- Maron, B.J.; Maron, M.S. LGE means better selection of HCM patients for primary prevention implantable defibrillators. JACC Cardiovasc. Imaging 2016, 9, 1403–1406. [Google Scholar] [CrossRef] [PubMed]
- Ismail, T.F.; Jabbour, A.; Gulati, A.; Mallorie, A.; Raza, S.; Cowling, T.E.; Das, B.; Khwaja, J.; Alpendurada, F.D.; Wage, R.; et al. Role of late gadolinium enhancement cardiovascular magnetic resonance in the risk stratification of hypertrophic cardiomyopathy. Heart 2014, 100, 1851–1858. [Google Scholar] [CrossRef] [PubMed]
- Chan, R.H.; Maron, B.J.; Olivotto, I.; Pencina, M.J.; Assenza, G.E.; Haas, T.; Lesser, J.R.; Gruner, C.; Crean, A.M.; Rakowski, H.; et al. Prognostic value of quantitative contrast-enhanced cardiovascular magnetic resonance for the evaluation of sudden death risk in patients with hypertrophic cardiomyopathy. Circulation 2014, 130, 484–495. [Google Scholar] [CrossRef] [PubMed]
- Adabag, A.S.; Maron, B.J.; Appelbaum, E.; Harrigan, C.J.; Buros, J.L.; Gibson, C.M.; Lesser, J.R.; Hanna, C.A.; Udelson, J.E.; Manning, W.J.; et al. Occurrence and frequency of arrhythmias in hypertrophic cardiomyopathy in relation to delayed enhancement on cardiovascular magnetic resonance. J. Am. Coll. Cardiol. 2008, 51, 1369–1374. [Google Scholar] [CrossRef]
- Leonardi, S.; Raineri, C.; De Ferrari, G.M.; Ghio, S.; Scelsi, L.; Pasotti, M.; Tagliani, M.; Valentini, A.; Dore, R.; Raisaro, A.; et al. Usefulness of cardiac magnetic resonance in assessing the risk of ventricular arrhythmias and sudden death in patients with hypertrophic cardiomyopathy. Eur. Heart J. 2009, 30, 2003–2010. [Google Scholar] [CrossRef]
- Moon, J.C.; McKenna, W.J.; McCrohon, J.A.; Elliott, P.M.; Smith, G.C.; Pennell, D.J. Toward clinical risk assessment in hypertrophic cardiomyopathy with gadolinium cardiovascular magnetic resonance. J. Am. Coll. Cardiol. 2003, 41, 1561–1567. [Google Scholar] [CrossRef]
- Bruder, O.; Wagner, A.; Jensen, C.J.; Schneider, S.; Ong, P.; Kispert, E.M.; Nassenstein, K.; Schlosser, T.; Sabin, G.V.; Sechtem, U.; et al. Myocardial scar visualized by cardiovascular magnetic resonance imaging predicts major adverse events in patients with hypertrophic cardiomyopathy. J. Am. Coll. Cardiol. 2010, 56, 875–887. [Google Scholar] [CrossRef]
- Todiere, G.; Nugara, C.; Gentile, G.; Negri, F.; Bianco, F.; Falletta, C.; Novo, G.; Di Bella, G.; De Caterina, R.; Zachara, E.; et al. Prognostic role of late gadolinium enhancement in patients with hypertrophic cardiomyopathy and low-to-intermediate sudden cardiac death risk score. Am. J. Cardiol. 2019, 124, 1286–1292. [Google Scholar] [CrossRef]
- McLELLAN, A.J.; Ellims, A.H.; Prabhu, S.; Voskoboinik, A.; Iles, L.M.; Hare, J.L.; Kaye, D.M.; Macciocca, I.; Mariani, J.A.; Kalman, J.M.; et al. Diffuse ventricular fibrosis on cardiac magnetic resonance imaging associates with ventricular tachycardia in patients with hypertrophic cardiomyopathy. J. Cardiovasc. Electrophysiol. 2016, 27, 571–580. [Google Scholar] [CrossRef]
- Hen, Y.; Iguchi, N.; Machida, H.; Takada, K.; Utanohara, Y.; Sumiyoshi, T. High signal intensity on T2-weighted cardiac magnetic resonance imaging correlates with the ventricular tachyarrhythmia in hypertrophic cardiomyopathy. Heart Vessel. 2013, 28, 742–749. [Google Scholar] [CrossRef] [PubMed]
- Avanesov, M.; Münch, J.; Weinrich, J.; Well, L.; Säring, D.; Stehning, C.; Tahir, E.; Bohnen, S.; Radunski, U.K.; Muellerleile, K.; et al. Prediction of the estimated 5-year risk of sudden cardiac death and syncope or non-sustained ventricular tachycardia in patients with hypertrophic cardiomyopathy using late gadolinium enhancement and extracellular volume CMR. Eur. Radiol. 2017, 27, 5136–5145. [Google Scholar] [CrossRef] [PubMed]
- Mavrogeni, S.I.; Sfikakis, P.P.; Markousis-Mavrogenis, G.; Bournia, V.K.; Poulos, G.; Koutsogeorgopoulou, L.; Karabela, G.; Stavropoulos, E.; Katsifis, G.; Boki, K.; et al. Cardiovascular magnetic resonance imaging pattern in patients with autoimmune rheumatic diseases and ventricular tachycardia with preserved ejection fraction. Int. J. Cardiol. 2019, 284, 105–109. [Google Scholar] [CrossRef] [PubMed]
- Lazzerini, P.E.; Bertolozzi, I.; Acampa, M.; Fulceri, R.; Laghi-Pasini, F.; Capecchi, P.L. Torsades de pointes in patients with polymyalgia rheumatica. Curr. Pharm. Des. 2018, 24, 323–340. [Google Scholar] [CrossRef] [PubMed]
- Lazzerini, P.E.; Capecchi, P.L.; Laghi-Pasini, F. Systemic inflammation and arrhythmic risk: Lessons from rheumatoid arthritis. Eur. Heart J. 2017, 38, 1717–1727. [Google Scholar] [CrossRef]
- El-Sayed, Z.A.; Mostafa, G.A.; Aly, G.S.; El-Shahed, G.S.; El-Aziz, M.M.; El-Emam, S.M. Cardiovascular autonomic function assessed by autonomic function tests and serum autonomic neuropeptides in Egyptian children and adolescents with rheumatic diseases. Rheumatology 2009, 48, 843–848. [Google Scholar] [CrossRef][Green Version]
- Metwalley, K.A.; Hamed, S.A.; Farghaly, H.S. Cardiac autonomic function in children with type 1 diabetes. Eur. J. Pediatr. 2018, 177, 805–813. [Google Scholar] [CrossRef]
- Dilaveris, P.; Pietri, P.; Tsiachris, D.; Gatzoulis, K.; Stefanadis, C. Inducible ventricular tachycardia due to dermatomyositis-related cardiomyopathy in the era of implantable cardioverter-defibrillator therapy. Circulation 2012, 125, 967–969. [Google Scholar] [CrossRef][Green Version]
- Lubitz, S.A.; Goldbarg, S.H.; Mehta, D. Sudden cardiac death in infiltrative cardiomyopathies: Sarcoidosis, scleroderma, amyloidosis, hemachromatosis. Prog. Cardiovasc. Dis. 2008, 51, 58–73. [Google Scholar] [CrossRef]
- Mavrogeni, S.; Gargani, L.; Pepe, A.; Monti, L.; Markousis-Mavrogenis, G.; De Santis, M.; Marchi, D.; Koutsogeorgopoulou, L.; Karabela, G.; Stavropoulos, E.; et al. Cardiac magnetic resonance predicts ventricular arrhythmias in scleroderma: The Scleroderma Arrhythmia Clinical Utility Study (SAnCtUS). Rheumatology 2019, kez494. [Google Scholar] [CrossRef]
- Dinov, B.; Fiedler, L.; Schönbauer, R.; Bollmann, A.; Rolf, S.; Piorkowski, C.; Hindricks, G.; Arya, A. Outcomes in catheter ablation of ventricular tachycardia in dilated nonischemic cardiomyopathy compared with ischemic cardiomyopathy: Results from the Prospective Heart Centre of Leipzig VT (HELP-VT) Study. Circulation 2014, 129, 728–736. [Google Scholar] [CrossRef] [PubMed]
- Shirai, Y.; Liang, J.J.; Santangeli, P.; Arkles, J.S.; Schaller, R.D.; Supple, G.E.; Nazarian, S.; Garcia, F.C.; Lin, D.; Dixit, S.; et al. Comparison of the ventricular tachycardia circuit between patients with ischemic and nonischemic cardiomyopathies: Detailed characterization by entrainment. Circ. Arrhythmia Electrophysiol. 2019, 12, e007249. [Google Scholar] [CrossRef] [PubMed]
- Koplan, B.A.; Stevenson, W.G. Ventricular tachycardia and sudden cardiac death. Mayo Clin. Proc. 2009, 84, 289–297. [Google Scholar] [CrossRef] [PubMed]
- Antonopoulos, A.S.; Tsiachris, D.; Economou, E.K.; Alexopoulos, N.; Goliopoulou, A.; Gatzoulis, K.A. Behçet’s disease cardiomyopathy: The role of magnetic resonance imaging and electroanatomical mapping in diagnosis and treatment. Case report. Curr. Cardiol. 2017, 1, 42–45. [Google Scholar]
- Mukherjee, R.K.; Chubb, H.; Roujol, S.; Razavi, R.; O’Neill, M.D. Advances in real-time MRI-guided electrophysiology. Version 2. Curr. Cardiovasc. Imaging Rep. 2019, 12, 6. [Google Scholar] [CrossRef]
- Wijnmaalen, A.P.; van der Geest, R.J.; van Huls van Taxis, C.F.; Siebelink, H.M.; Kroft, L.J.; Bax, J.J.; Reiber, J.H.; Schalij, M.J.; Zeppenfeld, K. Head-to-head comparison of contrast-enhanced magnetic resonance imaging and electroanatomical voltage mapping to assess post-infarct scar characteristics in patients with ventricular tachycardias: Real-time image integration and reversed registration. Eur. Heart J. 2011, 32, 104–114. [Google Scholar] [CrossRef]
- Fernández-Armenta, J.; Berruezo, A.; Andreu, D.; Camara, O.; Silva, E.; Serra, L.; Barbarito, V.; Carotenutto, L.; Evertz, R.; Ortiz-Pérez, J.T.; et al. Three-dimensional architecture of scar and conducting channels based on high resolution ce-CMR: Insights for ventricular tachycardia ablation. Circ. Arrhythmia Electrophysiol. 2013, 6, 528–537. [Google Scholar] [CrossRef]
- Andreu, D.; Penela, D.; Acosta, J.; Fernández-Armenta, J.; Perea, R.J.; Soto-Iglesias, D.; de Caralt, T.M.; Ortiz-Perez, J.T.; Prat-González, S.; Borràs, R.; et al. Cardiac magnetic resonance-aided scar dechanneling: Influence on acute and long-term outcomes. Heart Rhythm 2017, 14, 1121–1128. [Google Scholar] [CrossRef]
- Chubb, H.; Harrison, J.L.; Weiss, S.; Krueger, S.; Koken, P.; Bloch, L.Ø.; Kim, W.Y.; Stenzel, G.S.; Wedan, S.R.; Weisz, J.L.; et al. Development, preclinical validation, and clinical translation of a cardiac magnetic resonance—Electrophysiology system with active catheter tracking for ablation of cardiac arrhythmia. JACC Clin. Electrophysiol. 2017, 3, 89–103. [Google Scholar] [CrossRef]
- Mukherjee, R.K.; Costa, C.M.; Neji, R.; Harrison, J.L.; Sim, I.; Williams, S.E.; Whitaker, J.; Chubb, H.; O’Neill, L.; Schneider, R.; et al. Evaluation of a real-time magnetic resonance imaging-guided electrophysiology system for structural and electrophysiological ventricular tachycardia substrate assessment. Europace 2019, 21, 1432–1441. [Google Scholar] [CrossRef]
- Chubb, H.; Williams, S.E.; Whitaker, J.; Harrison, J.L.; Razavi, R.; O’Neill, M. Cardiac electrophysiology under MRI guidance: An Emerging technology. Arrhythmia Electrophysiol. Rev. 2017, 6, 85–93. [Google Scholar] [CrossRef]
- Ranjan, R.; Kholmovski, E.G.; Blauer, J.; Vijayakumar, S.; Volland, N.A.; Salama, M.E.; Parker, D.L.; MacLeod, R.; Marrouche, N.F. Identification and acute targeting of gaps in atrial ablation lesion sets using a real-time magnetic resonance imaging system. Circ. Arrhythmia Electrophysiol. 2012, 5, 1130–1135. [Google Scholar] [CrossRef] [PubMed]
- Celik, H.; Ramanan, V.; Barry, J.; Ghate, S.; Leber, V.; Oduneye, S.; Gu, Y.; Jamali, M.; Ghugre, N.; Stainsby, J.A.; et al. Intrinsic contrast for characterization of acute radiofrequency ablation lesions. Circ. Arrhythmia Electrophysiol. 2014, 7, 718–727. [Google Scholar] [CrossRef] [PubMed]
- Dickfeld, T.; Kato, R.; Zviman, M.; Nazarian, S.; Dong, J.; Ashikaga, H.; Lardo, A.C.; Berger, R.D.; Calkins, H.; Halperin, H. Characterization of acute and subacute radiofrequency ablation lesions with nonenhanced magnetic resonance imaging. Heart Rhythm 2007, 4, 208–214. [Google Scholar] [CrossRef] [PubMed]
- Arujuna, A.; Karim, R.; Caulfield, D.; Knowles, B.; Rhode, K.; Schaeffter, T.; Kato, B.; Rinaldi, C.A.; Cooklin, M.; Razavi, R.; et al. Acute pulmonary vein isolation is achieved by a combination of reversible and irreversible atrial injury after catheter ablation: Evidence from magnetic resonance imaging. Circ. Arrhythmia Electrophysiol. 2012, 5, 691–700. [Google Scholar] [CrossRef] [PubMed]
- Harrison, J.L.; Jensen, H.K.; Peel, S.A.; Chiribiri, A.; Grøndal, A.K.; Bloch, L.Ø.; Pedersen, S.F.; Bentzon, J.F.; Kolbitsch, C.; Karim, R.; et al. Cardiac magnetic resonance and electroanatomical mapping of acute and chronic atrial ablation injury: A histological validation study. Eur. Heart J. 2014, 35, 1486–1495. [Google Scholar] [CrossRef]
- Vergara, G.R.; Vijayakumar, S.; Kholmovski, E.G.; Blauer, J.J.; Guttman, M.A.; Gloschat, C.; Payne, G.; Vij, K.; Akoum, N.W.; Daccarett, M.; et al. Real-time magnetic resonance imaging-guided radiofrequency atrial ablation and visualization of lesion formation at 3 Tesla. Heart Rhythm 2011, 8, 295–303. [Google Scholar] [CrossRef]
- Tao, S.; Guttman, M.A.; Fink, S.; Elahi, H.; Patil, K.D.; Ashikaga, H.; Kolandaivelu, A.D.; Berger, R.D.; Halushka, M.K.; Schmidt, E.J.; et al. Ablation lesion characterization in scarred substrate assessed using cardiac magnetic resonance. JACC Clin. Electrophysiol. 2019, 5, 91–100. [Google Scholar] [CrossRef]
- Toupin, S.; Bour, P.; Lepetit-Coiffé, M.; Ozenne, V.; Denis de Senneville, B.; Schneider, R.; Vaussy, A.; Chaumeil, A.; Cochet, H.; Sacher, F.; et al. Feasibility of real-time MR thermal dose mapping for predicting radiofrequency ablation outcome in the myocardium in vivo. J. Cardiovasc. Magn. Reson. 2017, 19, 14. [Google Scholar] [CrossRef]
- Wang, P. Evaluation of MR thermometry with proton resonance frequency method at 7T. Quant. Imaging Med. Surg. 2017, 7, 259–266. [Google Scholar] [CrossRef]
- Gatzoulis, K.A.; Vouliotis, A.I.; Tsiachris, D.; Salourou, M.; Archontakis, S.; Dilaveris, P.; Gialernios, T.; Arsenos, P.; Karystinos, G.; Sideris, S.; et al. Primary prevention of sudden cardiac death in a nonischemic dilated cardiomyopathy population: Reappraisal of the role of programmed ventricular stimulation. Circ. Arrhythmia Electrophysiol. 2013, 6, 504–512. [Google Scholar] [CrossRef] [PubMed]
- Haugaa, K.H.; Goebel, B.; Dahlslett, T.; Meyer, K.; Jung, C.; Lauten, A.; Figulla, H.R.; Poerner, T.C.; Edvardsen, T. Risk assessment of ventricular arrhythmias in patients with nonischemic dilated cardiomyopathy by strain echocardiography. J. Am. Soc. Echocardiogr. 2012, 25, 667–673. [Google Scholar] [CrossRef] [PubMed]
- Haugaa, K.H.; Grenne, B.L.; Eek, C.H.; Ersbøll, M.; Valeur, N.; Svendsen, J.H.; Florian, A.; Sjøli, B.; Brunvand, H.; Køber, L.; et al. Strain echocardiography improves risk prediction of ventricular arrhythmias after myocardial infarction. JACC Cardiovasc. Imaging 2013, 6, 841–850. [Google Scholar] [CrossRef] [PubMed]
- Travin, M.I.; Feng, D.; Taub, C.C. Novel imaging approaches for predicting arrhythmic risk. Circ. Cardiovasc. Imaging 2015, 8, e003019. [Google Scholar] [CrossRef]
- Li, X.; Fan, X.; Li, S.; Sun, W.; Shivkumar, K.; Zhao, S.; Lu, M.; Yao, Y. A novel risk stratification score for sudden cardiac death prediction in middle-aged, nonischemic dilated cardiomyopathy patients: The ESTIMATED score. Can. J. Cardiol. 2020, 36, 1121–1129. [Google Scholar] [CrossRef]
- Selvanayagam, J.B.; Hartshorne, T.; Billot, L.; Grover, S.; Hillis, G.S.; Jung, W.; Krum, H.; Prasad, S.; McGavigan, A.D. Cardiovascular magnetic resonance-GUIDEd management of mild to moderate left ventricular systolic dysfunction (CMR GUIDE): Study protocol for a randomized controlled trial. Ann. Noninvasive Electrocardiol. 2017, 22, e12420. [Google Scholar] [CrossRef]
- Gatzoulis, K.A.; Dilaveris, P.; Arsenos, P.; Tsiachris, D.; Antoniou, C.K.; Sideris, S.; Kolettis, T.; Kanoupakis, E.; Sideris, A.; Flevari, P.; et al. Arrhythmic risk stratification in nonischemic dilated cardiomyopathy: The ReCONSIDER study design—A two-step, multifactorial, electrophysiology-inclusive approach. Hell. J. Cardiol. 2020, 21, S1109-9666(20)30075-0. [Google Scholar] [CrossRef]
- Monfredi, O.; Calkins, H. Was a mistake made when programmed electrical stimulation was eliminated as a sudden death risk marker in hypertrophic cardiomyopathy? Int. J. Cardiol. 2018, 254, 238–239. [Google Scholar] [CrossRef]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kariki, O.; Antoniou, C.-K.; Mavrogeni, S.; Gatzoulis, K.A. Updating the Risk Stratification for Sudden Cardiac Death in Cardiomyopathies: The Evolving Role of Cardiac Magnetic Resonance Imaging. An Approach for the Electrophysiologist. Diagnostics 2020, 10, 541. https://doi.org/10.3390/diagnostics10080541
Kariki O, Antoniou C-K, Mavrogeni S, Gatzoulis KA. Updating the Risk Stratification for Sudden Cardiac Death in Cardiomyopathies: The Evolving Role of Cardiac Magnetic Resonance Imaging. An Approach for the Electrophysiologist. Diagnostics. 2020; 10(8):541. https://doi.org/10.3390/diagnostics10080541
Chicago/Turabian StyleKariki, Ourania, Christos-Konstantinos Antoniou, Sophie Mavrogeni, and Konstantinos A. Gatzoulis. 2020. "Updating the Risk Stratification for Sudden Cardiac Death in Cardiomyopathies: The Evolving Role of Cardiac Magnetic Resonance Imaging. An Approach for the Electrophysiologist" Diagnostics 10, no. 8: 541. https://doi.org/10.3390/diagnostics10080541
APA StyleKariki, O., Antoniou, C.-K., Mavrogeni, S., & Gatzoulis, K. A. (2020). Updating the Risk Stratification for Sudden Cardiac Death in Cardiomyopathies: The Evolving Role of Cardiac Magnetic Resonance Imaging. An Approach for the Electrophysiologist. Diagnostics, 10(8), 541. https://doi.org/10.3390/diagnostics10080541






