Concurrent Validity of Digital Vascular Auscultation for the Assessment of Blood Flow Obliteration on the Radial Artery in Healthy Subjects
Abstract
1. Introduction
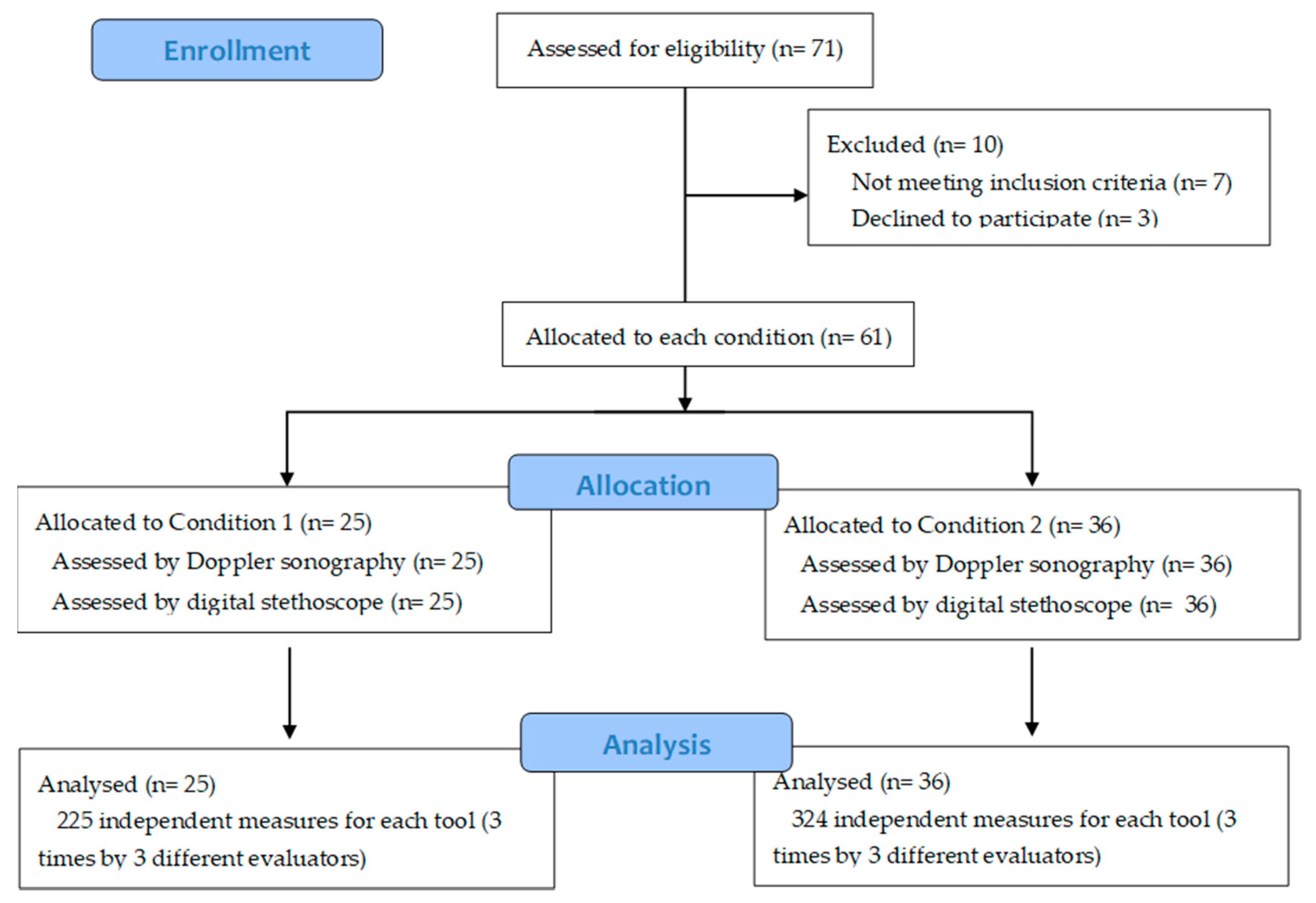
2. Materials and Methods
2.1. Subjects
2.2. Instruments
2.3. Procedure
Assessment
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Laulan, J.; Fouquet, B.; Rodaix, C.; Jauffret, P.; Roquelaure, Y.; Descatha, A. Thoracic outlet syndrome: Definition, aetiological factors, diagnosis, management and occupational impact. J. Occup. Rehabil. 2011, 21, 366–373. [Google Scholar] [CrossRef]
- Illig, K.A.; Donahue, D.; Duncan, A.; Freischlag, J.; Gelabert, H.; Johansen, K.; Jordan, S.; Sanders, R.; Thompson, R. Reporting standards of the Society for Vascular Surgery for thoracic outlet syndrome. J. Vasc. Surg. 2016, 64, e23–e35. [Google Scholar] [CrossRef]
- Ferrante, M.A.; Ferrante, N.D. The thoracic outlet syndromes: Part 1. Overview of the thoracic outlet syndromes and review of true neurogenic thoracic outlet syndrome. Muscle Nerve 2017, 55, 782–793. [Google Scholar] [CrossRef] [PubMed]
- Ferrante, M.A.; Ferrante, N.D. The thoracic outlet syndromes: Part 2. The Arterial, Venous, Neurovascular and Disputed Thoracic Outlet Syndrome. Muscle Nerve 2017, 56, 663–673. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Xu, Z.; Chen, J.; Liu, Z.; Wang, T.; Hu, Y.; Shen, L.; Xue, F. A novel approach for imaging of thoracic outlet syndrome using contrast-enhanced magnetic resonance angiography (CE-MRA), short inversion time inversion recovery sampling perfection with application-optimized contrasts using different flip angle evolution. Med. Sci. Monit. 2019, 25, 7617–7623. [Google Scholar] [CrossRef] [PubMed]
- Weaver, M.; Lum, Y. New Diagnostic and Treatment Modalities for Neurogenic Thoracic Outlet Syndrome. Diagnostics 2017, 7, 28. [Google Scholar] [CrossRef] [PubMed]
- Povlsen, S.; Povlsen, B. Diagnosing thoracic outlet syndrome: Current approaches and future directions. Diagnostics 2018, 8, 21. [Google Scholar] [CrossRef]
- Masocatto, N.O.; Da-Matta, T.; Prozzo, T.G.; Couto, W.J.; Porfirio, G. Thoracic outlet syndrome: A narrative review. Revista do Colégio Brasileiro de Cirurgiões 2019, 46, 1–7. [Google Scholar]
- Hixson, K.; Horris, H.; Valovich-McLeod, T.; Welch-Bacon, C. The Diagnostic Accuracy of Clinical Diagnostic Tests for Thoracic Outlet Syndrome. J. Sport Rehabil. 2017, 26, 456–465. [Google Scholar] [CrossRef]
- Dessureault-Dober, I.; Bronchti, G.; Bussières, A. Diagnostic Accuracy of Clinical Tests for Neurogenic and Vascular Thoracic Outlet Syndrome: A Systematic Review. J. Manip. Physiol. Ther. 2018, 41, 789–799. [Google Scholar] [CrossRef]
- Jones, M.R.; Prabhakar, A.; Viswanath, O.; Urits, I.; Green, J.B.; Kendrick, J.B.; Brunk, A.J.; Eng, M.R.; Orhurhu, V.; Cornett, E.M.; et al. Thoracic Outlet Syndrome: A Comprehensive Review of Pathophysiology, Diagnosis, and Treatment. Pain Ther. 2019, 8, 5–18. [Google Scholar] [CrossRef]
- Gillard, J.; Pérez-Cousin, M.; Hachulla, É.; Remy, J.; Hurtevent, J.F.; Vinckier, L.; Thevénon, A.; Duquesnoy, B. Diagnosing thoracic outlet syndrome: Contribution of provocative tests, ultrasonography, electrophysiology, and helical computed tomography in 48 patients. Jt. Bone Spine 2001, 68, 416–424. [Google Scholar] [CrossRef]
- Likes, K.; Rochlin, D.H.; Salditch, Q.; Dapash, T.; Baker, Y.; Deguzman, R.; Selvarajah, S.; Freischlag, J.A. Diagnostic accuracy of physician and self-referred patients for thoracic outlet syndrome is excellent. Ann. Vasc. Surg. 2014, 28, 1100–1105. [Google Scholar] [CrossRef] [PubMed]
- Hardy, A.; Pougès, C.; Wavreille, G.; Behal, H.; Demondion, X.; Lefebvre, G. Thoracic Outlet Syndrome: Diagnostic Accuracy of MRI. Orthop. Traumatol. Surg. Res. 2019, 105, 1563–1569. [Google Scholar] [CrossRef] [PubMed]
- Molina, J.E.; D’Cunha, J. The vascular component in neurogenic-arterial thoracic outlet syndrome. Int. J. Angiol. 2008, 17, 83–87. [Google Scholar] [CrossRef] [PubMed]
- Özcan, H.N.; Kara, M.; Özcan, F.; Bostanoǧlu, S.; Karademir, M.A.; Erkin, G.; Özçakar, L. Dynamic doppler evaluation of the radial and ulnar arteries in patients with carpal tunnel syndrome. Am. J. Roentgenol. 2011, 197, 817–820. [Google Scholar] [CrossRef]
- Hartley, C.; Reddy, A.; Madala, S.; Entman, M.; Taffet, G. Feasibility of dual Doppler velocity measurements to estimate volume pulsations of an arterial segment. Ultrasound Med. Biol. 2010, 36, 1169–1175. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3624763/pdf/nihms412728.pdf (accessed on 18 March 2020). [CrossRef]
- Demondion, X.; Vidal, C.; Herbinet, P.; Gautier, C.; Duquesnoy, B.; Cotton, A. Ultrasonographic assessment of arterial cross-sectional area in the thoracic outlet on postural maneuvers measured with power Doppler ultrasonography in both asymptomatic and symptomatic populations. J. Ultrasound Med. 2006, 25, 217–224. [Google Scholar] [CrossRef]
- Makaryus, A.N.; Makaryus, J.N.; Figgatt, A.; Mulholland, D.; Kushner, H.; Semmlow, J.L.; Mieres, J.; Taylor, A.J. Utility of an advanced digital electronic stethoscope in the diagnosis of coronary artery disease compared with coronary computed tomographic angiography. Am. J. Cardiol. 2013, 111, 786–792. [Google Scholar] [CrossRef]
- Sztajzel, J.M.; Picard-Kossovsky, M.; Lerch, R.; Vuille, C.; Sarasin, F.P. Accuracy of cardiac auscultation in the era of Doppler-echocardiography: A comparison between cardiologists and internists. Int. J. Cardiol. 2010, 138, 308–310. [Google Scholar] [CrossRef]
- Takahashi, O.; Shimbo, T.; Rahman, M.; Musa, R.; Kurokawa, W.; Yoshinaka, T.; Fukui, T. Validation of the auscultatory method for diagnosing peripheral arterial disease. Fam. Pract. 2006, 23, 10–14. [Google Scholar] [CrossRef] [PubMed]
- Carmo, G.; Mandil, A.; Nascimento, B.R.; Arantes, B.D.; Bittencourt, J.C.; Falqueto, E.B.; Ribeiro, A.L. Can we measure the ankle—Brachial index using only a stethoscope? A pilot study. Fam. Pract. 2009, 26, 22–26. [Google Scholar] [PubMed]
- Jou, L.D.; Mawad, M.E. Indirect measurement of aneurysm wall thickness using digital stethoscope. Neurol. Res. 2010, 32, 661–665. [Google Scholar] [CrossRef] [PubMed]
- Van Der Hoeven, N.V.; Van Den Born, B.J.H.; Van Montfrans, G.A. Reliability of palpation of the radial artery compared with auscultation of the brachial artery in measuring SBP. J. Hypertens. 2011, 29, 51–55. [Google Scholar] [CrossRef] [PubMed]
- Whiting, P.; Rutjes, W.; Westwood, M.; Mallet, S.; Deeks, J.; Reitsma, J.; Leeflang, M.M.G.; Sterne, J.A.C.; Bossuyt, P.M.M. QUADAS-2: A Revised Tool for the Quality Assessment of Diagnostic Accuracy Studies. Ann. Intern. Med. 2011, 55, 529–538. [Google Scholar] [CrossRef]
- Mokkink, L.B.; Terwee, C.B.; Patrick, D.L.; Alonso, J.; Stratford, P.W.; Knol, D.L.; Bouter, L.M.; de Vet, H.C.W. The COSMIN checklist for assessing the methodological quality of studies on measurement properties of health status measurement instruments: An international Delphi study. Qual. Life Res. 2010, 19, 539–549. [Google Scholar] [CrossRef] [PubMed]
- Perloff, D.; Grim, C.; Flack, J.; Frohlich, E.D.; Hill, M.; McDonald, M.; Morgenstern, B.Z. Human Blood Pressure Determination by Sphygmomanometry. Circulation 1993, 88, 2460–2470. [Google Scholar] [CrossRef]
- Endres, H.G.; Hucke, C.; Holland-Letz, T.; Trampisch, J. A new efficient trial design for assessing reliability of ankle-brachial index measures by three different observer groups. BMC Cardiovasc. Disord. 2006, 6, 1–10. [Google Scholar] [CrossRef]
- Chesbro, S.B.; Asongwed, E.T.; Brown, J.; John, E.B. Reliability of doppler and stethoscope methods of determining systolic blood pressures: Considerations for calculating an ankle-brachial index. J. Natl. Med. Assoc. 2011, 103, 863–869. [Google Scholar] [CrossRef]
- Kaufmann, C.; Jacomella, V.; Kovacicova, L.; Husmann, M.; Clemens, R.K.; Thalhammer, C.; Amannvesti, B. Predictive value of auscultation of femoropopliteal arteries. Swiss Med. Wkly. 2013, 143, 1–5. [Google Scholar] [CrossRef]
| Sample Characteristics | Study Group | Total | Chi-Squared | p-Value | ||
|---|---|---|---|---|---|---|
| Condition 1 | Condition 2 | |||||
| Gender | Male | 8 | 10 | 18 | 0.126 | 0.722 |
| Female | 17 | 26 | 43 | |||
| Aged | 20–29 | 3 | 7 | 10 | 2.622 | 0.758 |
| 30–39 | 1 | 2 | 3 | |||
| 40–49 | 4 | 5 | 9 | |||
| 50–59 | 2 | 6 | 8 | |||
| 60–69 | 13 | 15 | 28 | |||
| 70–79 | 2 | 1 | 3 | |||
| Handedness | Right | 23 | 34 | 57 | 0.114 | 0.704 |
| Left | 2 | 2 | 4 | |||
| Tobacco consumption | Smoker | 3 | 3 | 6 | 1.740 | 0.419 |
| Former smoker | 4 | 11 | 15 | |||
| Non-smoker | 18 | 22 | 40 | |||
| Treatment for Hypertension | Yes | 13 | 17 | 30 | 0.135 | 0.714 |
| No | 12 | 19 | 31 | |||
| Diabetes | Yes | 4 | 7 | 11 | 0.118 | 0.731 |
| No | 21 | 29 | 50 | |||
| Hypertension non-diabetic(BP of [140/90] mmHg) | Yes | 13 | 16 | 29 | 0.338 | 0.561 |
| No | 12 | 20 | 32 | |||
| Hypertension diabetic(BP of [130/80] mmHg) | Yes | 4 | 6 | 10 | 0.005 | 0.945 |
| No | 21 | 30 | 51 | |||
| Tool | Study Group | p-Value | ||
|---|---|---|---|---|
| Condition 1 | Condition 2 | |||
| Doppler | No change | 220 | 1 | <0.0001 |
| Change | 5 | 323 | ||
| Stethoscope | No change | 224 | 12 | <0.0001 |
| Change | 1 | 312 | ||
| Tool | Doppler | Total | Kappa | ASE | p-Value | ||
|---|---|---|---|---|---|---|---|
| No Change | Change | ||||||
| Stethoscope | No change | 220 (40.1%) | 16 (2.9%) | 236 | 0.936 | 0.015 | <0.0001 |
| Change | 1 (2%) | 312 (56.8%) | 313 | ||||
| Total | 221 (40.3%) | 328 (59.7%) | 549 | ||||
| Tool | Doppler | ||||
|---|---|---|---|---|---|
| Yes | No | Total | |||
| Stethoscope | Yes | 312 | 1 | 313 | |
| No | 16 | 220 | 236 | ||
| Total | 328 | 221 | 549 | ||
| Se 0.95 (95%) | Sp 0.99 (99%) | PPV 0.99 (99%) | NPV 0.93 (93%) | LR+ 95 | LR− 0.05 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cortés-Vega, M.-D.; Casuso-Holgado, M.J.; Oliva-Pascual-Vaca, Á.; García-Bernal, M.-I.; González-García, P.; Rodríguez-Blanco, C. Concurrent Validity of Digital Vascular Auscultation for the Assessment of Blood Flow Obliteration on the Radial Artery in Healthy Subjects. Diagnostics 2020, 10, 494. https://doi.org/10.3390/diagnostics10070494
Cortés-Vega M-D, Casuso-Holgado MJ, Oliva-Pascual-Vaca Á, García-Bernal M-I, González-García P, Rodríguez-Blanco C. Concurrent Validity of Digital Vascular Auscultation for the Assessment of Blood Flow Obliteration on the Radial Artery in Healthy Subjects. Diagnostics. 2020; 10(7):494. https://doi.org/10.3390/diagnostics10070494
Chicago/Turabian StyleCortés-Vega, María-Dolores, María Jesús Casuso-Holgado, Ángel Oliva-Pascual-Vaca, María-Isabel García-Bernal, Paula González-García, and Cleofás Rodríguez-Blanco. 2020. "Concurrent Validity of Digital Vascular Auscultation for the Assessment of Blood Flow Obliteration on the Radial Artery in Healthy Subjects" Diagnostics 10, no. 7: 494. https://doi.org/10.3390/diagnostics10070494
APA StyleCortés-Vega, M.-D., Casuso-Holgado, M. J., Oliva-Pascual-Vaca, Á., García-Bernal, M.-I., González-García, P., & Rodríguez-Blanco, C. (2020). Concurrent Validity of Digital Vascular Auscultation for the Assessment of Blood Flow Obliteration on the Radial Artery in Healthy Subjects. Diagnostics, 10(7), 494. https://doi.org/10.3390/diagnostics10070494








