Bioanalytical Performance of a New Particle-Enhanced Method for Measuring Procalcitonin
Abstract
1. Introduction
2. Material and Methods
2.1. PETIA (Particle-Enhanced) Methods for PCT Measurements
2.2. BRAHMS PCT Kryptor CompactPlus© Method for PCT Measurements
2.3. Analytical Performances of the PETIA PCT Immunoassays
2.4. Interference Studies on PETIA Methods for PCT Quantification
2.5. Comparison Studies
2.6. Statistical Analysis
3. Results
3.1. Analytical Performances
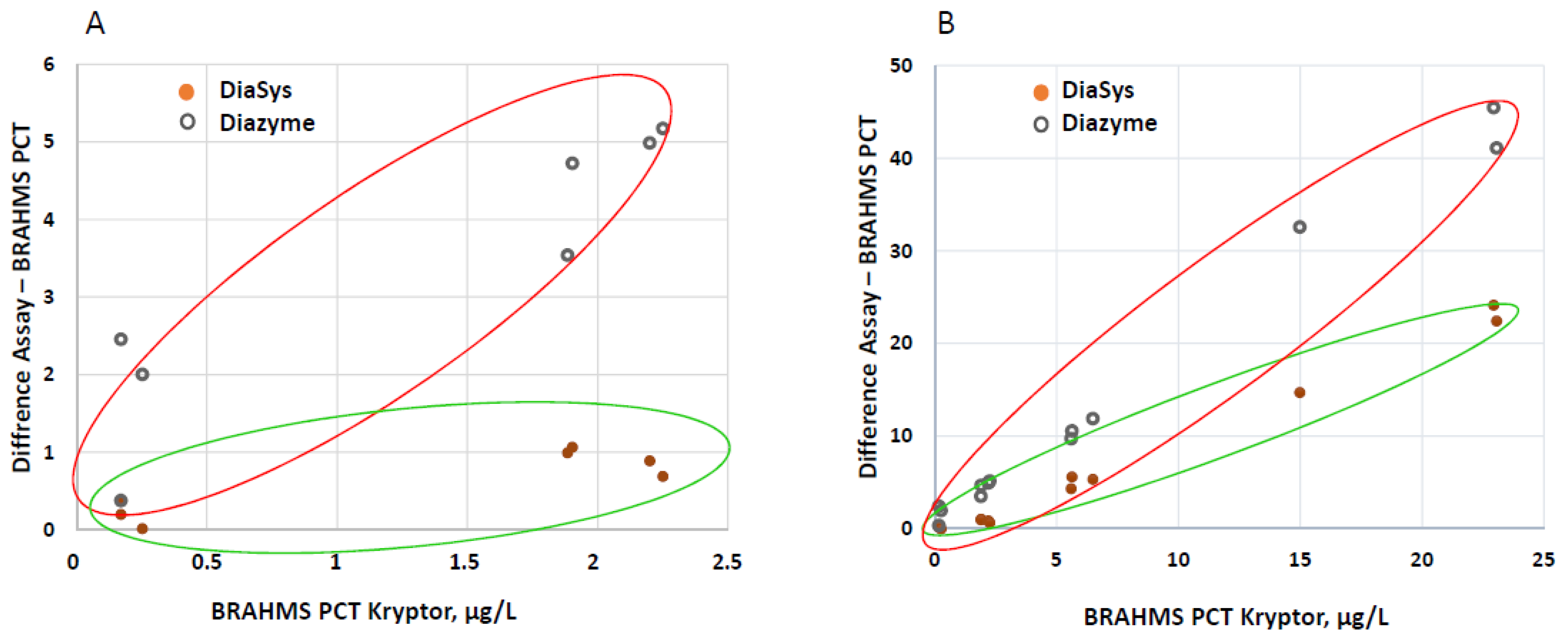
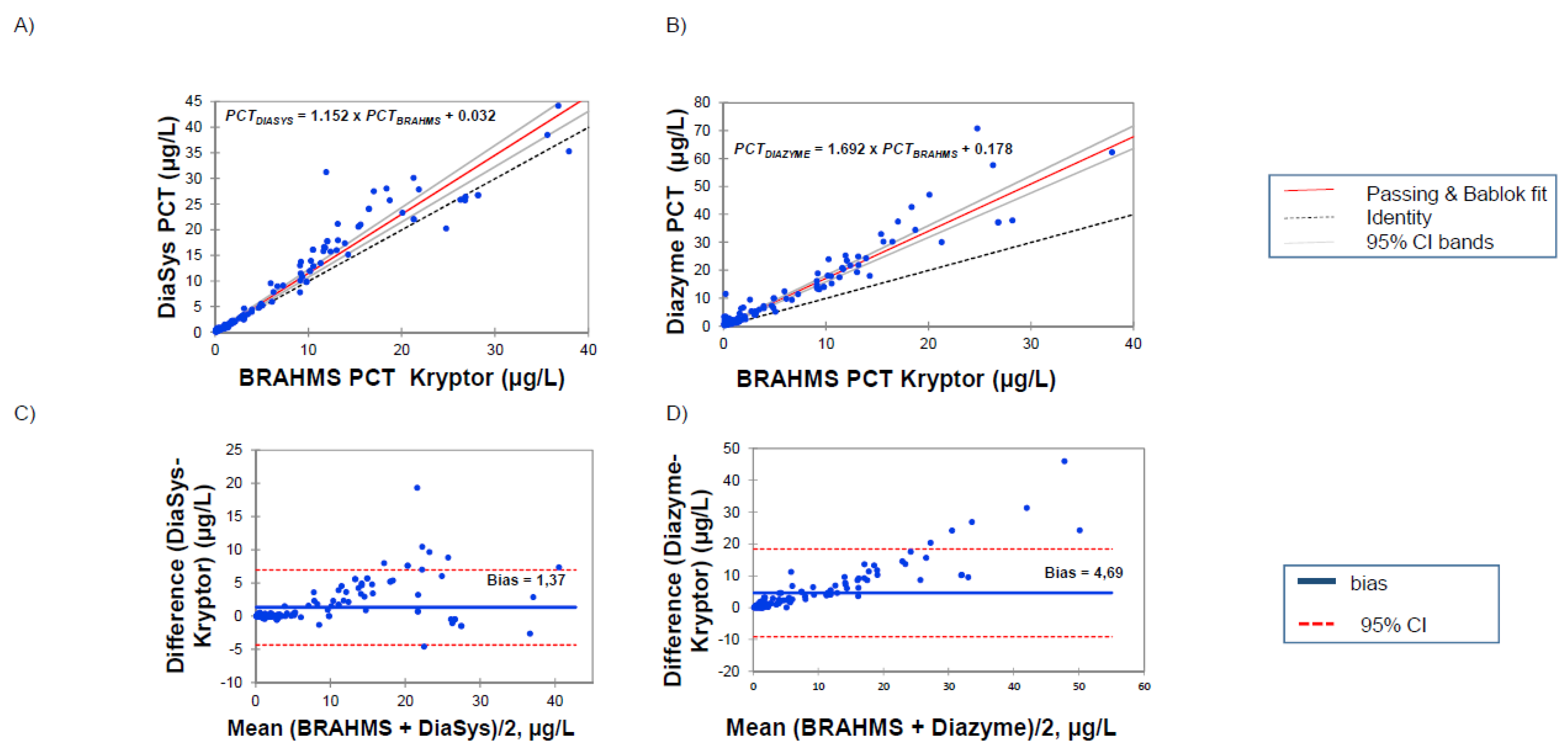
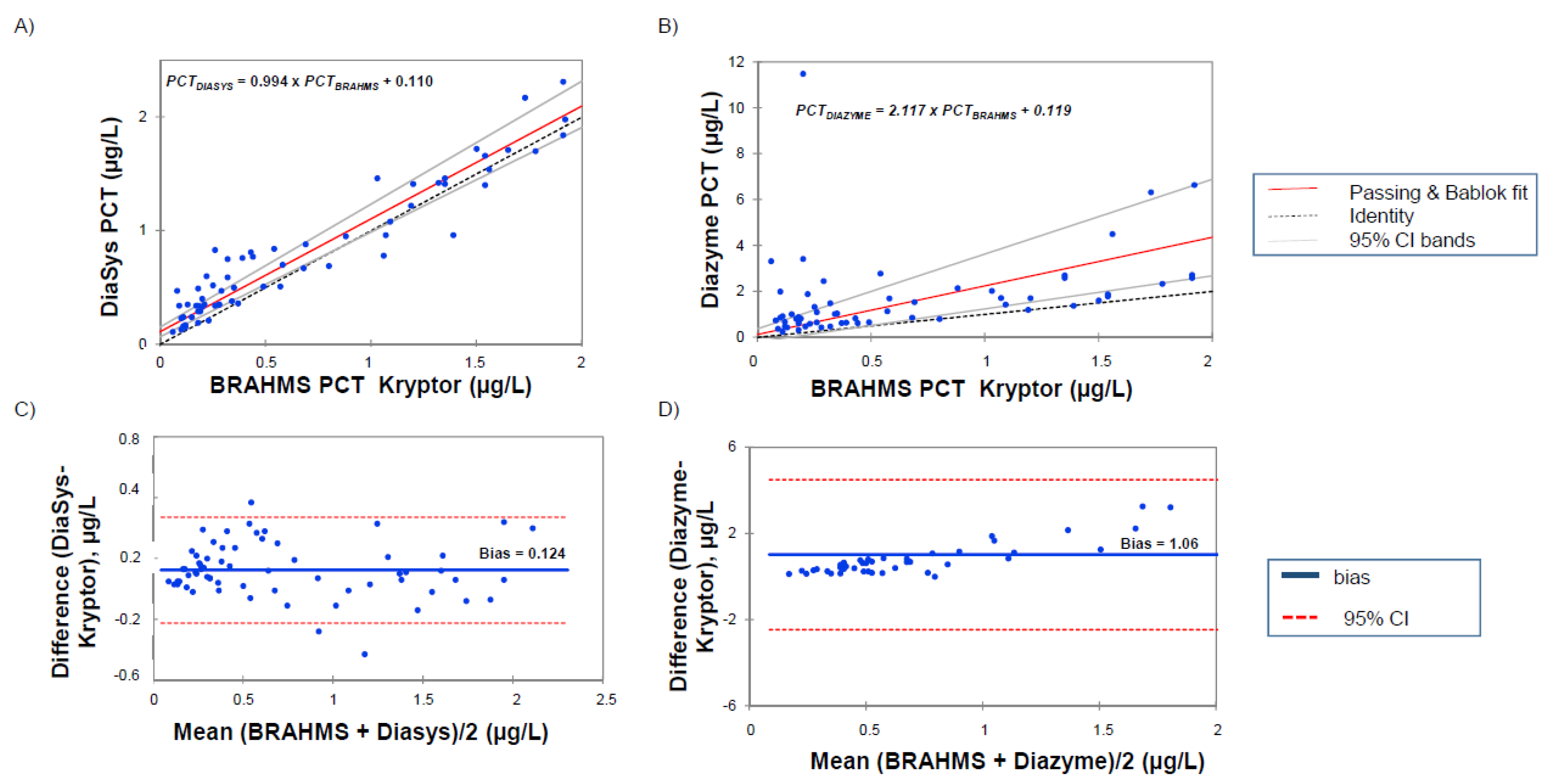
3.2. Correlations between PETIA and BRAHMS PCT Assays
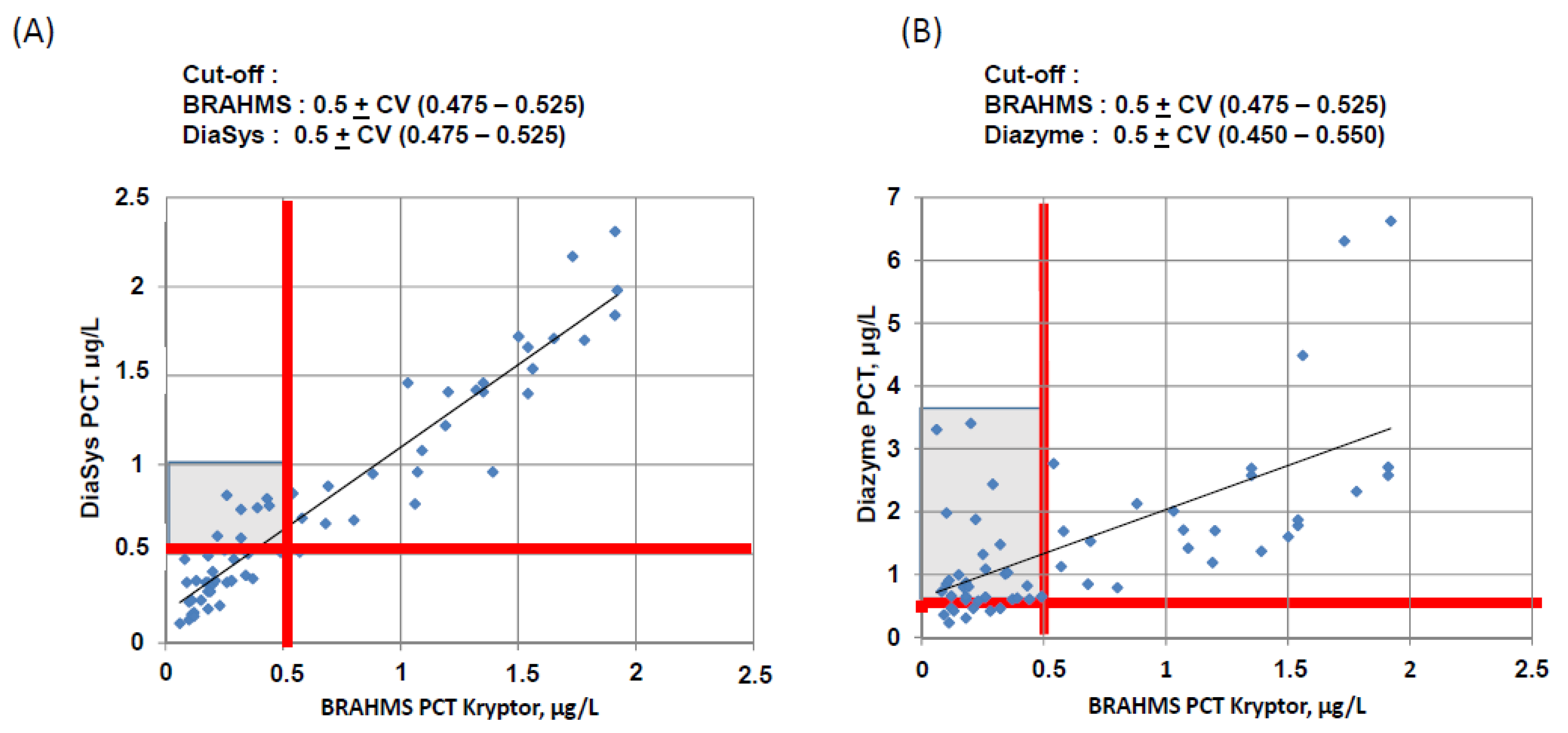
3.3. Concordance at the Cut-Off Levels
3.4. Analysis of the EQA Samples
4. Discussion
4.1. Performance Assessment
4.2. Comparison with BRAHMS PCT Kryptor and Clinical Concordance
4.3. Lack of Standardization
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| (i) | PCT DiaSys Assay | BRAHMS PCT Assay | ||||
| PCT < 0.25 | 0.25 ≤ PCT < 0.5 | 0.5 ≤ PCT < 2 | PCT ≥ 2 | Kappa Test | ||
| PCT < 0.25 | 10 | 2 | 0 | 0 | 0.695 (95%CI, 0.605 to 0.786) | |
| 0.25 ≤ PCT < 0.5 | 11 | 2 | 0 | 0 | ||
| 0.5 ≤ PCT < 2 | 2 | 9 | 25 | 0 | ||
| PCT ≥ 2 | 0 | 0 | 2 | 73 | ||
| (ii) | PCT Diazyme Assay | BRAHMS PCT Assay | ||||
| PCT < 0.25 | 0.25 ≤ PCT < 0.5 | 0.5 ≤ PCT < 2 | PCT ≥ 2 | Kappa Test | ||
| PCT < 0.25 | 3 | 0 | 0 | 0 | 0.401 (95%CI, 0.306 to 0.496) | |
| 0.25 ≤ PCT < 0.5 | 7 | 2 | 0 | 0 | ||
| 0.5 ≤ PCT < 2 | 15 | 12 | 14 | 0 | ||
| PCT ≥ 2 | 4 | 2 | 12 | 65 | ||
References
- Schuetz, P.; Wirz, Y.; Sager, R.; Christ-Crain, M.; Stolz, D.; Tamm, M.; Bouadma, L.; Luyt, C.E.; Wolff, M.; Chastre, J.; et al. Effect of procalcitonin-guided antibiotic treatment on mortality in acute respiratory infections: A patient level meta-analysis. Lancet Infect. Dis. 2018, 18, 95–107. [Google Scholar] [CrossRef]
- Schuetz, P.; Beishuizen, A.; Broyles, M.; Ferrer, R.; Gavazzi, G.; Gluck, E.H.; Del Castillo, J.G.; Jensen, J.-U.; Kanizsai, P.L.; Kwa, A.L.H.; et al. Procalcitonin (PCT)-guided antibiotic stewardship: An international experts consensus on optimized clinical use. Clin. Chem. Lab. Med. 2019, 27, 1308–1318. [Google Scholar] [CrossRef] [PubMed]
- Lippi, G.; Plebani, M. Laboratory abnormalities in patients with COVID-2019 infection. Clin. Chem. Lab. Med. 2020, 58, 1131–1134. [Google Scholar] [CrossRef] [PubMed]
- Lippi, G.; Plebani, M. Procalcitonin in patients with severe coronavirus disease 2019 (COVID-19): A meta-analysis. Clin. Chim. Acta 2020, 505, 190–191. [Google Scholar] [CrossRef]
- Sager, R.; Wirz, Y.; Amin, D.; Amin, A.; Hausfater, P.; Huber, A.; Haubitz, S.; Kutz, A.; Mueller, B.; Schuetz, P. Are admission procalcitonin levels universal mortality predictors across different medical emergency patient populations? Results from the multi-national, prospective, observational TRIAGE study. Clin. Chem. Lab. Med. 2017, 55, 1873–1880. [Google Scholar] [CrossRef]
- Viallon, A.; Desseigne, N.; Marjollet, O.; Birynczyk, A.; Belin, M.; Guyomarch, S.; Borg, J.; Pozetto, B.; Bertrand, J.C.; Zeni, F. Meningitis in adult patients with a negative direct cerebrospinal fluid examination: Value of cytochemical markers for differential diagnosis. Crit. Care 2011, 15, R136. [Google Scholar] [CrossRef]
- McGill, F.; Heyderman, R.S.; Michael, B.D.; Defres, S.; Beeching, N.J.; Borrow, R.; Glennie, L.; Gaillemin, O.; Wyncoll, D.; Kaczmarski, E.; et al. The UK joint specialist societies guideline on the diagnosis and management of acute meningitis and meningococcal sepsis in immunocompetent adults. J. Infect. 2016, 72, 405–438. [Google Scholar] [CrossRef]
- Bouadma, L.; Luyt, C.E.; Tubach, F.; Cracco, C.; Alvarez, A.; Schwebel, C.; Schortgen, F.; Lasocki, S.; Veber, B.; Dehoux, M.; et al. Use of procalcitonin to reduce patients’ exposure to antibiotics in intensive care units (PRORATA trial): A multicentre randomised controlled trial. Lancet 2010, 375, 463–474. [Google Scholar] [CrossRef]
- Pepper, D.J.; Sun, J.; Rhee, C.; Welsh, J.; Powers, J.H., III; Danner, R.L.; Kadri, S.S. Procalcitonin-guided antibiotic discontinuation and mortality in critically ill adults: A systematic review and meta-analysis. Chest 2019, 155, 1109–1118. [Google Scholar] [CrossRef]
- Dupuy, A.M.; Chevrier, Q.; Olejnik, Y.; Bargnoux, A.S.; Badiou, S.; Cristol, J.P. Analytical evaluation of point-of-care procalcitonin (PCT) and clinical performances in an unselected population as compared with central lab PCT assay. Clin. Chem. Lab. Med. 2017, 55, e167–e171. [Google Scholar] [CrossRef]
- Dupuy, A.M.; Bargnoux, A.S.; Andreeva, A.; Zins, C.; Kuster, N.; Badiou, S.; Cristol, J.P. Analytical performances of a novel point-of-care procalcitonin assay. Pract. Lab. Med. 2019, 26, e00145. [Google Scholar] [CrossRef] [PubMed]
- Dipalo, M.; Buonocore, R.; Gnocchi, C.; Picanza, A.; Aloe, R.; Lippi, G. Analytical evaluation of Diazyme procalcitonin (PCT) latex-enhanced immunoturbidimetric assay on Beckman Coulter AU5800. Clin. Chem. Lab. Med. 2015, 53, 593–597. [Google Scholar] [CrossRef] [PubMed]
- Ceriotti, F.; Marino, I.; Motta, A.; Carobene, A. Analytical evaluation of the performances of Diazyme and BRAHMS procalcitonin applied to Roche Cobas in comparison with BRAHMS PCT-sensitive Kryptor. Clin. Chem. Lab. Med. 2017, 56, 162–169. [Google Scholar] [CrossRef]
- Dipalo, M.; Guido, L.; Micca, G.; Pittalis, S.; Locatelli, M.; Motta, A.; Bianchi, V.; Callegari, T.; Aloe, R.; Da Rin, G.; et al. Multicenter comparison of automated procalcitonin immunoassays. Pract. Lab. Med. 2015, 2, 22–28. [Google Scholar] [CrossRef]
- Clinical and Laboratory Standards Institute. EP15-A3. User Verification of Precision and Estimation of Bias-Approved Guideline; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2014. [Google Scholar]
- Clinical and Laboratory Standards Institute. EP17-A2. Protocols for Determination of Limits of Detection and Limits of Quantitation; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2004. [Google Scholar]
- Putheti, R.R.; Okigbo, R.N.; Patil, S.C.; Advanapu, M.S.; Leburu, R. Method development and validations: Characterization of critical elements in the development of pharmaceuticals. Int. J. Health Res. 2008, 1, 5–14. [Google Scholar] [CrossRef]
- Clinical and Laboratory Standards Institute. EP07-A2. Interference Testing in Clinical Chemistry; Approved Guideline, 2nd ed.; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2015. [Google Scholar]
- Bland, J.M.; Altman, D.G. Measuring agreement in method comparison studies. Stat. Methods Med. Res. 1999, 8, 135–160. [Google Scholar] [CrossRef]
- Emerson, J.F.; Ngo, G.; Emerson, S.S. Screening for interference in immunoassays. Clin. Chem. 2003, 49, 1163–1169. [Google Scholar] [CrossRef]
- Guggenmoos-Holzman, I. The meaning of kappa: Probabilistic concepts of reliability and validity revisited. J. Clin. Epidemiol. 1996, 49, 775–782. [Google Scholar] [CrossRef]
- Technical Sheet DZ062. Available online: https://www.diazyme.com/procalcitonin-pct-assay (accessed on 1 January 2020).
- Yuan, C. Reply to: Analytical evaluation of the performances of Diazyme and BRAHMS procalcitonin applied to Roche Cobas in comparison with BRAHMS PCT-sensitive Kryptor. Clin. Chem. Lab. Med. 2017, 56, e5–e6. [Google Scholar] [CrossRef]
- Steinbach, G.; Rau, B.; Debard, A.L.; Javourez, J.F.; Bienvenu, J.; Ponzio, A.; Bonfà, A.; Hubl, W.; Demant, T.; Külpmann, W.R.; et al. Multicenter evaluation of a new immunoassay for procalcitonin measurement on the Kryptor. System. Clin. Chem. Lab. Med. 2004, 42, 440–449. [Google Scholar] [CrossRef]
- Hiroyuki, Y.; Shinjiro, M.; Yoshihiro, U.; Nakamura, K.; Kobatake, S.; Satomura, S.; Matsuura, S. Determination of procalcitonin concentration using the Sphere Light 180 clinical auto-analyzer. Clin. Chim. Acta 2008, 388, 38–40. [Google Scholar]
- Agarwal, S.; Akbas, N.; Soundar, E.P.; Gonzalez, G.; Devaraj, S. Validation of the procalcitonin (PCT) assay: Experience in a pediatric hospital. Clin. Biochem. 2015, 48, 886–890. [Google Scholar] [CrossRef] [PubMed]
- Lippi, G.; Salvagno, G.L.; Gelati, M.; Pucci, M.; Lo Cascio, C.; Demonte, D.; Faggian, D.; Plebani, M. Two-center comparison of 10 fully-automated commercial procalcitonin (PCT) immunoassays. Clin. Chem. Lab. Med. 2019, 58, 77–84. [Google Scholar] [CrossRef] [PubMed]
| DiaSys Assay | Diazyme Assay | |||
|---|---|---|---|---|
| Total CV imprecision results | Mean, µg/L | CV, % | Mean, µg/L | CV, % |
| Control level 1 | 0.99 | 5.42 | 1.50 | 10.70 |
| Control level 2 | 11.80 | 3.30 | 15.20 | 2.90 |
| Serum pool | 0.61 | 7.53 | 0.77 | 13.23 |
| LoB | 0.019 | 0.030 | ||
| LoD | 0.053 | 0.140 | ||
| LoQ | 0.095 | 0.150 | ||
| Linearity | ||||
| Theoretical values from DiaSys measurement, µg/L | Mean of observed values with DiaSys assay, µg/L (% of mean recovery) | Theoretical values from Diazyme measurement, µg/L | Mean of observed values with Diazyme assay, µg/L (% of mean recovery) | |
| 7.08 | 7.08 (100.0) | 6.99 | 6.99 (100.0) | |
| 3.54 | 2.58 (72.8) | 3.50 | 2.70 (77.2) | |
| 1.77 | 1.17 (66.1) | 1.75 | 1.25 (71.5) | |
| 0.71 | 0.51 (72.0) | 0.70 | 0.67 (95.8) | |
| 0.35 | 0.19 (53.6) | 0.35 | 0.52 (148.7) | |
| 0.18 | 0.09 (50.8) | 0.17 | 0.46 (263.2) | |
| 0.04 | 0.03 (67.8) | 0.04 | 0.41 (938.4) | |
| Discordants | BRAHMS PCT Values, µg/L | PCT DiaSys Values, µg/L | PCT Diazyme Values, µg/L | Final Diagnosis |
|---|---|---|---|---|
| 1 | 0.08 | 0.47 | 0.73 | Infectious endocarditis a,d |
| 2 | 0.10 | 0.13 | 1.98 | Pharmaco-resistant epilepsies c |
| 3 | 0.11 | 0.24 | 0.91 | Cirrhosis c |
| 4 | 0.12 | 0.15 | 0.66 | ARDS c |
| 5 | 0.15 | 0.24 | 1.00 | Polytrauma c |
| 6 | 0.17 | 0.34 | 0.8 | Bronchial surinfection a,d |
| 7 | 0.18 | 0.34 | 0.60 | Repeated fall c |
| 8 | 0.18 | 0.49 | 0.87 | ICU without Antibiotherapy c |
| 9 | 0.19 | 0.29 | 0.81 | Leg fracture c |
| 10 | 0.22 | 0.6 | 1.88 | Suspected meningitis a |
| 11 | 0.25 | 0.52 | 1.32 | ARDS b,c |
| 12 | 0.26 | 0.34 | 0.64 | Cirrhosis decompensation c |
| 13 | 0.26 | 0.83 | 1.09 | Heart stroke b,c |
| 14 | 0.29 | 0.47 | 2.44 | Complicated pneumonia with ARDS a,d |
| 15 | 0.32 | 0.75 | 1.48 | ICU without Antibiotherapy b,c |
| 16 | 0.35 | 0.50 | 1.03 | Sepsis from parietal origin a |
| 17 | 0.37 | 0.36 | 0.61 | Complication following cardiac surgery c |
| 18 | 0.39 | 0.76 | 0.63 | ICU with Antibiotherapy a |
| 19 | 0.43 | 0.81 | 0.82 | Lung Adenocarcinoma b,c |
| 20 | 0.44 | 0.77 | 0.61 | ICU without Antibiotherapy b,c |
| 21 | 0.49 | 0.51 | 0.65 | Decompensated respiratory acidosis b,c |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dupuy, A.M.; Bargnoux, A.S.; Larcher, R.; Merindol, A.; Masetto, T.; Badiou, S.; Cristol, J.P. Bioanalytical Performance of a New Particle-Enhanced Method for Measuring Procalcitonin. Diagnostics 2020, 10, 461. https://doi.org/10.3390/diagnostics10070461
Dupuy AM, Bargnoux AS, Larcher R, Merindol A, Masetto T, Badiou S, Cristol JP. Bioanalytical Performance of a New Particle-Enhanced Method for Measuring Procalcitonin. Diagnostics. 2020; 10(7):461. https://doi.org/10.3390/diagnostics10070461
Chicago/Turabian StyleDupuy, Anne Marie, Anne Sophie Bargnoux, Romaric Larcher, Antoine Merindol, Thomas Masetto, Stéphanie Badiou, and Jean Paul Cristol. 2020. "Bioanalytical Performance of a New Particle-Enhanced Method for Measuring Procalcitonin" Diagnostics 10, no. 7: 461. https://doi.org/10.3390/diagnostics10070461
APA StyleDupuy, A. M., Bargnoux, A. S., Larcher, R., Merindol, A., Masetto, T., Badiou, S., & Cristol, J. P. (2020). Bioanalytical Performance of a New Particle-Enhanced Method for Measuring Procalcitonin. Diagnostics, 10(7), 461. https://doi.org/10.3390/diagnostics10070461






