Diagnostic Roles of Immunohistochemistry in Thymic Tumors: Differentiation between Thymic Carcinoma and Thymoma
Abstract
1. Introduction
2. Materials and Methods
2.1. Published Study Search and Selection Criteria
2.2. Data Extraction
2.3. Statistical Analyses
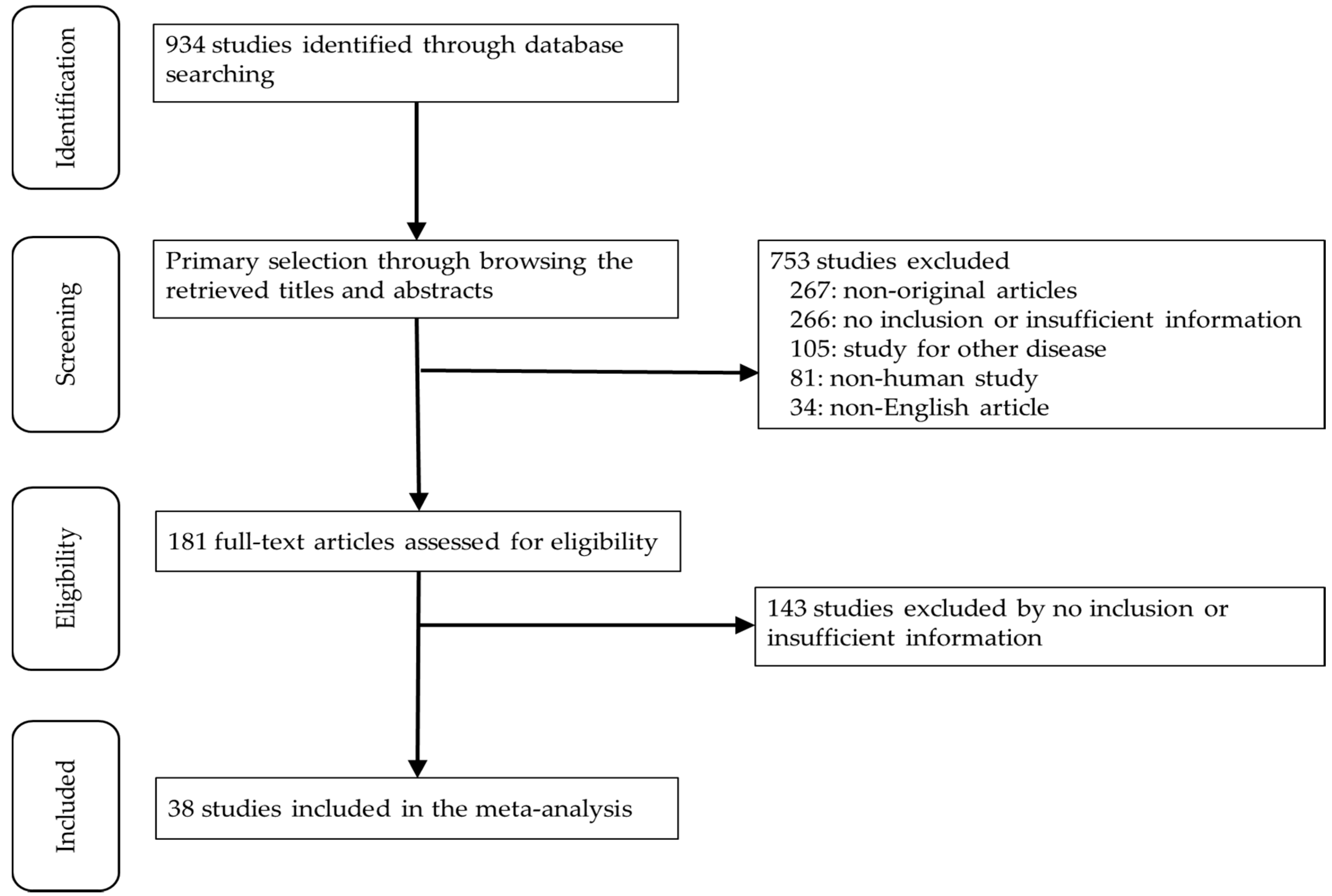
3. Results
3.1. Selection and Characteristics of the Studies
3.2. Comparison of Immunohistochemical Expressions between Thymic Carcinoma and Thymoma
3.3. Diagnostic Test Accuracy Review for Immunohistochemical Markers
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Travis, W.D.; Brambilla, E.; Burke, A.P.; Marx, A.; Nicholson, A.G. WHO Classification of Tumours of the Lung, Pleura, Thymus and Heart, 4th ed.; International Agency for Research on Cancer: Lyon, France, 2015. [Google Scholar]
- Wu, Z.; Xue, S.; Zheng, B.; Ye, R.; Xu, G.; Zhang, S.; Zeng, T.; Zheng, W.; Chen, C. Expression and significance of c-kit and epithelial-mesenchymal transition (EMT) molecules in thymic epithelial tumors (TETs). J. Thorac. Dis. 2019, 11, 4602–4612. [Google Scholar] [CrossRef]
- Kelly, R.J.; Petrini, I.; Rajan, A.; Wang, Y.; Giaccone, G. Thymic malignancies: From clinical management to targeted therapies. J. Clin. Oncol. 2011, 29, 4820–4827. [Google Scholar] [CrossRef]
- Marx, A.; Strobel, P.; Badve, S.S.; Chalabreysse, L.; Chan, J.K.; Chen, G.; de Leval, L.; Detterbeck, F.; Girard, N.; Huang, J.; et al. ITMIG consensus statement on the use of the WHO histological classification of thymoma and thymic carcinoma: Refined definitions, histological criteria, and reporting. J. Thorac. Oncol. 2014, 9, 596–611. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Zhao, H.; Hu, D.; Fan, L.; Shi, J.; Fang, W. Surgical treatment and prognosis of thymic squamous cell carcinoma: A retrospective analysis of 105 cases. Ann. Thorac. Surg. 2013, 96, 1019–1024. [Google Scholar] [CrossRef] [PubMed]
- Okereke, I.C.; Kesler, K.A.; Freeman, R.K.; Rieger, K.M.; Birdas, T.J.; Ascioti, A.J.; Badve, S.; Nelson, R.P.; Loehrer, P.J. Thymic carcinoma: Outcomes after surgical resection. Ann. Thorac. Surg. 2012, 93, 1668–1672. [Google Scholar] [CrossRef] [PubMed]
- Rashid, O.M.; Cassano, A.D.; Takabe, K. Thymic neoplasm: A rare disease with a complex clinical presentation. J. Thorac. Dis. 2013, 5, 173–183. [Google Scholar] [PubMed]
- Nakagawa, K.; Matsuno, Y.; Kunitoh, H.; Maeshima, A.; Asamura, H.; Tsuchiya, R. Immunohistochemical KIT (CD117) expression in thymic epithelial tumors. Chest 2005, 128, 140–144. [Google Scholar] [CrossRef]
- Adam, P.; Hakroush, S.; Hofmann, I.; Reidenbach, S.; Marx, A.; Strobel, P. Thymoma with loss of keratin expression (and giant cells): A potential diagnostic pitfall. Virchows Arch. 2014, 465, 313–320. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.F.; Yan, J.J.; Chang, K.C.; Lai, W.W.; Chen, R.M.; Jin, Y.T. Immunohistochemical localization of Mcl-1 and bcl-2 proteins in thymic epithelial tumours. Histopathology 1996, 29, 541–547. [Google Scholar] [CrossRef] [PubMed]
- Cui, F.; He, J.; Liu, J.; Chen, Y. Protein expression status of p53 and epidermal growth factor receptor in thymoma. Oncol. Lett. 2011, 2, 459–463. [Google Scholar] [CrossRef] [PubMed]
- Dorfman, D.M.; Shahsafaei, A.; Chan, J.K. Thymic carcinomas, but not thymomas and carcinomas of other sites, show CD5 immunoreactivity. Am. J. Surg. Pathol. 1997, 21, 936–940. [Google Scholar] [CrossRef] [PubMed]
- Du, M.J.; Shen, Q.; Yin, H.; Rao, Q.; Zhou, M.X. Diagnostic roles of MUC1 and GLUT1 in differentiating thymic carcinoma from type B3 thymoma. Pathol. Res. Pract. 2016, 212, 1048–1051. [Google Scholar] [CrossRef] [PubMed]
- Comprehensive genomic analysis reveals clinically relevant molecular distinctions between thymic carcinomas and thymomas. Clin. Cancer Res. 2009, 15, 6790–6799. [CrossRef]
- Girard, N.; Teruya-Feldstein, J.; Payabyab, E.C.; Riely, G.J.; Rusch, V.W.; Kris, M.G.; Zakowski, M.F. Insulin-like growth factor-1 receptor expression in thymic malignancies. J. Thorac. Oncol. 2010, 5, 1439–1446. [Google Scholar] [CrossRef]
- Hayashi, A.; Fumon, T.; Miki, Y.; Sato, H.; Yoshino, T.; Takahashi, K. The evaluation of immunohistochemical markers and thymic cortical microenvironmental cells in distinguishing thymic carcinoma from type b3 thymoma or lung squamous cell carcinoma. J. Clin. Exp. Hematop. 2013, 53, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Henley, J.D.; Koukoulis, G.K.; Loehrer, P.J. Epidermal growth factor receptor expression in invasive thymoma. J. Cancer Res. Clin. Oncol. 2002, 128, 167–170. [Google Scholar] [CrossRef]
- Hino, N.; Kondo, K.; Miyoshi, T.; Uyama, T.; Monden, Y. High frequency of p53 protein expression in thymic carcinoma but not in thymoma. Br. J. Cancer 1997, 76, 1361–1366. [Google Scholar] [CrossRef][Green Version]
- Hirabayashi, H.; Fujii, Y.; Sakaguchi, M.; Tanaka, H.; Yoon, H.E.; Komoto, Y.; Inoue, M.; Miyoshi, S. p16INK4, pRB, p53 and cyclin D1 expression and hypermethylation of CDKN2 gene in thymoma and thymic carcinoma. Int. J. Cancer 1997, 73, 639–644. [Google Scholar] [CrossRef]
- Hiroshima, K.; Iyoda, A.; Toyozaki, T.; Supriatna, Y.; Shibuya, K.; Shimamura, F.; Haga, Y.; Yoshida, S.; Fujisawa, T.; Ohwada, H. Proliferative activity and apoptosis in thymic epithelial neoplasms. Mod. Pathol. 2002, 15, 1326–1332. [Google Scholar] [CrossRef]
- Kaira, K.; Murakami, H.; Serizawa, M.; Koh, Y.; Abe, M.; Ohde, Y.; Takahashi, T.; Kondo, H.; Nakajima, T.; Yamamoto, N. MUC1 expression in thymic epithelial tumors: MUC1 may be useful marker as differential diagnosis between type B3 thymoma and thymic carcinoma. Virchows Arch. 2011, 458, 615–620. [Google Scholar] [CrossRef]
- Khoury, T.; Chandrasekhar, R.; Wilding, G.; Tan, D.; Cheney, R.T. Tumour eosinophilia combined with an immunohistochemistry panel is useful in the differentiation of type B3 thymoma from thymic carcinoma. Int. J. Exp. Pathol. 2011, 92, 87–96. [Google Scholar] [CrossRef]
- Kornstein, M.J.; Rosai, J. CD5 labeling of thymic carcinomas and other nonlymphoid neoplasms. Am. J. Clin. Pathol. 1998, 109, 722–726. [Google Scholar] [CrossRef] [PubMed]
- Laury, A.R.; Perets, R.; Piao, H.; Krane, J.F.; Barletta, J.A.; French, C.; Chirieac, L.R.; Lis, R.; Loda, M.; Hornick, J.L.; et al. A comprehensive analysis of PAX8 expression in human epithelial tumors. Am. J. Surg. Pathol. 2011, 35, 816–826. [Google Scholar] [CrossRef]
- Lee, G.J.; Lee, H.; Woo, I.S.; Kim, T.; An, H.J.; Choi, H.J.; Lee, Y.S.; Lee, K.Y.; Lee, J.; Kang, J.H. High expression level of SOX2 is significantly associated with shorter survival in patients with thymic epithelial tumors. Lung Cancer 2019, 132, 9–16. [Google Scholar] [CrossRef]
- Mimae, T.; Tsuta, K.; Takahashi, F.; Yoshida, A.; Kondo, T.; Murakami, Y.; Okada, M.; Takeuchi, M.; Asamura, H.; Tsuda, H. Steroid receptor expression in thymomas and thymic carcinomas. Cancer 2011, 117, 4396–4405. [Google Scholar] [CrossRef] [PubMed]
- Mimae, T.; Tsuta, K.; Kondo, T.; Nitta, H.; Grogan, T.M.; Okada, M.; Asamura, H.; Tsuda, H. Protein expression and gene copy number changes of receptor tyrosine kinase in thymomas and thymic carcinomas. Ann. Oncol. 2012, 23, 3129–3137. [Google Scholar] [CrossRef]
- Nonaka, D.; Henley, J.D.; Chiriboga, L.; Yee, H. Diagnostic utility of thymic epithelial markers CD205 (DEC205) and Foxn1 in thymic epithelial neoplasms. Am. J. Surg. Pathol. 2007, 31, 1038–1044. [Google Scholar] [CrossRef] [PubMed]
- Omatsu, M.; Kunimura, T.; Mikogami, T.; Hamatani, S.; Shiokawa, A.; Masunaga, A.; Kitami, A.; Suzuki, T.; Kadokura, M.; Morohoshi, T. Immunohistochemical analysis of thymic carcinoma focusing on the possibility of molecular targeted and hormonal therapies. Gen. Thorac. Cardiovasc. Surg. 2012, 60, 803–810. [Google Scholar] [CrossRef]
- Pan, C.C.; Chen, P.C.; Chou, T.Y.; Chiang, H. Expression of calretinin and other mesothelioma-related markers in thymic carcinoma and thymoma. Hum. Pathol. 2003, 34, 1155–1162. [Google Scholar] [CrossRef]
- Petrini, I.; Zucali, P.A.; Lee, H.S.; Pineda, M.A.; Meltzer, P.S.; Walter-Rodriguez, B.; Roncalli, M.; Santoro, A.; Wang, Y.; Giaccone, G. Expression and mutational status of c-kit in thymic epithelial tumors. J. Thorac. Oncol. 2010, 5, 1447–1453. [Google Scholar] [CrossRef]
- Remon, J.; Abedallaa, N.; Taranchon-Clermont, E.; Bluthgen, V.; Lindsay, C.R.; Besse, B.; Thomas de Montpreville, V. CD52, CD22, CD26, EG5 and IGF-1R expression in thymic malignancies. Lung Cancer 2017, 108, 168–172. [Google Scholar] [CrossRef] [PubMed]
- Rieker, R.J.; Joos, S.; Mechtersheimer, G.; Blaeker, H.; Schnabel, P.A.; Morresi-Hauf, A.; Hecker, E.; Thomas, M.; Dienemann, H.; Schirmacher, P.; et al. COX-2 upregulation in thymomas and thymic carcinomas. Int. J. Cancer 2006, 119, 2063–2070. [Google Scholar] [CrossRef] [PubMed]
- Song, N.; Chen, G.; Zhang, P.; Liu, M.; He, W.X.; Jiang, G.N. Diagnostic and clinical significance of KIT(CD117) expression in thymic epithelial tumors in China. Asian Pac. J. Cancer Prev. 2012, 13, 2745–2748. [Google Scholar] [CrossRef]
- Stefanaki, K.; Rontogianni, D.; Kouvidou, C.H.; Bolioti, S.; Delides, G.; Pantelidaki, A.; Sotsiou, F.; Kanavaros, P. Expression of p53, mdm2, p21/waf1 and bcl-2 proteins in thymomas. Histopathology 1997, 30, 549–555. [Google Scholar] [CrossRef] [PubMed]
- Su, X.Y.; Wang, W.Y.; Li, J.N.; Liao, D.Y.; Wu, W.L.; Li, G.D. Immunohistochemical differentiation between type B3 thymomas and thymic squamous cell carcinomas. Int. J. Clin. Exp. Pathol. 2015, 8, 5354–5362. [Google Scholar]
- Suzuki, A.; Hirokawa, M.; Takada, N.; Higuchi, M.; Tanaka, A.; Hayashi, T.; Kuma, S.; Miyauchi, A. Utility of monoclonal PAX8 antibody for distinguishing intrathyroid thymic carcinoma from follicular cell-derived thyroid carcinoma. Endocr. J. 2018, 65, 1171–1175. [Google Scholar] [CrossRef] [PubMed]
- Tateyama, H.; Eimoto, T.; Tada, T.; Hattori, H.; Murase, T.; Takino, H. Immunoreactivity of a new CD5 antibody with normal epithelium and malignant tumors including thymic carcinoma. Am. J. Clin. Pathol. 1999, 111, 235–240. [Google Scholar] [CrossRef]
- Thomas, A.; Chen, Y.; Berman, A.; Schrump, D.S.; Giaccone, G.; Pastan, I.; Venzon, D.J.; Liewehr, D.J.; Steinberg, S.M.; Miettinen, M.; et al. Expression of mesothelin in thymic carcinoma and its potential therapeutic significance. Lung Cancer 2016, 101, 104–110. [Google Scholar] [CrossRef]
- Thomas de Montpreville, V.; Quilhot, P.; Chalabreysse, L.; De Muret, A.; Hofman, V.; Lantuejoul, S.; Parrens, M.; Payan, M.J.; Rouquette, I.; Secq, V.; et al. Glut-1 intensity and pattern of expression in thymic epithelial tumors are predictive of WHO subtypes. Pathol. Res. Pract. 2015, 211, 996–1002. [Google Scholar] [CrossRef]
- Tsuchida, M.; Umezu, H.; Hashimoto, T.; Shinohara, H.; Koike, T.; Hosaka, Y.; Eimoto, T.; Hayashi, J.I. Absence of gene mutations in KIT-positive thymic epithelial tumors. Lung Cancer 2008, 62, 321–325. [Google Scholar] [CrossRef]
- Weissferdt, A.; Moran, C.A. Pax8 expression in thymic epithelial neoplasms: An immunohistochemical analysis. Am. J. Surg. Pathol. 2011, 35, 1305–1310. [Google Scholar] [CrossRef]
- Yamada, Y.; Tomaru, U.; Ishizu, A.; Kiuchi, T.; Marukawa, K.; Matsuno, Y.; Kasahara, M. Expression of proteasome subunit beta5t in thymic epithelial tumors. Am. J. Surg. Pathol. 2011, 35, 1296–1304. [Google Scholar] [CrossRef]
- Zucali, P.A.; Petrini, I.; Lorenzi, E.; Merino, M.; Cao, L.; Di Tommaso, L.; Lee, H.S.; Incarbone, M.; Walter, B.A.; Simonelli, M.; et al. Insulin-like growth factor-1 receptor and phosphorylated AKT-serine 473 expression in 132 resected thymomas and thymic carcinomas. Cancer 2010, 116, 4686–4695. [Google Scholar] [CrossRef]
- Kojika, M.; Ishii, G.; Yoshida, J.; Nishimura, M.; Hishida, T.; Ota, S.J.; Murata, Y.; Nagai, K.; Ochiai, A. Immunohistochemical differential diagnosis between thymic carcinoma and type B3 thymoma: Diagnostic utility of hypoxic marker, GLUT-1, in thymic epithelial neoplasms. Mod. Pathol. 2009, 22, 1341–1350. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.S.; Kim, J.K.; Kang, C.H.; Kim, Y.T.; Jung, K.C.; Won, J.K. An immunohistochemical panel consisting of EZH2, C-KIT, and CD205 is useful for distinguishing thymic squamous cell carcinoma from type B3 thymoma. Pathol. Res. Pract. 2018, 214, 343–349. [Google Scholar] [CrossRef] [PubMed]
- Berezowski, K.; Grimes, M.M.; Gal, A.; Kornstein, M.J. CD5 immunoreactivity of epithelial cells in thymic carcinoma and CASTLE using paraffin-embedded tissue. Am. J. Clin. Pathol. 1996, 106, 483–486. [Google Scholar] [CrossRef] [PubMed]
- Hishima, T.; Fukayama, M.; Fujisawa, M.; Hayashi, Y.; Arai, K.; Funata, N.; Koike, M. CD5 expression in thymic carcinoma. Am. J. Pathol. 1994, 145, 268–275. [Google Scholar] [PubMed]
- Thomas de Montpreville, V.; Ghigna, M.R.; Lacroix, L.; Besse, B.; Broet, P.; Dartevelle, P.; Fadel, E.; Dorfmuller, P. Thymic carcinomas: Clinicopathologic study of 37 cases from a single institution. Virchows Arch. 2013, 462, 307–313. [Google Scholar] [CrossRef] [PubMed]
- Detterbeck, F.C.; Asamura, H.; Crowley, J.; Falkson, C.; Giaccone, G.; Giroux, D.; Huang, J.; Kim, J.; Kondo, K.; Lucchi, M.; et al. The IASLC/ITMIG thymic malignancies staging project: Development of a stage classification for thymic malignancies. J. Thorac. Oncol. 2013, 8, 1467–1473. [Google Scholar] [CrossRef]
- Girard, N.; Ruffini, E.; Marx, A.; Faivre-Finn, C.; Peters, S. Thymic epithelial tumours: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2015, 26 (Suppl. 5), v40–v55. [Google Scholar] [CrossRef]
| Study | Location | Number of Patients | ||||||
|---|---|---|---|---|---|---|---|---|
| Thymic Carcinoma | Thymoma | Type A | Type AB | Type B1 | Type B2 | Type B3 | ||
| Adam 2014 [9] | Germany | 24 | 45 | |||||
| Chen 1996 [10] | Taiwan | 26 | 15 | |||||
| Cui 2011 [11] | China | 4 | 39 | 2 | 5 | 7 | 14 | 11 |
| Dorfman 1997 [12] | USA | 24 | 41 | |||||
| Du 2016 [13] | China | 22 | 21 | 21 | ||||
| Girard 2009 [14] | USA | 7 | 38 | 8 | 22 | 8 | ||
| Girard 2010 [15] | USA | 7 | 56 | 5 | 12 | 8 | 21 | 10 |
| Hayashi 2013 [16] | Japan | 18 | 17 | 17 | ||||
| Henley 2002 [17] | USA | 6 | 36 | |||||
| Hino 1997 [18] | Japan | 19 | 17 | |||||
| Hirabayashi 1997 [19] | Japan | 4 | 36 | |||||
| Hiroshima 2002 [20] | Japan | 10 | 36 | 8 | 8 | 7 | 7 | 6 |
| Kaira 2011 [21] | Japan | 17 | 5 | 5 | ||||
| Khoury 2010 [22] | USA | 12 | 54 | 17 | ||||
| Kornstein 1997 [23] | USA | 24 | 85 | |||||
| Laury 2011 [24] | USA | 5 | 9 | |||||
| Lee 2019 [25] | Korea | 30 | 110 | 11 | 31 | 28 | 16 | 19 |
| Mimae 2011 [26] | Japan | 37 | 103 | 6 | ||||
| Mimae 2012 [27] | USA | 37 | 103 | 6 | ||||
| Nakagawa 2005 [8] | Japan | 20 | 50 | 10 | 10 | 10 | 10 | 10 |
| Nonaka 2007 [28] | USA | 16 | 58 | 9 | 19 | 7 | 16 | 7 |
| Omatsu 2012 [29] | Japan | 22 | 22 | 1 | 1 | 7 | 7 | 6 |
| Pan 2003 [30] | Taiwan | 22 | 35 | 9 | 10 | 4 | 7 | 5 |
| Petrini 2010 [31] | Italy | 13 | 105 | |||||
| Remon 2017 [32] | France | 12 | 84 | 4 | 25 | 8 | 27 | 20 |
| Rieker 2006 [33] | Germany | 4 | 30 | 8 | 6 | 5 | 6 | 5 |
| Song 2012 [34] | China | 15 | 87 | 3 | 29 | 5 | 22 | 28 |
| Stefanaki 1997 [35] | Greece | 2 | 29 | |||||
| Su 2015 [36] | China | 20 | 16 | 16 | ||||
| Suzuki 2018 [37] | Japan | 10 | 7 | |||||
| Tateyama 1999 [38] | Japan | 7 | 18 | |||||
| Thomas 2016 [39] | USA | 34 | 29 | |||||
| Thomas de Montpréville 2015 [40] | France | 16 | 75 | 5 | 17 | 11 | 25 | 17 |
| Tsuchida 2008 [41] | Japan | 17 | 20 | 5 | 4 | 6 | 5 | |
| Weissferdt 2011 [42] | USA | 31 | 60 | 30 | ||||
| Wu 2019 [2] | China | 22 | 128 | 11 | 35 | 19 | 40 | 23 |
| Yamada 2011 [43] | Japan | 13 | 41 | 3 | 17 | 7 | 10 | 4 |
| Zucali 2010 [44] | Italy | 8 | 101 | 15 | 28 | 24 | 8 | 24 |
| Marker | Number of Subsets | Fixed Effect [95% CI] | Heterogeneity Test [p-Value] | Random Effect [95% CI] | Egger’s Test [p-Value] |
|---|---|---|---|---|---|
| Androgen receptor | 3 | 0.362 [0.120, 1.091] | 0.063 | 0.740 [0.065, 8.450] | 0.480 |
| beta-5t | 2 | 0.002 [0.000, 0.030] | 0.564 | 0.002 [0.000, 0.030] | - |
| beta-catenin | 2 | 0.829 [0.254, 2.704] | 0.022 | 0.512 [0.027, 9.722] | - |
| Bcl-2 | 4 | 2.461 [1.043, 5.807] | 0.637 | 2.461 [1.043, 5.807] | 0.871 |
| Calretinin | 1 | 19.429 [2.218, 170.165] | 1.000 | 19.429 [2.218, 170.165] | - |
| CD15 | 2 | 4.139 [1.413, 12.127] | 0.022 | 2.263 [0.130, 39.382] | - |
| CD1a | 2 | 0.052 [0.012, 0.223] | 0.073 | 0.028 [0.001, 0.623] | - |
| CD205 | 2 | 0.221 [0.064, 0.759] | 0.019 | 0.137 [0.006, 3.046] | - |
| CD5 | 11 | 52.560 [26.424, 104.547] | 0.972 | 52.560 [26.424, 104.547] | 0.034 |
| CEA | 2 | 45.273 [5.567, 368.160] | 0.505 | 45.273 [5.567, 368.160] | - |
| CK19 | 2 | 0.061 [0.016, 0.224] | 0.364 | 0.061 [0.016, 0.224] | - |
| CK5/6 | 4 | 0.191 [0.080, 0.459] | 0.022 | 0.294 [0.054, 1.607] | 0.283 |
| c-Kit | 12 | 41.444 [23.767, 72.267] | 0.771 | 41.444 [23.767, 72.267] | 0.024 |
| Cyclin D1 | 2 | 0.407 [0.128, 1.298] | 0.006 | 1.140 [0.022, 58.476] | - |
| E-cadherin | 3 | 0.340 [0.170, 0.680] | 0.001 | 0.400 [0.064, 2.516] | 0.167 |
| EGFR | 6 | 0.311 [0.130, 0.741] | 0.014 | 0.314 [0.066, 1.493] | 0.964 |
| Estrogen receptor | 1 | 0.319 [0.012, 8.254] | 1.000 | 0.319 [0.012, 8.254] | - |
| Glut-1 | 4 | 11.607 [3.003, 44.862] | 0.100 | 15.187 [2.082, 110.780] | 0.019 |
| HBME | 2 | 2.776 [0.337, 22.853] | 0.088 | 2.763 [0.076, 100.781] | - |
| IGF-1R | 6 | 10.216 [5.611, 18.602] | 0.005 | 9.465 [2.869, 31.221] | 0.806 |
| Mesothelin | 3 | 39.842 [12.067, 131.542] | 0.876 | 39.842 [12.067, 131.542] | 0.386 |
| MOC31 | 2 | 18.019 [4.366, 75.113] | 0.874 | 18.019 [4.366, 75.113] | - |
| MUC1 | 3 | 44.866 [11.273, 178.576] | 0.786 | 44.866 [11.273, 178.576] | 0.249 |
| p21 | 2 | 10.270 [2.862, 36.849] | 0.716 | 10.270 [2.862, 36.849] | - |
| p53 | 7 | 2.554 [1.077, 6.055] | 0.029 | 3.199 [0.759, 13.481] | 0.487 |
| p63 | 3 | 0.239 [0.094, 0.610] | 0.013 | 0.264 [0.028, 2.482] | 0.924 |
| PAX8 | 3 | 0.371 [0.107, 1.288] | 0.065 | 0.539 [0.058, 4.989] | 0.505 |
| Progesterone receptor | 2 | 1.681 [0.170, 16.597] | 0.597 | 1.681 [0.170, 16.597] | - |
| Survivin | 2 | 1.251 [0.358, 4.378] | 0.103 | 0.733 [0.056, 9.558] | - |
| TdT | 2 | 0.015 [0.003, 0.085] | 0.206 | 0.014 [0.001, 0.126] | - |
| Thrombomodulin | 1 | 0.449 [0.023, 8.896] | 1.000 | 0.449 [0.023, 8.896] | - |
| WT-1 | 1 | 4.953 [0.193, 127.130] | 1.000 | 4.953 [0.193, 127.130] | - |
| Marker | Type | Number of Subsets | Fixed Effect [95% CI] | Heterogeneity Test [p-Value] | Random Effect [95% CI] | Egger’s Test [p-Value] |
|---|---|---|---|---|---|---|
| beta-5t | Thymic carcinoma | 2 | 0.031 [0.004, 0.188] | 0.877 | 0.031 [0.004, 0.188] | - |
| Thymoma type B3 | 2 | 0.948 [0.706, 0.993] | 0.511 | 0.948 [0.706, 0.993] | - | |
| beta-catenin | Thymic carcinoma | 1 | 0.750 [0.448, 0.917] | 1.000 | 0.750 [0.448, 0.917] | - |
| Thymoma type B3 | 1 | 0.118 [0.030, 0.368] | 1.000 | 0.118 [0.030, 0.368] | - | |
| CD1a | Thymic carcinoma | 2 | 0.127 [0.036, 0.360] | 0.155 | 0.096 [0.012, 0.489] | - |
| Thymoma type B3 | 2 | 0.847 [0.680, 0.935] | 0.682 | 0.847 [0.680, 0.935] | - | |
| CD205 | Thymic carcinoma | 2 | 0.650 [0.461, 0.801] | 0.371 | 0.650 [0.461, 0.801] | - |
| Thymoma type B3 | 2 | 0.958 [0.757, 0.994] | 0.679 | 0.958 [0.757, 0.994] | - | |
| CD5 | Thymic carcinoma | 5 | 0.722 [0.610, 0.812] | 0.678 | 0.722 [0.610, 0.812] | 0.318 |
| Thymoma type B3 | 5 | 0.100 [0.039, 0.233] | 0.358 | 0.096 [0.035, 0.236] | 0.110 | |
| CEA | Thymic carcinoma | 1 | 0.750 [0.522, 0.892] | 1.000 | 0.750 [0.522, 0.892] | - |
| Thymoma type B3 | 1 | 0.029 [0.002, 0.336] | 1.000 | 0.029 [0.002, 0.336] | - | |
| c-Kit | Thymic carcinoma | 11 | 0.688 [0.607, 0.759] | 0.142 | 0.692 [0.591, 0.778] | 0.532 |
| Thymoma type B3 | 11 | 0.099 [0.060, 0.160] | 0.944 | 0.099 [0.060, 0.160] | 0.005 | |
| Glut-1 | Thymic carcinoma | 4 | 0.952 [0.862, 0.985] | 0.827 | 0.952 [0.862, 0.985] | 0.017 |
| Thymoma type B3 | 4 | 0.495 [0.351, 0.640] | 0.105 | 0.526 [0.296, 0.745] | 0.621 | |
| IGF-1R | Thymic carcinoma | 5 | 0.820 [0.720, 0.890] | 0.580 | 0.820 [0.720, 0.890] | 0.147 |
| Thymoma type B3 | 5 | 0.632 [0.495, 0.751] | 0.179 | 0.646 [0.468, 0.791] | 0.573 | |
| Mesothelin | Thymic carcinoma | 1 | 0.417 [0.185, 0.692] | 1.000 | 0.417 [0.185, 0.692] | - |
| Thymoma type B3 | 1 | 0.028 [0.002, 0.322] | 1.000 | 0.028 [0.002, 0.322] | - | |
| MOC31 | Thymic carcinoma | 1 | 0.500 [0.244, 0.756] | 1.000 | 0.500 [0.244, 0.756] | - |
| Thymoma type B3 | 1 | 0.118 [0.030, 0.368] | 1.000 | 0.118 [0.030, 0.368] | - | |
| MUC1 | Thymic carcinoma | 3 | 0.849 [0.706, 0.930] | 0.140 | 0.897 [0.666, 0.975] | 0.034 |
| Thymoma type B3 | 3 | 0.270 [0.144, 0.449] | 0.051 | 0.198 [0.048, 0.549] | 0.462 | |
| p21 | Thymic carcinoma | 1 | 0.667 [0.376, 0.869] | 1.000 | 0.667 [0.376, 0.869] | - |
| Thymoma type B3 | 1 | 0.118 [0.030, 0.368] | 1.000 | 0.118 [0.030, 0.368] | - | |
| TdT | Thymic carcinoma | 2 | 0.070 [0.018, 0.242] | 0.611 | 0.070 [0.018, 0.242] | - |
| Thymoma type B3 | 2 | 0.865 [0.690, 0.949] | 0.336 | 0.865 [0.690, 0.949] | - |
| Marker | Included Studies | Sensitivity (%) [95% CI] | Specificity (%) [95% CI] | Diagnostic OR [95% CI] | AUC on SROC | |
|---|---|---|---|---|---|---|
| Thymic carcinoma | CD5 | 5 | 0.731 [0.622, 0.817] | 0.967 [0.756, 0.996] | 23.936 [7.693, 74.478] | 0.725 |
| c-kit | 11 | 0.709 [0.613, 0.790] | 0.925 [0.873, 0.957] | 23.623 [11.900, 46.894] | 0.910 | |
| Glut-1 | 4 | 0.942 [0.856, 0.978] | 0.464 [0.225, 0.720] | 11.823 [2.879, 48.549] | 0.916 | |
| IGF-1R | 3 | 0.875 [0.760, 0.939] | 0.250 [0.136, 0.415] | 4.050 [1.087, 15.085] | 0.758 | |
| MUC1 | 3 | 0.932 [0.686, 0.988] | 0.847 [0.505, 0.968] | 46.251 [11.634, 183.877] | 0.921 | |
| Thymoma type B3 | beta-5t | 2 | 1.000 [0.927, 1.000] | 1.000 [0.942, 1.000] | 571.396 [33.356, 9788.053] | 0.985 |
| CD1a | 2 | 0.743 [0.628, 0.832] | 0.952 [0.504, 0.997] | 35.919 [1.606, 803.371] | 0.871 | |
| CD205 | 2 | 1.000 [0.931, 1.000] | 0.335 [0.165, 0.504] | 11.735 [1.368, 100.632] | 0.785 | |
| TdT | 2 | 0.879 [0.718, 0.954] | 0.933 [0.769, 0.983] | 93.458 [14.682, 594.912] | 0.958 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jeong, J.-H.; Pyo, J.-S.; Kim, N.-Y.; Kang, D.-W. Diagnostic Roles of Immunohistochemistry in Thymic Tumors: Differentiation between Thymic Carcinoma and Thymoma. Diagnostics 2020, 10, 460. https://doi.org/10.3390/diagnostics10070460
Jeong J-H, Pyo J-S, Kim N-Y, Kang D-W. Diagnostic Roles of Immunohistochemistry in Thymic Tumors: Differentiation between Thymic Carcinoma and Thymoma. Diagnostics. 2020; 10(7):460. https://doi.org/10.3390/diagnostics10070460
Chicago/Turabian StyleJeong, Jae-Han, Jung-Soo Pyo, Nae-Yu Kim, and Dong-Wook Kang. 2020. "Diagnostic Roles of Immunohistochemistry in Thymic Tumors: Differentiation between Thymic Carcinoma and Thymoma" Diagnostics 10, no. 7: 460. https://doi.org/10.3390/diagnostics10070460
APA StyleJeong, J.-H., Pyo, J.-S., Kim, N.-Y., & Kang, D.-W. (2020). Diagnostic Roles of Immunohistochemistry in Thymic Tumors: Differentiation between Thymic Carcinoma and Thymoma. Diagnostics, 10(7), 460. https://doi.org/10.3390/diagnostics10070460






