Partial Pancreatic Parenchymal Atrophy Is a New Specific Finding to Diagnose Small Pancreatic Cancer (≤10 mm) Including Carcinoma in Situ: Comparison with Localized Benign Main Pancreatic Duct Stenosis Patients
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Selection
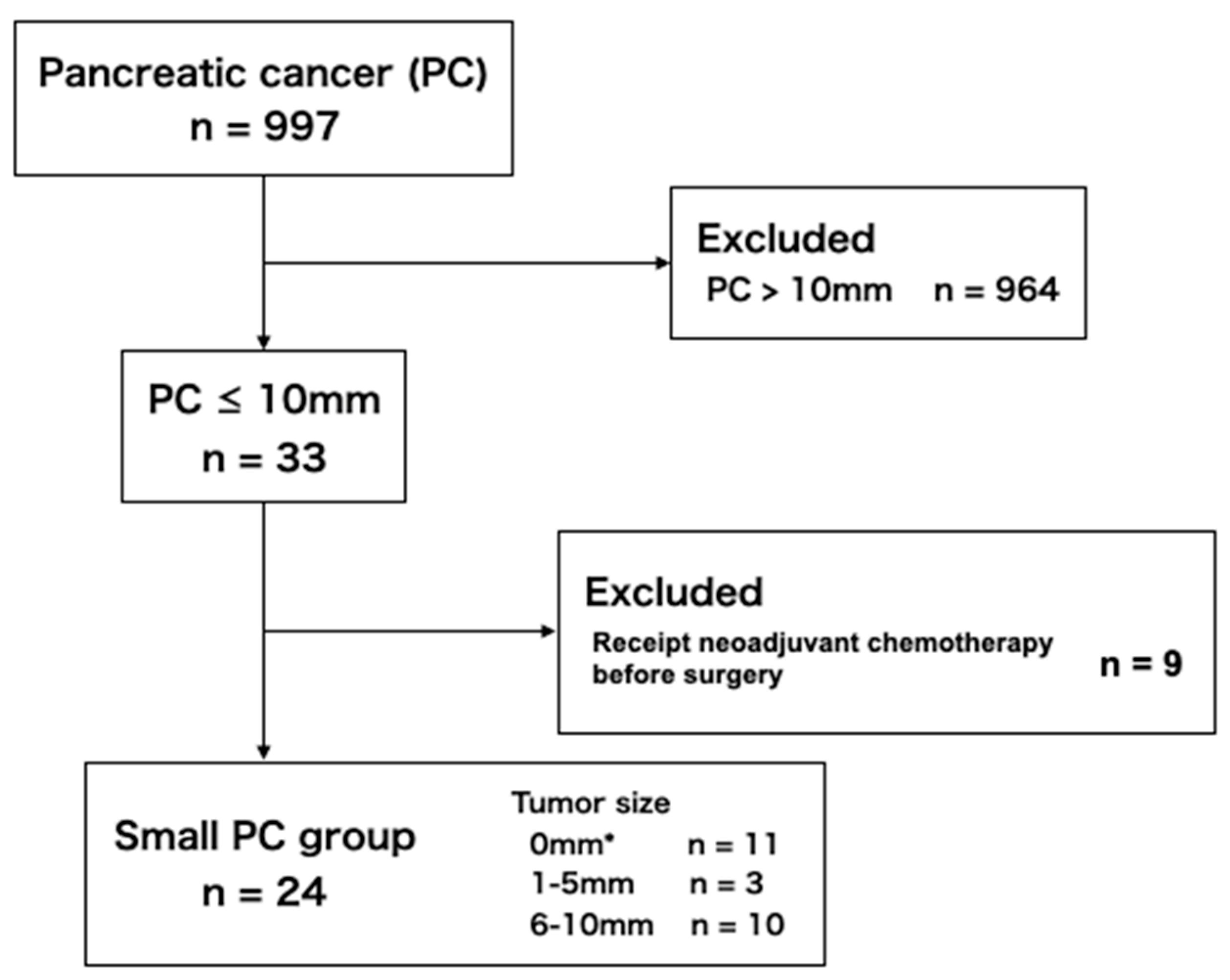
2.1.1. Small PC Group
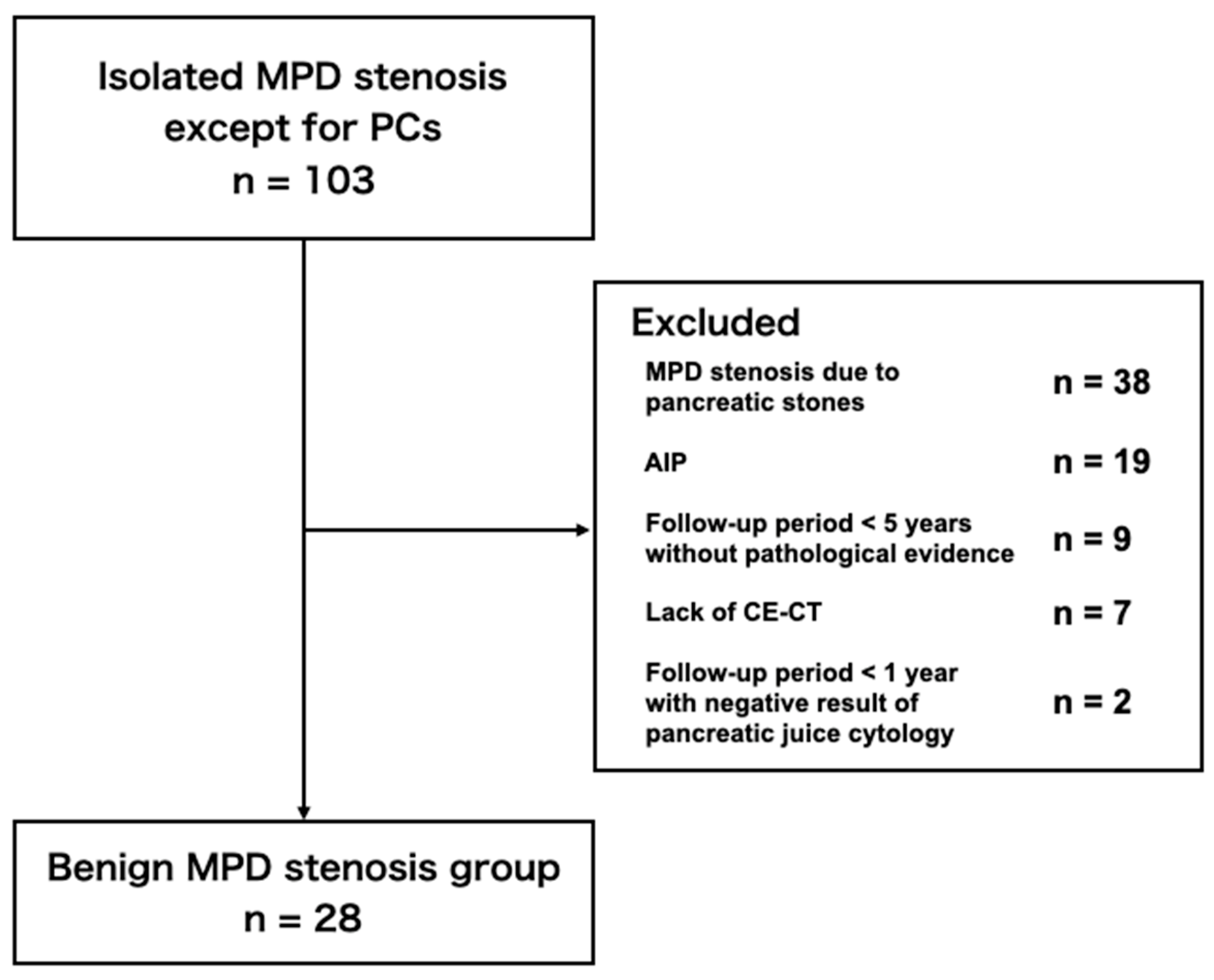
2.1.2. Benign MPD Stenosis Group
2.2. Imaging Technique
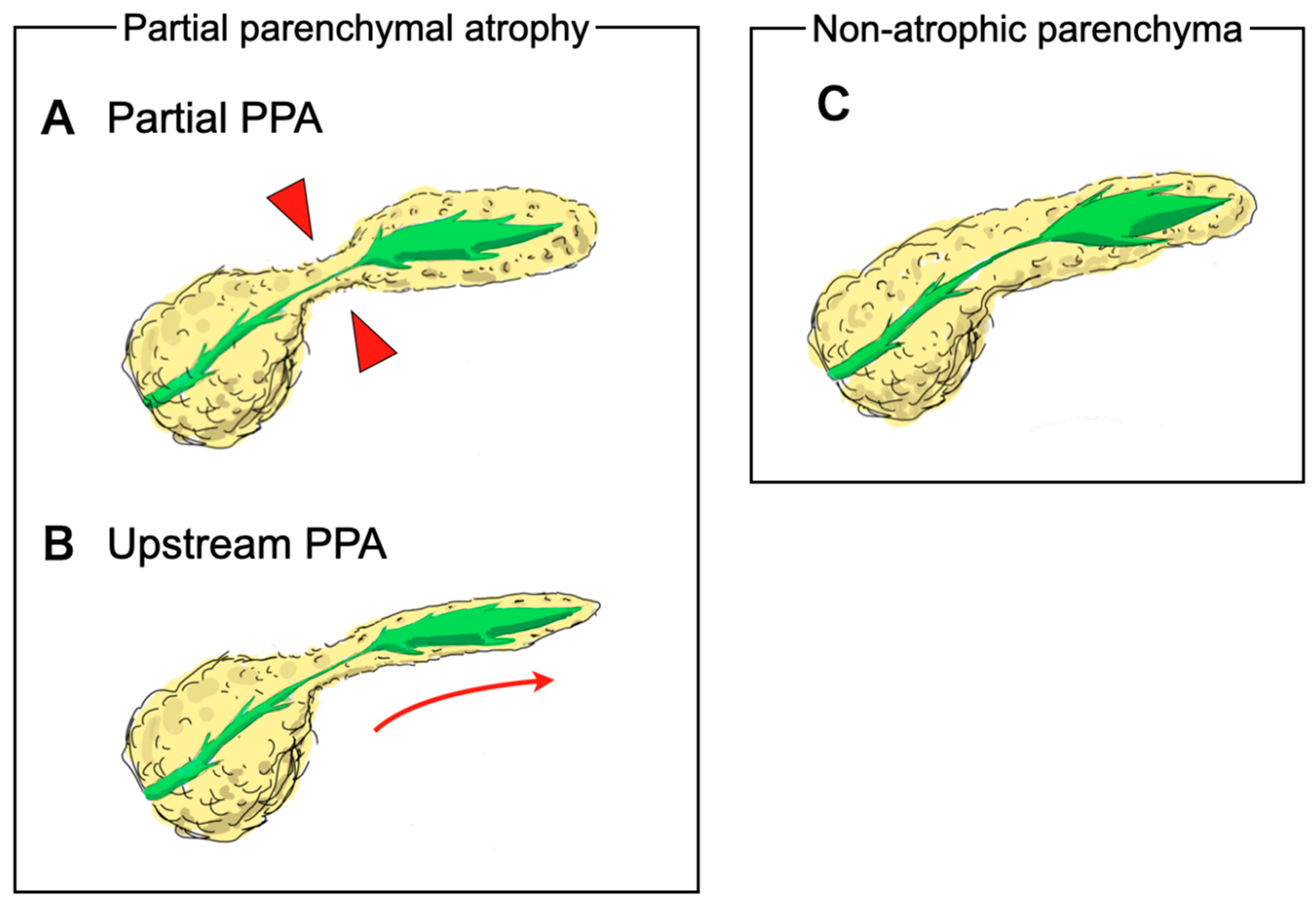
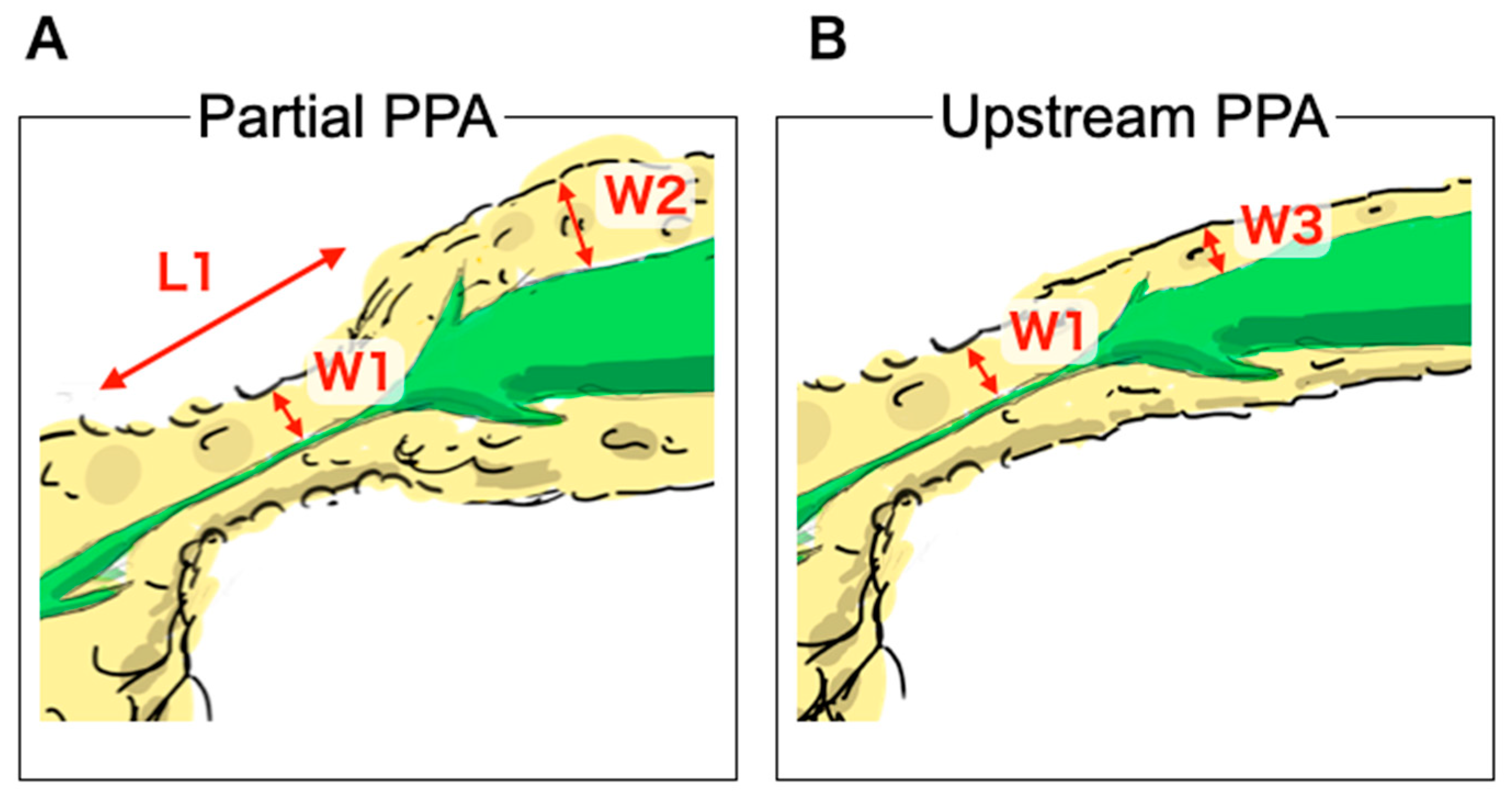
2.3. Outcome Measurements and Definitions
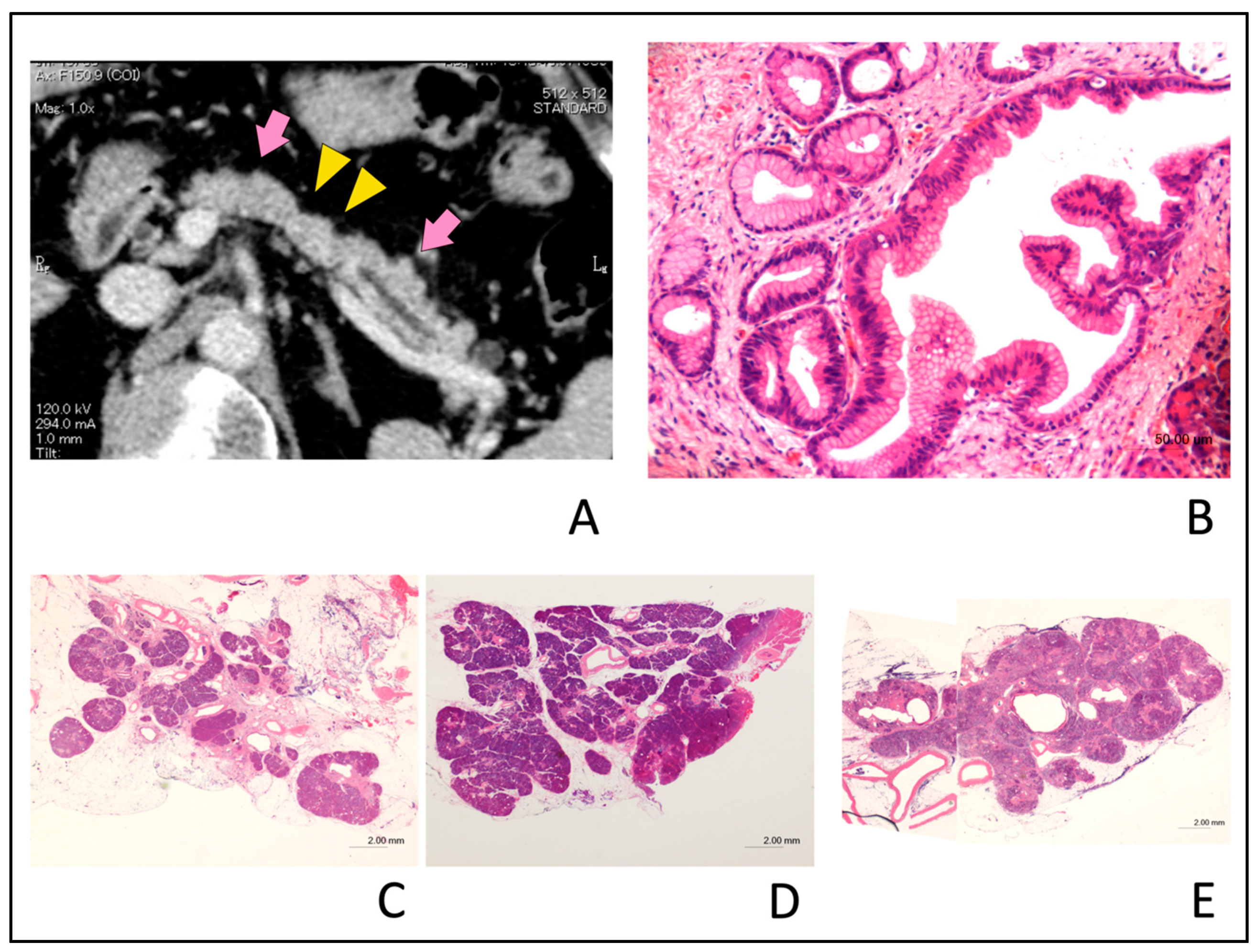
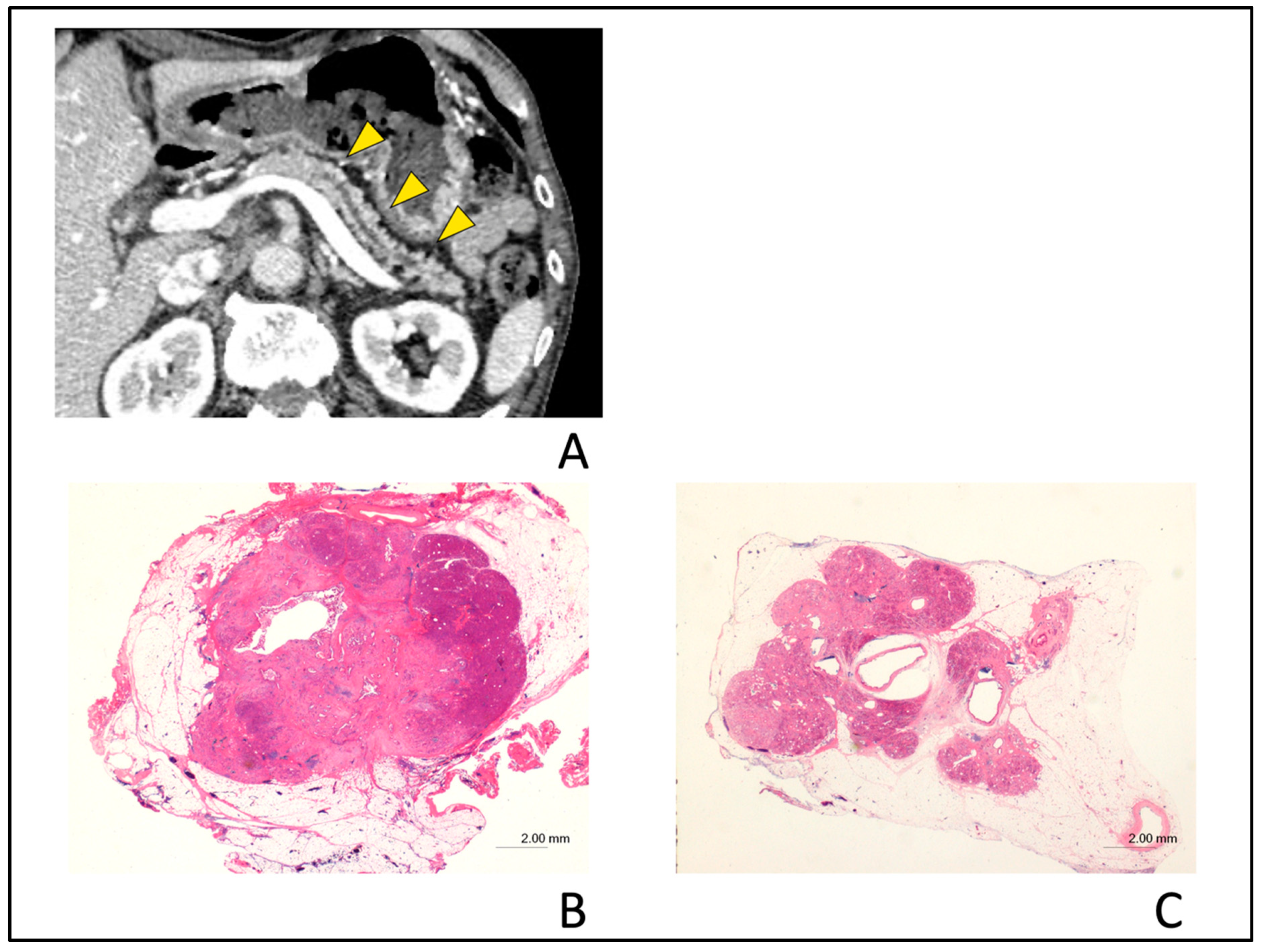
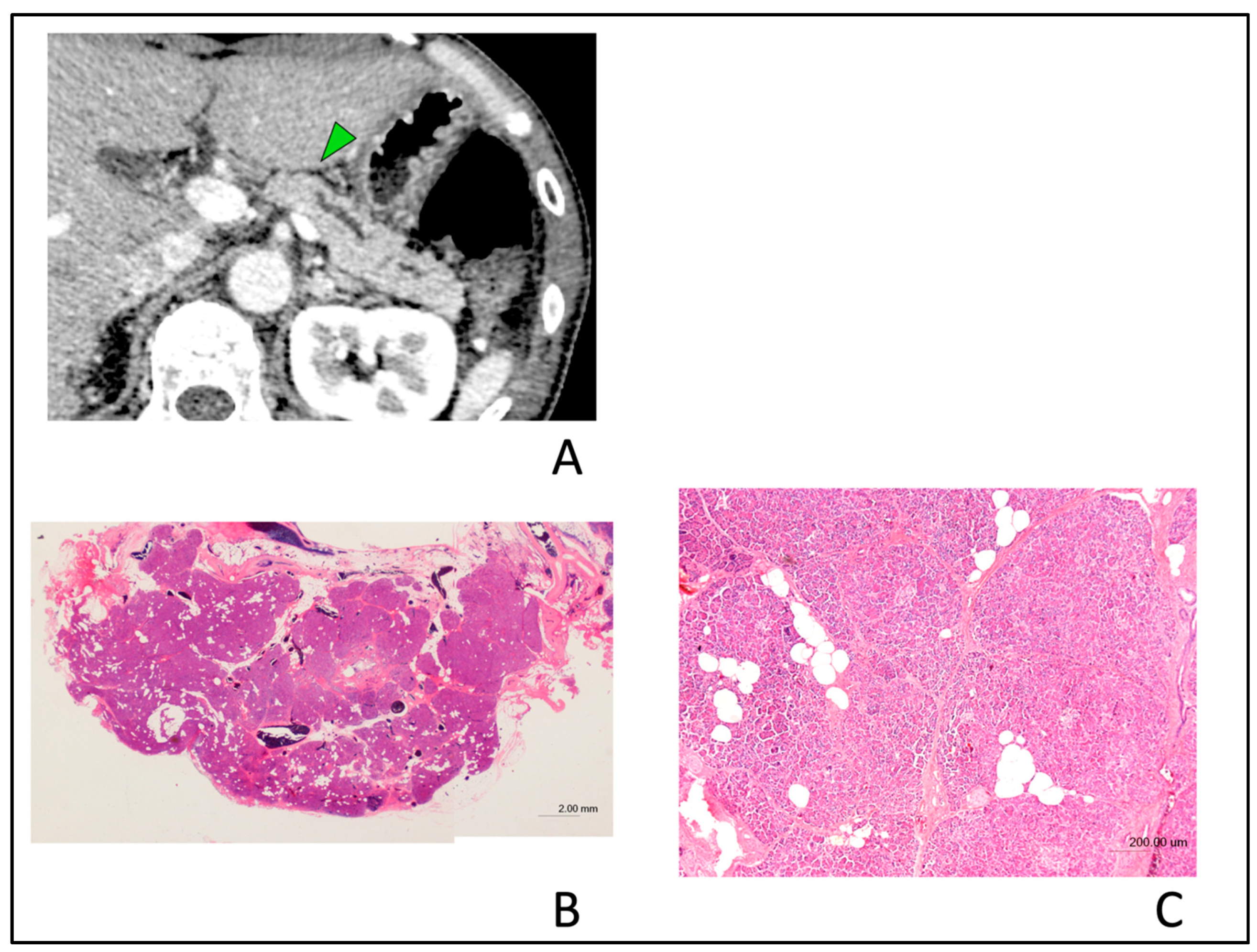
2.4. CT Findings
2.5. Statistical Analysis
3. Results
3.1. Patient Selections
3.2. Patient Characteristics
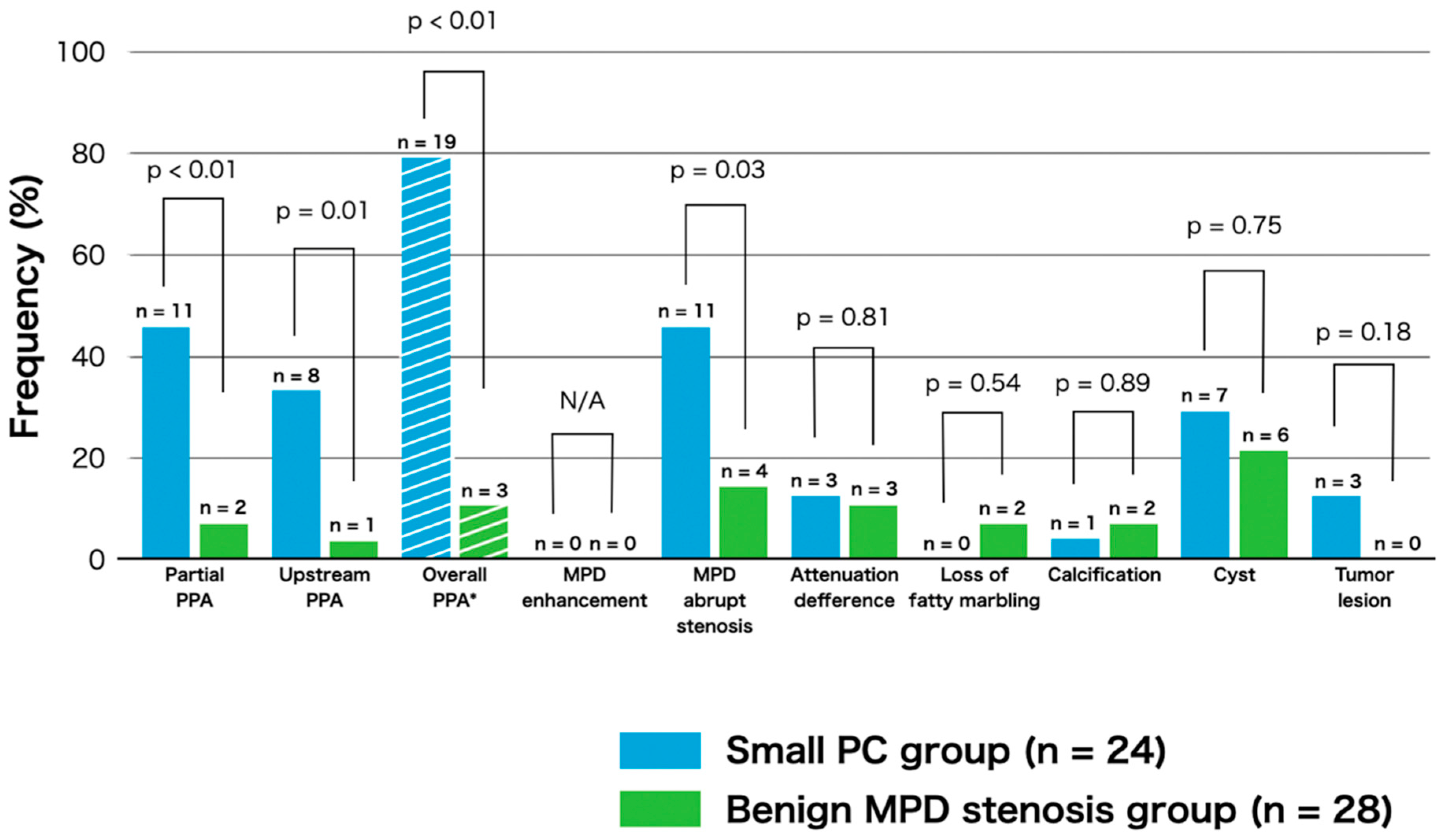
3.3. Frequency of CT Findings
3.4. Interobserver Agreement
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Egawa, S.; Toma, H.; Ohigashi, H.; Okusaka, T.; Nakao, A.; Hatori, T.; Maguchi, H.; Yanagisawa, A.; Tanaka, M. Japan pancreatic cancer registry; 30th year anniversary: Japan pancreas society. Pancreas 2012, 41, 985–992. [Google Scholar] [CrossRef] [PubMed]
- Kanno, A.; Masamune, A.; Hanada, K.; Maguchi, H.; Shimizu, Y.; Ueki, T.; Hasebe, O.; Ohtsuka, T.; Nakamura, M.; Takenaka, M.; et al. Multicenter study of early pancreatic cancer in japan. Pancreatology 2018, 18, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, K.; Okusaka, T.; Shimizu, K.; Furuse, J.; Ito, Y.; Hanada, K.; Shimosegawa, T.; Okazaki, K. Clinical practice guidelines for pancreatic cancer 2016 from the japan pancreas society: A synopsis. Pancreas 2017, 46, 595–604. [Google Scholar] [CrossRef] [PubMed]
- Dewitt, J.; Devereaux, B.M.; Lehman, G.A.; Sherman, S.; Imperiale, T.F. Comparison of endoscopic ultrasound and computed tomography for the preoperative evaluation of pancreatic cancer: A systematic review. Clin. Gastroenterol. Hepatol. 2006, 4, 717–725. [Google Scholar] [CrossRef]
- Sakamoto, H.; Kitano, M.; Suetomi, Y.; Maekawa, K.; Takeyama, Y.; Kudo, M. Utility of contrast-enhanced endoscopic ultrasonography for diagnosis of small pancreatic carcinomas. Ultrasound Med. Biol. 2008, 34, 525–532. [Google Scholar] [CrossRef]
- Kim, J.H.; Park, S.H.; Yu, E.S.; Kim, M.H.; Kim, J.; Byun, J.H.; Lee, S.S.; Hwang, H.J.; Hwang, J.Y.; Lee, S.S.; et al. Visually isoattenuating pancreatic adenocarcinoma at dynamic-enhanced ct: Frequency, clinical and pathologic characteristics, and diagnosis at imaging examinations. Radiology 2010, 257, 87–96. [Google Scholar] [CrossRef]
- Yoon, S.H.; Lee, J.M.; Cho, J.Y.; Lee, K.B.; Kim, J.E.; Moon, S.K.; Kim, S.J.; Baek, J.H.; Kim, S.H.; Kim, S.H.; et al. Small (≤20 mm) pancreatic adenocarcinomas: Analysis of enhancement patterns and secondary signs with multiphasic multidetector ct. Radiology 2011, 259, 442–452. [Google Scholar] [CrossRef]
- Zaheer, A.; Singh, V.K.; Akshintala, V.S.; Kawamoto, S.; Tsai, S.D.; Gage, K.L.; Fishman, E.K. Differentiating autoimmune pancreatitis from pancreatic adenocarcinoma using dual-phase computed tomography. J. Comput. Assist. Tomogr. 2014, 38, 146–152. [Google Scholar] [CrossRef]
- Kim, S.W.; Kim, S.H.; Lee, D.H.; Lee, S.M.; Kim, Y.S.; Jang, J.Y.; Han, J.K. Isolated main pancreatic duct dilatation: Ct differentiation between benign and malignant causes. AJR Am. J. Roentgenol. 2017, 209, 1046–1055. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, S.; Nakao, M.; Ioka, T.; Takakura, R.; Takano, Y.; Tsukuma, H.; Uehara, H.; Suzuki, R.; Fukuda, J. Slight dilatation of the main pancreatic duct and presence of pancreatic cysts as predictive signs of pancreatic cancer: A prospective study. Radiology 2010, 254, 965–972. [Google Scholar] [CrossRef] [PubMed]
- Kanno, A.; Masamune, A.; Hanada, K.; Kikuyama, M.; Kitano, M. Advances in early detection of pancreatic cancer. Diagnostics (Basel) 2019, 9, 18. [Google Scholar] [CrossRef]
- Satoh, T.; Kikuyama, M.; Kawaguchi, S.; Kanemoto, H.; Muro, H.; Hanada, K. Acute pancreatitis-onset carcinoma in situ of the pancreas with focal fat replacement diagnosed using serial pancreatic-juice aspiration cytologic examination (space). Clin. J. Gastroenterol. 2017, 10, 541–545. [Google Scholar] [CrossRef] [PubMed]
- Gangi, S.; Fletcher, J.G.; Nathan, M.A.; Christensen, J.A.; Harmsen, W.S.; Crownhart, B.S.; Chari, S.T. Time interval between abnormalities seen on ct and the clinical diagnosis of pancreatic cancer: Retrospective review of ct scans obtained before diagnosis. AJR Am. J. Roentgenol. 2004, 182, 897–903. [Google Scholar] [CrossRef] [PubMed]
- Ahn, S.S.; Kim, M.J.; Choi, J.Y.; Hong, H.S.; Chung, Y.E.; Lim, J.S. Indicative findings of pancreatic cancer in prediagnostic ct. Eur. Radiol. 2009, 19, 2448–2455. [Google Scholar] [CrossRef] [PubMed]
- Tamada, T.; Ito, K.; Kanomata, N.; Sone, T.; Kanki, A.; Higaki, A.; Hayashida, M.; Yamamoto, A. Pancreatic adenocarcinomas without secondary signs on multiphasic multidetector ct: Association with clinical and histopathologic features. Eur. Radiol. 2016, 26, 646–655. [Google Scholar] [CrossRef]
- Gonoi, W.; Hayashi, T.Y.; Okuma, H.; Akahane, M.; Nakai, Y.; Mizuno, S.; Tateishi, R.; Isayama, H.; Koike, K.; Ohtomo, K. Development of pancreatic cancer is predictable well in advance using contrast-enhanced ct: A case-cohort study. Eur. Radiol. 2017, 27, 4941–4950. [Google Scholar] [CrossRef]
- Higashi, M.; Tanabe, M.; Onoda, H.; Nakao, S.; Miyoshi, K.; Iida, E.; Okada, M.; Furukawa, M.; Ito, K. Incidentally detected pancreatic adenocarcinomas on computed tomography obtained during the follow-up for other diseases. Abdom. Radiol. (NY) 2020, 45, 774–781. [Google Scholar] [CrossRef]
- Lee, E.S.; Lee, J.M. Imaging diagnosis of pancreatic cancer: A state-of-the-art review. World J. Gastroenterol. 2014, 20, 7864–7877. [Google Scholar] [CrossRef]
- Detlefsen, S.; Sipos, B.; Feyerabend, B.; Kloppel, G. Pancreatic fibrosis associated with age and ductal papillary hyperplasia. Virchows Arch. 2005, 447, 800–805. [Google Scholar] [CrossRef]
- Inagaki, M.; Goto, J.; Tokusashi, Y.; Miyokawa, N.; Yokoyama, K.; Ikeue, S.; Kasai, S. Multifocal pancreatic intraepithelial neoplasia (panin) lesions of the branch ducts associated with lobular parenchymal atrophy in a japanese patient diagnosed to have familial pancreatic cancer. Clin. J. Gastroenterol. 2009, 2, 103–108. [Google Scholar] [CrossRef]
- Brune, K.; Abe, T.; Canto, M.; O’Malley, L.; Klein, A.P.; Maitra, A.; Volkan Adsay, N.; Fishman, E.K.; Cameron, J.L.; Yeo, C.J.; et al. Multifocal neoplastic precursor lesions associated with lobular atrophy of the pancreas in patients having a strong family history of pancreatic cancer. Am. J. Surg. Pathol. 2006, 30, 1067–1076. [Google Scholar] [PubMed]
- Tomita, Y.; Azuma, K.; Nonaka, Y.; Kamada, Y.; Tomoeda, M.; Kishida, M.; Tanemura, M.; Miyoshi, E. Pancreatic fatty degeneration and fibrosis as predisposing factors for the development of pancreatic ductal adenocarcinoma. Pancreas 2014, 43, 1032–1041. [Google Scholar] [CrossRef] [PubMed]
- Hori, M.; Onaya, H.; Hiraoka, N.; Yamaji, T.; Kobayashi, H.; Takahashi, M.; Mutoh, M.; Shimada, K.; Nakagama, H. Evaluation of the degree of pancreatic fatty infiltration by area-based assessment of ct images: Comparison with histopathology-based and ct attenuation index-based assessments. Jpn. J. Radiol. 2016, 34, 667–676. [Google Scholar] [CrossRef] [PubMed]
- Yachida, S.; Jones, S.; Bozic, I.; Antal, T.; Leary, R.; Fu, B.; Kamiyama, M.; Hruban, R.H.; Eshleman, J.R.; Nowak, M.A.; et al. Distant metastasis occurs late during the genetic evolution of pancreatic cancer. Nature 2010, 467, 1114–1117. [Google Scholar] [CrossRef] [PubMed]
- Yamao, K.; Takenaka, M.; Nakai, A.; Omoto, S.; Kamata, K.; Minaga, K.; Miyata, T.; Imai, H.; Sakurai, T.; Watanabe, T.; et al. Detection of high-grade pancreatic intraepithelial neoplasia without morphological changes of the main pancreatic duct over a long period: Importance for close follow-up for confirmation. Oncology 2017, 93 (Suppl. S1), 81–86. [Google Scholar] [CrossRef] [PubMed]
- Kuruma, S.; Kikuyama, M.; Chiba, K.; Yoshimoto, K.; Kamisawa, T.; Honda, G.; Horiguchi, S.; Nakahodo, J. Carcinoma in situ of the pancreas with pancreatic duct stricture persistent for 4 years diagnosed by serial pancreatic juice aspiration cytologic examination (space). Clin. J. Gastroenterol. 2020, 13, 443–447. [Google Scholar] [CrossRef] [PubMed]
- Izumi, Y.; Hanada, K.; Okazaki, A.; Minami, T.; Hirano, N.; Ikemoto, J.; Kanemitsu, K.; Nakadoi, K.; Shishido, T.; Katamura, Y.; et al. Endoscopic ultrasound findings and pathological features of pancreatic carcinoma in situ. Endosc. Int. Open 2019, 7, E585–E593. [Google Scholar] [CrossRef]
- Terada, S.; Kikuyama, M.; Kawaguchi, S.; Kanemoto, H.; Yokoi, Y.; Kamisawa, T.; Kuruma, S.; Chiba, K.; Honda, G.; Horiguchi, S.; et al. Proposal for endoscopic ultrasonography classification for small pancreatic cancer. Diagnostics (Basel) 2019, 9, 15. [Google Scholar] [CrossRef]
- Banafea, O.; Mghanga, F.P.; Zhao, J.; Zhao, R.; Zhu, L. Endoscopic ultrasonography with fine-needle aspiration for histological diagnosis of solid pancreatic masses: A meta-analysis of diagnostic accuracy studies. BMC Gastroenterol. 2016, 16, 108. [Google Scholar] [CrossRef]
- Chen, J.; Yang, R.; Lu, Y.; Xia, Y.; Zhou, H. Diagnostic accuracy of endoscopic ultrasound-guided fine-needle aspiration for solid pancreatic lesion: A systematic review. J. Cancer Res. Clin. Oncol. 2012, 138, 1433–1441. [Google Scholar] [CrossRef]
- Chen, G.; Liu, S.; Zhao, Y.; Dai, M.; Zhang, T. Diagnostic accuracy of endoscopic ultrasound-guided fine-needle aspiration for pancreatic cancer: A meta-analysis. Pancreatology 2013, 13, 298–304. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, A.A.; Brown, L.J.; Hong, S.K.; Draganova-Tacheva, R.A.; Korenblit, J.; Loren, D.E.; Kowalski, T.E.; Solomides, C. Relationship of pancreatic mass size and diagnostic yield of endoscopic ultrasound-guided fine needle aspiration. Dig. Dis. Sci. 2011, 56, 3370–3375. [Google Scholar] [CrossRef] [PubMed]
- Uehara, H.; Ikezawa, K.; Kawada, N.; Fukutake, N.; Katayama, K.; Takakura, R.; Takano, Y.; Ishikawa, O.; Takenaka, A. Diagnostic accuracy of endoscopic ultrasound-guided fine needle aspiration for suspected pancreatic malignancy in relation to the size of lesions. J. Gastroenterol. Hepatol. 2011, 26, 1256–1261. [Google Scholar] [CrossRef] [PubMed]
- Ramesh, J.; Kim, H.; Reddy, K.; Eltoum, I.E. Performance characteristic of endoscopic ultrasound-guided fine needle aspiration is unaffected by pancreatic mass size. Endosc. Int. Open 2016, 4, E434–E438. [Google Scholar] [CrossRef][Green Version]
- Iiboshi, T.; Hanada, K.; Fukuda, T.; Yonehara, S.; Sasaki, T.; Chayama, K. Value of cytodiagnosis using endoscopic nasopancreatic drainage for early diagnosis of pancreatic cancer: Establishing a new method for the early detection of pancreatic carcinoma in situ. Pancreas 2012, 41, 523–529. [Google Scholar] [CrossRef]
| Small PC Group | Benign MPD Stenosis Group | p Value | |
|---|---|---|---|
| (n = 24) | (n = 28) | ||
| Age in years, median (range) | 73 (47–85) | 69.5 (45–86) | 0.15 |
| Sex, n (%) | |||
| Male | 14 (58.3) | 16 (57.1) | 0.93 |
| Female | 10 (41.7) | 12 (42.9) | |
| Diabetes mellitus, n (%) | 6 (25.0) | 6 (21.4) | 0.98 |
| Chronic pancreatitis *, n (%) | 1 (4.2) | 0 (0) | 0.28 |
| Body mass index ≥ 30 kg/m2, n (%) | 0 (0) | 1 (3.6) | 0.94 |
| Smoker (current or previous), n (%) | 10 (41.7) | 15 (53.6) | 0.39 |
| Heavy alcohol consumption **, n (%) | 4 (16.7) | 9 (32.1) | 0.34 |
| Main location of MPD stenosis, n (%) | |||
| Pancreatic head | 5 (20.1) | 8 (28.6) | 0.16 |
| Pancreatic body | 16 (66.7) | 13 (46.4) | |
| Pancreatic tail | 3 (12.5) | 7 (25.0) | |
| Final confirmed detection method, n (%) | |||
| Histological analysis | 24 (100) | 21 (75.0) | |
| Surgery | 24 (100) | 6 (21.4) | |
| Pancreatic juice cytology | 0 (0) | 15 (53.6) | |
| Follow up † | 0 (0) | 7 (25.0) |
| Findings | Kappa Value |
|---|---|
| Partial PPA | 0.74 |
| Upstream PPA | 0.68 |
| MPD enhanced with stenotic area | −0.02 |
| MPD with abrupt stenosis | 0.57 |
| Attenuation difference | 0.70 |
| Loss of fatty marbling | 0.65 |
| Calcification | 0.85 |
| Cyst | 0.88 |
| Tumor lesion | 0.64 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yamao, K.; Takenaka, M.; Ishikawa, R.; Okamoto, A.; Yamazaki, T.; Nakai, A.; Omoto, S.; Kamata, K.; Minaga, K.; Matsumoto, I.; et al. Partial Pancreatic Parenchymal Atrophy Is a New Specific Finding to Diagnose Small Pancreatic Cancer (≤10 mm) Including Carcinoma in Situ: Comparison with Localized Benign Main Pancreatic Duct Stenosis Patients. Diagnostics 2020, 10, 445. https://doi.org/10.3390/diagnostics10070445
Yamao K, Takenaka M, Ishikawa R, Okamoto A, Yamazaki T, Nakai A, Omoto S, Kamata K, Minaga K, Matsumoto I, et al. Partial Pancreatic Parenchymal Atrophy Is a New Specific Finding to Diagnose Small Pancreatic Cancer (≤10 mm) Including Carcinoma in Situ: Comparison with Localized Benign Main Pancreatic Duct Stenosis Patients. Diagnostics. 2020; 10(7):445. https://doi.org/10.3390/diagnostics10070445
Chicago/Turabian StyleYamao, Kentaro, Mamoru Takenaka, Rei Ishikawa, Ayana Okamoto, Tomohiro Yamazaki, Atsushi Nakai, Shunsuke Omoto, Ken Kamata, Kosuke Minaga, Ippei Matsumoto, and et al. 2020. "Partial Pancreatic Parenchymal Atrophy Is a New Specific Finding to Diagnose Small Pancreatic Cancer (≤10 mm) Including Carcinoma in Situ: Comparison with Localized Benign Main Pancreatic Duct Stenosis Patients" Diagnostics 10, no. 7: 445. https://doi.org/10.3390/diagnostics10070445
APA StyleYamao, K., Takenaka, M., Ishikawa, R., Okamoto, A., Yamazaki, T., Nakai, A., Omoto, S., Kamata, K., Minaga, K., Matsumoto, I., Takeyama, Y., Numoto, I., Tsurusaki, M., Chikugo, T., Chiba, Y., Watanabe, T., & Kudo, M. (2020). Partial Pancreatic Parenchymal Atrophy Is a New Specific Finding to Diagnose Small Pancreatic Cancer (≤10 mm) Including Carcinoma in Situ: Comparison with Localized Benign Main Pancreatic Duct Stenosis Patients. Diagnostics, 10(7), 445. https://doi.org/10.3390/diagnostics10070445








