Platelet-Rich Plasma Ameliorates Cyclophosphamide-Induced Acute Interstitial Cystitis/Painful Bladder Syndrome in a Rat Model
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Statement
2.2. Preparation of PRP
2.3. Cell Culture and 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl Tetrazoliumbromide (MTT) Assay for Cell Proliferation
2.4. Animal Model and Cystometry
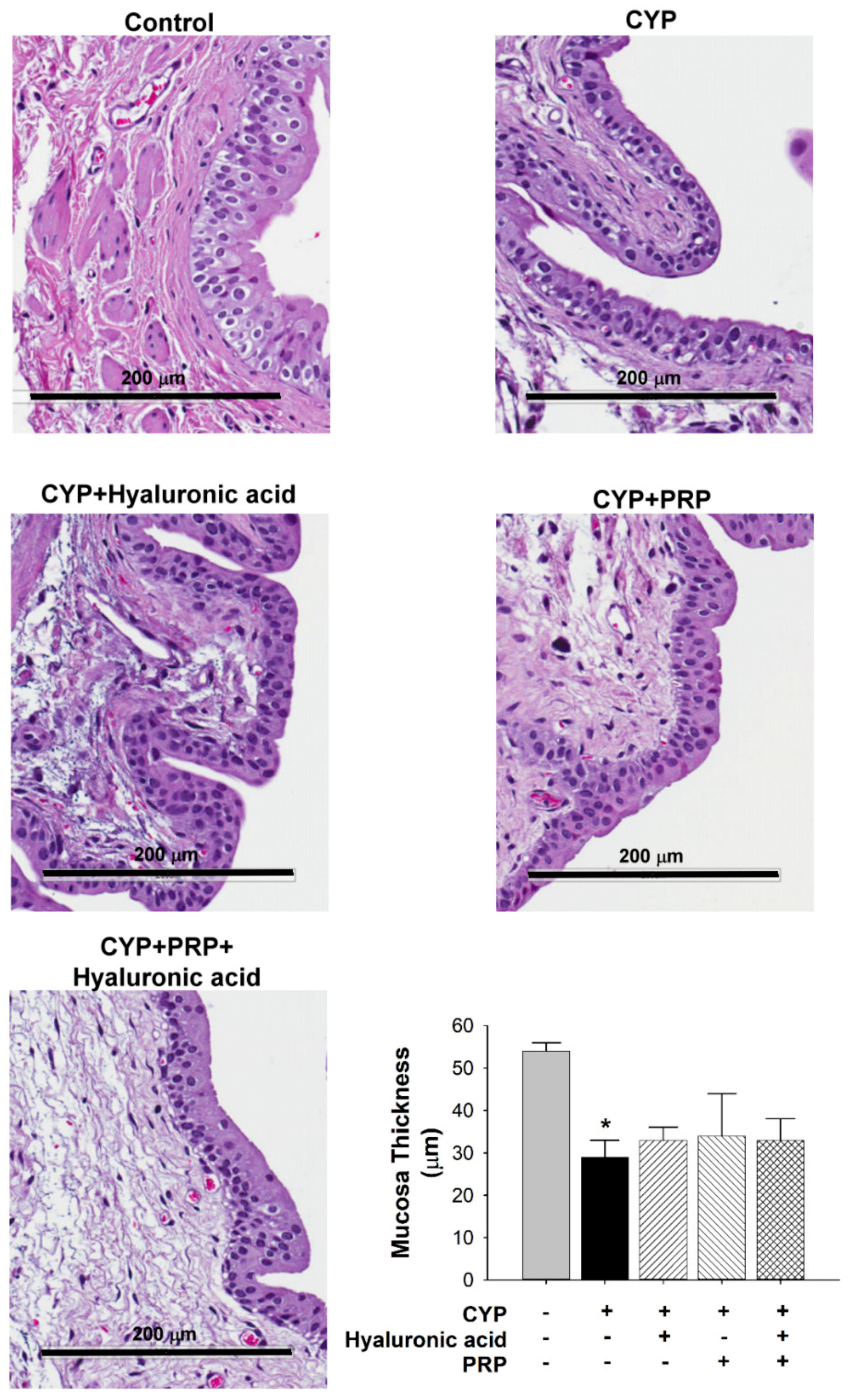
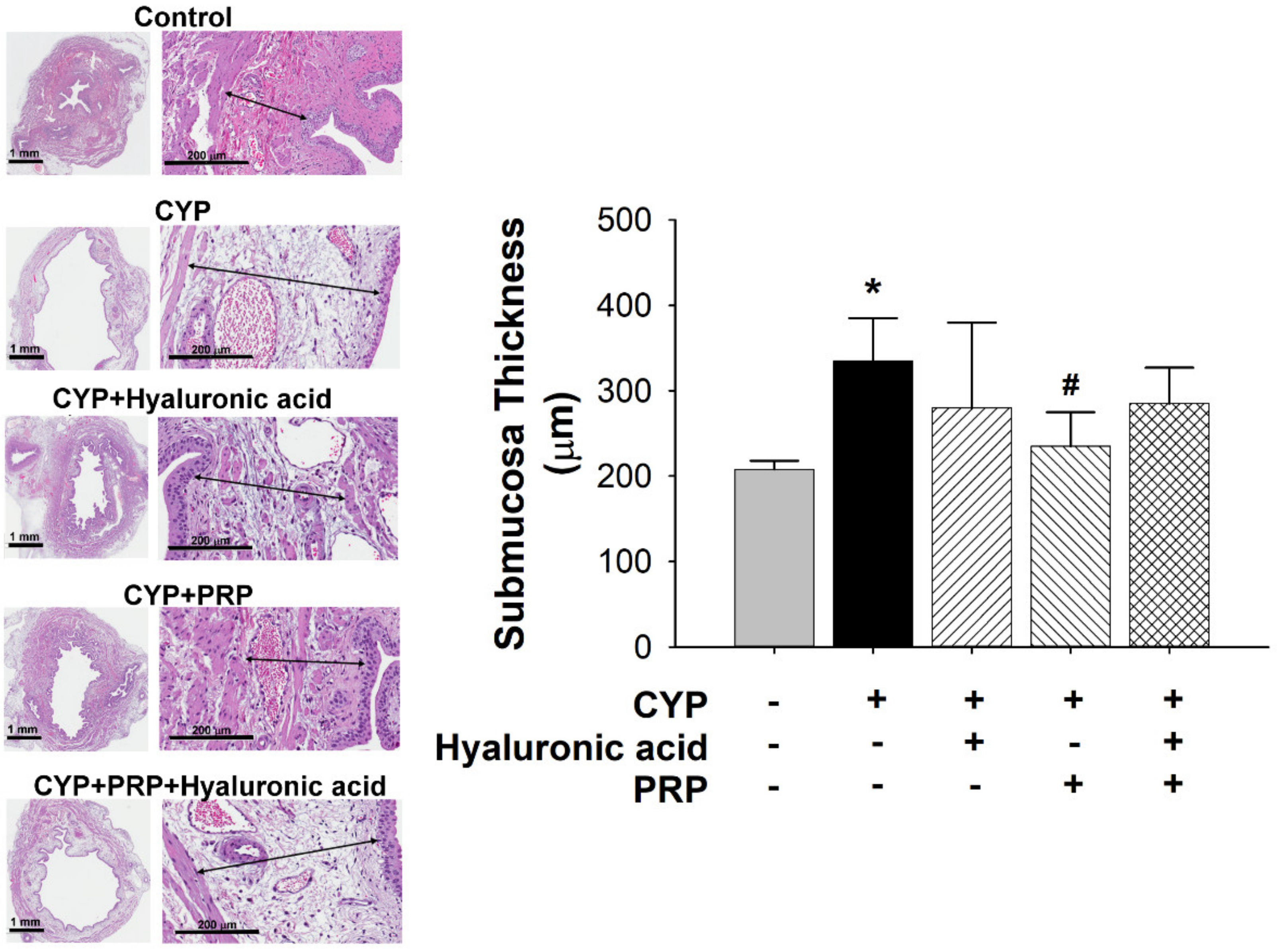
2.5. Histological Examination
2.6. Protein Preparation and Western Blot Analysis
2.7. Statistical Analysis
3. Results
3.1. Effects of PRP on Cell Proliferation of HFCs
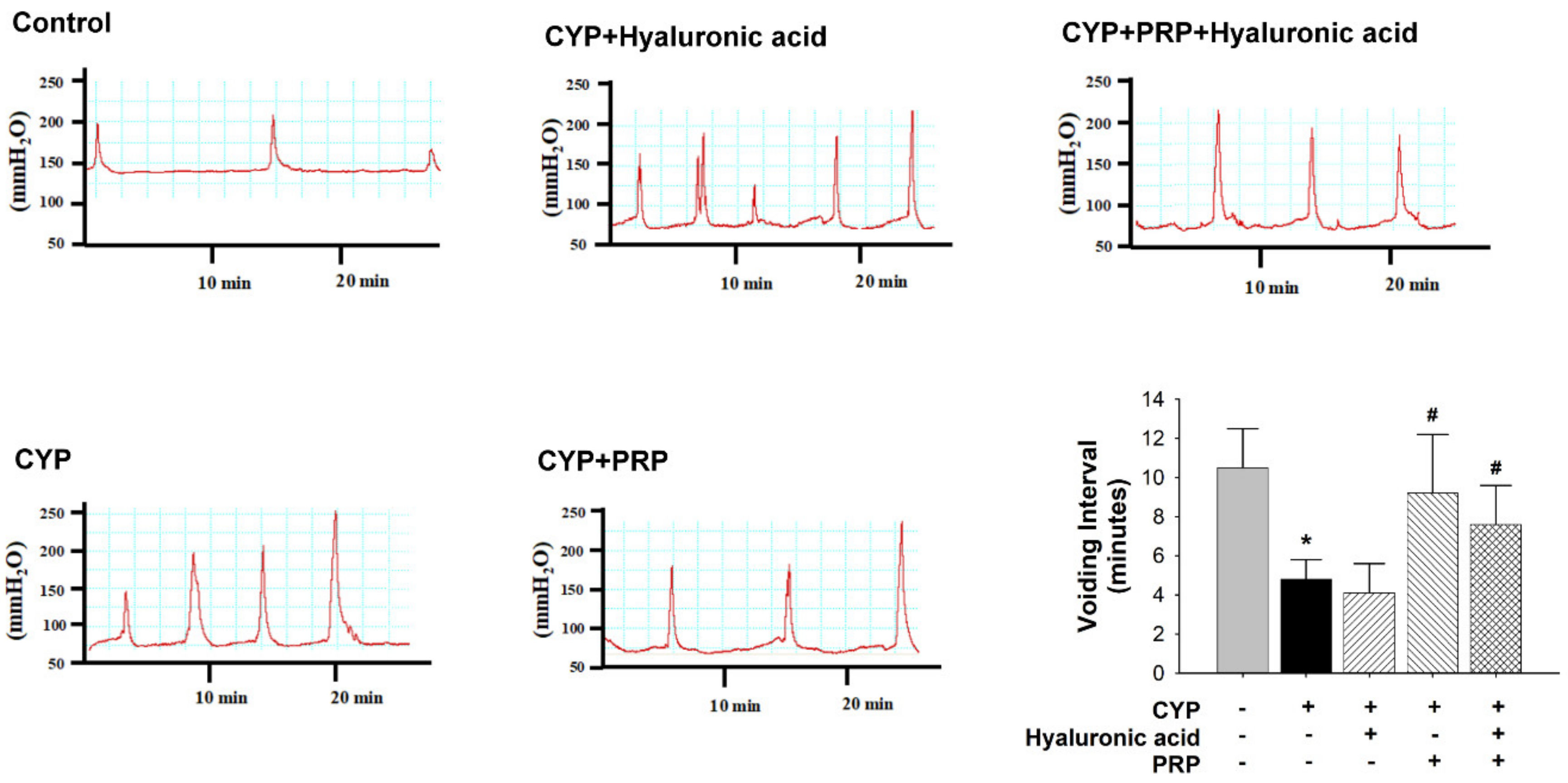
3.2. Bladder Effect of PRP in a Rat Model of CYP-induced Acute IC/PBS
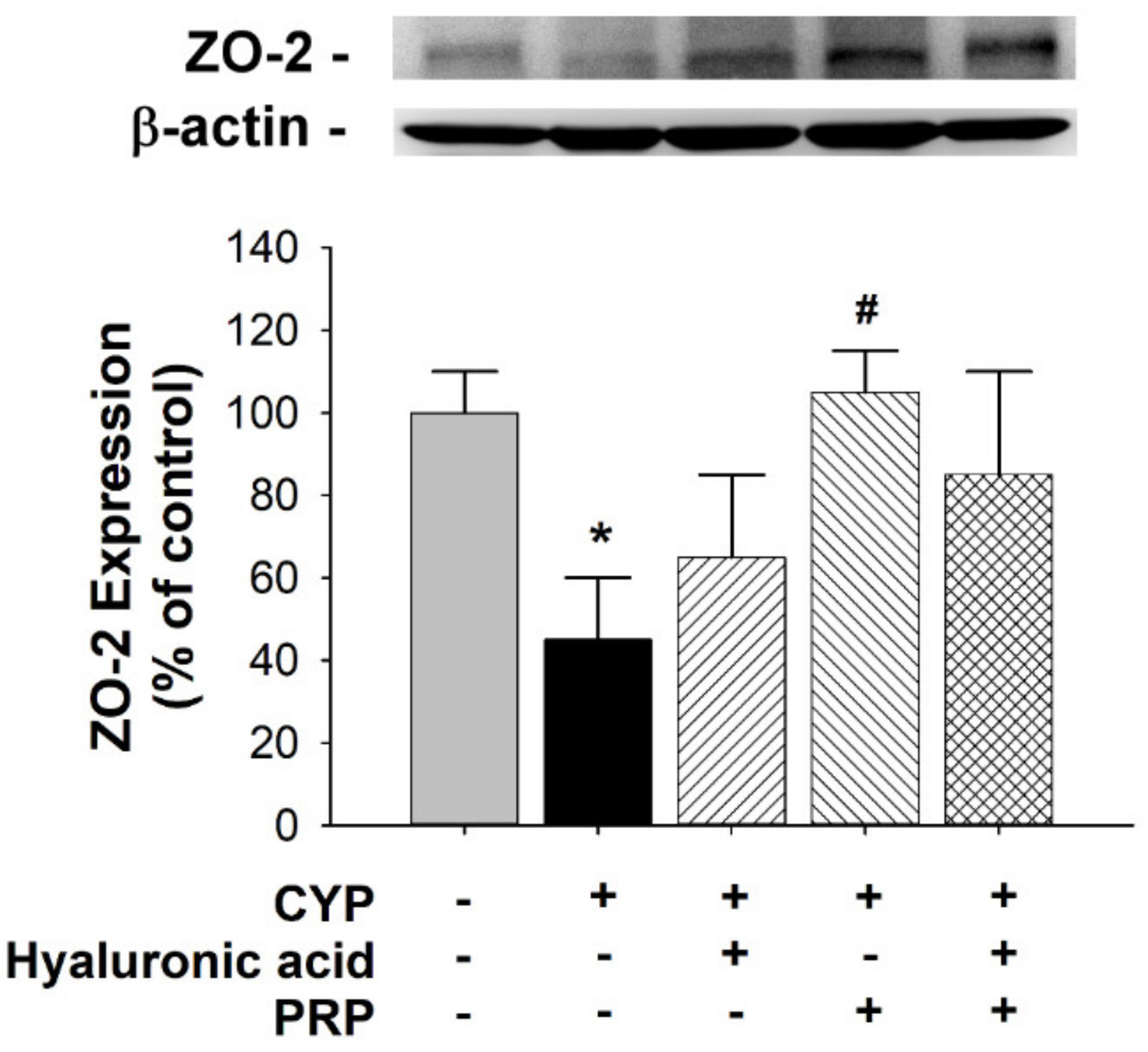
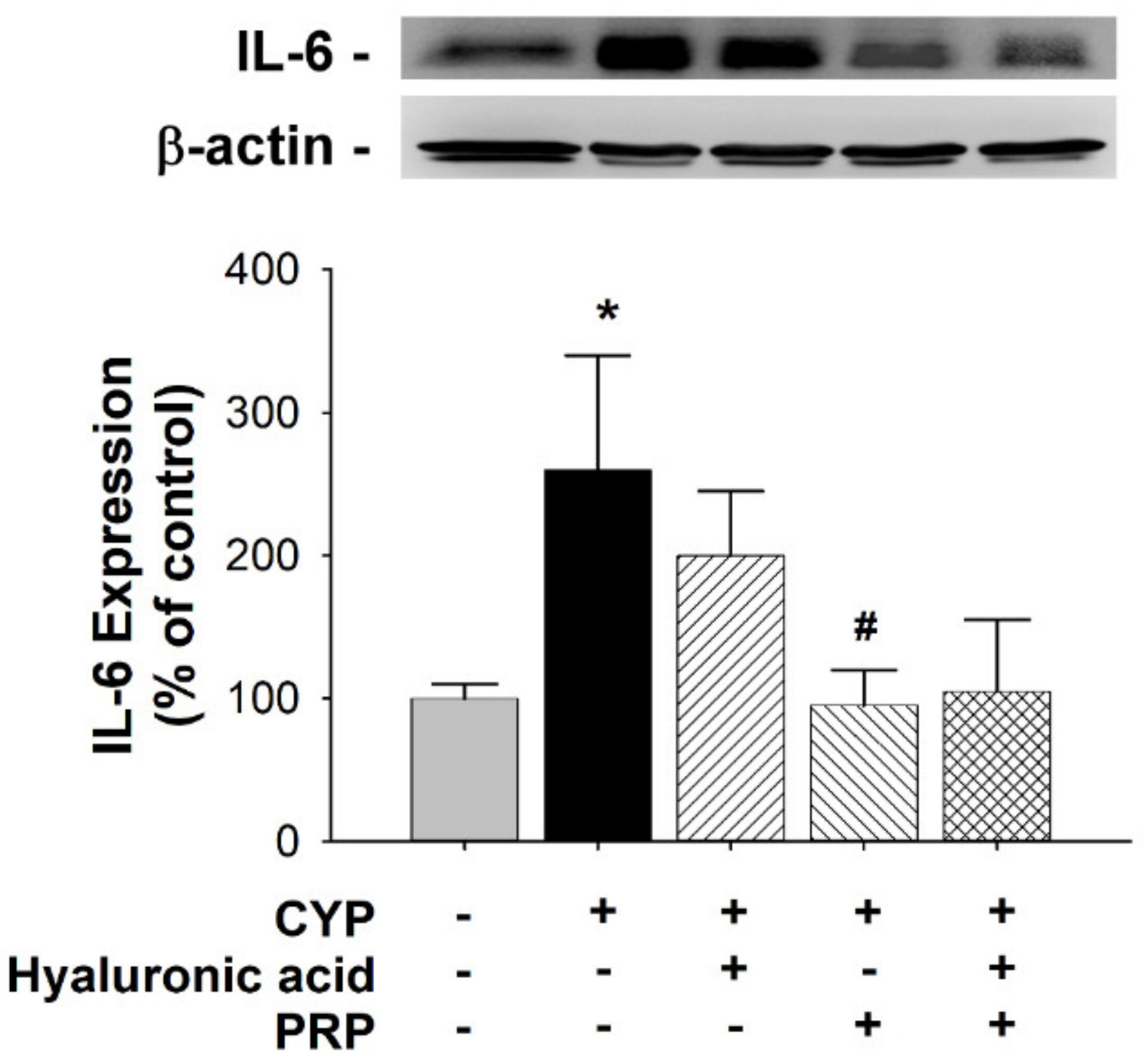
3.3. ZO-2 and IL-6 Expression in a Rat Model of CYP-induced Acute IC/PBS
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Batista, R.; Vinagre, N.; Meireles, S.; Vinagre, J.; Prazeres, H.; Leao, R.; Maximo, V.; Soares, P. Biomarkers for Bladder Cancer Diagnosis and Surveillance: A Comprehensive Review. Diagnostics 2020, 10. [Google Scholar] [CrossRef] [PubMed]
- Akiyama, Y.; Luo, Y.; Hanno, P.M.; Maeda, D.; Homma, Y. Interstitial cystitis/bladder pain syndrome: The evolving landscape, animal models and future perspectives. Int. J. Urol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Rahnama’i, M.S.; Marcelissen, T.; Apostolidis, A.; Veit-Rubin, N.; Schurch, B.; Cardozo, L.; Dmochowski, R. The efficacy of botulinum toxin A and sacral neuromodulation in the management of interstitial cystitis (IC)/bladder pain syndrome (BPS), what do we know? ICI-RS 2017 think thank, Bristol. Neurourol. Urodyn. 2018, 37, S99–S107. [Google Scholar] [CrossRef] [PubMed]
- Patnaik, S.S.; Lagana, A.S.; Vitale, S.G.; Buttice, S.; Noventa, M.; Gizzo, S.; Valenti, G.; Rapisarda, A.M.C.; La Rosa, V.L.; Magno, C.; et al. Etiology, pathophysiology and biomarkers of interstitial cystitis/painful bladder syndrome. Arch. Gynecol. Obstet. 2017, 295, 1341–1359. [Google Scholar] [CrossRef] [PubMed]
- Meng, E.; Hsu, Y.C.; Chuang, Y.C. Advances in intravesical therapy for bladder pain syndrome (BPS)/interstitial cystitis (IC). Low Urin Tract Symptoms 2018, 10, 3–11. [Google Scholar] [CrossRef]
- Auge, C.; Chene, G.; Dubourdeau, M.; Desoubzdanne, D.; Corman, B.; Palea, S.; Lluel, P.; Vergnolle, N.; Coelho, A.M. Relevance of the cyclophosphamide-induced cystitis model for pharmacological studies targeting inflammation and pain of the bladder. Eur. J. Pharmacol. 2013, 707, 32–40. [Google Scholar] [CrossRef]
- Boucher, M.; Meen, M.; Codron, J.P.; Coudore, F.; Kemeny, J.L.; Eschalier, A. Cyclophosphamide-induced cystitis in freely-moving conscious rats: Behavioral approach to a new model of visceral pain. J. Urol. 2000, 164, 203–208. [Google Scholar] [CrossRef]
- Felsen, D.; Frye, S.; Vaughan, E.D., Jr. Inflammatory mediators and interstitial cystitis. Semin. Urol. 1991, 9, 102–107. [Google Scholar]
- Juszczak, K.; Gil, K.; Wyczolkowski, M.; Thor, P.J. Functional, histological structure and mastocytes alterations in rat urinary bladders following acute and [corrected] chronic cyclophosphamide treatment. J. Physiol. Pharmacol. 2010, 61, 477–482. [Google Scholar]
- Smaldone, M.C.; Vodovotz, Y.; Tyagi, V.; Barclay, D.; Philips, B.J.; Yoshimura, N.; Chancellor, M.B.; Tyagi, P. Multiplex analysis of urinary cytokine levels in rat model of cyclophosphamide-induced cystitis. Urology 2009, 73, 421–426. [Google Scholar] [CrossRef]
- Simon, L.J.; Landis, J.R.; Erickson, D.R.; Nyberg, L.M. The Interstitial Cystitis Data Base Study: Concepts and preliminary baseline descriptive statistics. Urology 1997, 49, 64–75. [Google Scholar] [CrossRef]
- Cervigni, M. Interstitial cystitis/bladder pain syndrome and glycosaminoglycans replacement therapy. Transl. Androl. Urol. 2015, 4, 638–642. [Google Scholar] [CrossRef] [PubMed]
- Rickard, A.; Dorokhov, N.; Ryerse, J.; Klumpp, D.J.; McHowat, J. Characterization of tight junction proteins in cultured human urothelial cells. In Vitro Cell. Dev. Biol. Anim. 2008, 44, 261–267. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Donmez, M.I.; Inci, K.; Zeybek, N.D.; Dogan, H.S.; Ergen, A. The Early Histological Effects of Intravesical Instillation of Platelet-Rich Plasma in Cystitis Models. Int. Neurourol. J. 2016, 20, 188–196. [Google Scholar] [CrossRef]
- Garg, A.K. The use of platelet-rich plasma to enhance the success of bone grafts around dental implants. Dent. Implantol. Update 2000, 11, 17–21. [Google Scholar] [PubMed]
- Marx, R.E. Platelet-rich plasma: Evidence to support its use. J. Oral Maxillofac. Surg. 2004, 62, 489–496. [Google Scholar] [CrossRef]
- Jhang, J.F.; Lin, T.Y.; Kuo, H.C. Intravesical injections of platelet-rich plasma is effective and safe in treatment of interstitial cystitis refractory to conventional treatment—A prospective clinical trial. Neurourol. Urodyn. 2019, 38, 703–709. [Google Scholar] [CrossRef]
- Kallestrup, E.B.; Jorgensen, S.S.; Nordling, J.; Hald, T. Treatment of interstitial cystitis with Cystistat: A hyaluronic acid product. Scand. J. Urol. Nephrol. 2005, 39, 143–147. [Google Scholar] [CrossRef]
- Gulpinar, O.; Esen, B.; Kayis, A.; Gokce, M.I.; Suer, E. Clinical comparison of intravesical hyaluronic acid and chondroitin sulfate therapies in the treatment of bladder pain syndrome/interstitial cystitis. Neurourol. Urodyn. 2018, 37, 257–262. [Google Scholar] [CrossRef]
- Liang, C.C.; Lin, Y.H.; Hsieh, W.C.; Huang, L. Urinary and psychological outcomes in women with interstitial cystitis/bladder pain syndrome following hyaluronic acid treatment. Taiwan J. Obstet. Gynecol. 2018, 57, 360–363. [Google Scholar] [CrossRef]
- Pyo, J.S.; Cho, W.J. Systematic Review and Meta-Analysis of Intravesical Hyaluronic Acid and Hyaluronic Acid/Chondroitin Sulfate Instillation for Interstitial Cystitis/Painful Bladder Syndrome. Cell Physiol. Biochem. 2016, 39, 1618–1625. [Google Scholar] [CrossRef] [PubMed]
- Keay, S.K.; Birder, L.A.; Chai, T.C. Evidence for bladder urothelial pathophysiology in functional bladder disorders. Biomed. Res. Int. 2014, 2014, 865463. [Google Scholar] [CrossRef] [PubMed]
- Nagae, M.; Ikeda, T.; Mikami, Y.; Hase, H.; Ozawa, H.; Matsuda, K.; Sakamoto, H.; Tabata, Y.; Kawata, M.; Kubo, T. Intervertebral disc regeneration using platelet-rich plasma and biodegradable gelatin hydrogel microspheres. Tissue Eng. 2007, 13, 147–158. [Google Scholar] [CrossRef] [PubMed]
- Ozyuvali, E.; Yildirim, M.E.; Yaman, T.; Kosem, B.; Atli, O.; Cimentepe, E. Protective Effect of Intravesical Platelet-Rich Plasma on Cyclophosphamide-Induced Hemorrhagic Cystitis. Clin. Investig. Med. 2016, 39, 27514. [Google Scholar] [CrossRef]
- Chen, Y.H.; Chen, W.C.; Liu, P.L.; Chen, H.Y. Astragalus polysaccharides and astragaloside IV ameliorates cyclophosphamide-induced mouse model of overactive bladder. Taiwan J. Obstet. Gynecol. 2020, 59, 248–255. [Google Scholar] [CrossRef]
- Monjotin, N.; Farrie, M.; Vergnolle, N.; Le Grand, B.; Gillespie, J.; Junquero, D. Bladder telemetry: A new approach to evaluate micturition behavior under physiological and inflammatory conditions. Neurourol. Urodyn. 2017, 36, 308–315. [Google Scholar] [CrossRef]
- Wang, H.J.; Lee, W.C.; Tyagi, P.; Huang, C.C.; Chuang, Y.C. Effects of low energy shock wave therapy on inflammatory moleculars, bladder pain, and bladder function in a rat cystitis model. Neurourol. Urodyn. 2017, 36, 1440–1447. [Google Scholar] [CrossRef]
- Boudes, M.; Uvin, P.; Kerselaers, S.; Vennekens, R.; Voets, T.; De Ridder, D. Functional characterization of a chronic cyclophosphamide-induced overactive bladder model in mice. Neurourol. Urodyn. 2011, 30, 1659–1665. [Google Scholar] [CrossRef]
- Apodaca, G. The uroepithelium: Not just a passive barrier. Traffic 2004, 5, 117–128. [Google Scholar] [CrossRef]
- Lewis, S.A. Everything you wanted to know about the bladder epithelium but were afraid to ask. Am. J. Physiol. Renal Physiol. 2000, 278, F867–F874. [Google Scholar] [CrossRef]
- Fujimura, M.; Izumimoto, N.; Momen, S.; Yoshikawa, S.; Kobayashi, R.; Kanie, S.; Hirakata, M.; Komagata, T.; Okanishi, S.; Hashimoto, T.; et al. Characteristics of TRK-130 (Naltalimide), a novel opioid ligand, as a new therapeutic agent for overactive bladder. J. Pharmacol. Exp. Ther. 2014, 350, 543–551. [Google Scholar] [CrossRef]
- Schneider, M.P.; Hughes, F.M., Jr.; Engmann, A.K.; Purves, J.T.; Kasper, H.; Tedaldi, M.; Spruill, L.S.; Gullo, M.; Schwab, M.E.; Kessler, T.M. A novel urodynamic model for lower urinary tract assessment in awake rats. BJU Int. 2015, 115, 8–15. [Google Scholar] [CrossRef]
- Dhurat, R.; Sukesh, M. Principles and Methods of Preparation of Platelet-Rich Plasma: A Review and Author’s Perspective. J. Cutan. Aesthet. Surg. 2014, 7, 189–197. [Google Scholar] [CrossRef]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.-H.; Man, K.-M.; Chen, W.-C.; Liu, P.-L.; Tsai, K.-S.; Tsai, M.-Y.; Wu, Y.-T.; Chen, H.-Y. Platelet-Rich Plasma Ameliorates Cyclophosphamide-Induced Acute Interstitial Cystitis/Painful Bladder Syndrome in a Rat Model. Diagnostics 2020, 10, 381. https://doi.org/10.3390/diagnostics10060381
Chen Y-H, Man K-M, Chen W-C, Liu P-L, Tsai K-S, Tsai M-Y, Wu Y-T, Chen H-Y. Platelet-Rich Plasma Ameliorates Cyclophosphamide-Induced Acute Interstitial Cystitis/Painful Bladder Syndrome in a Rat Model. Diagnostics. 2020; 10(6):381. https://doi.org/10.3390/diagnostics10060381
Chicago/Turabian StyleChen, Yung-Hsiang, Kee-Ming Man, Wen-Chi Chen, Po-Len Liu, Kao-Sung Tsai, Ming-Yen Tsai, Yu-Tzu Wu, and Huey-Yi Chen. 2020. "Platelet-Rich Plasma Ameliorates Cyclophosphamide-Induced Acute Interstitial Cystitis/Painful Bladder Syndrome in a Rat Model" Diagnostics 10, no. 6: 381. https://doi.org/10.3390/diagnostics10060381
APA StyleChen, Y.-H., Man, K.-M., Chen, W.-C., Liu, P.-L., Tsai, K.-S., Tsai, M.-Y., Wu, Y.-T., & Chen, H.-Y. (2020). Platelet-Rich Plasma Ameliorates Cyclophosphamide-Induced Acute Interstitial Cystitis/Painful Bladder Syndrome in a Rat Model. Diagnostics, 10(6), 381. https://doi.org/10.3390/diagnostics10060381






