Impact of mRNA-Assessed Molecular Subtype Conversion, Intact and Apoptotic Circulating Tumor Cells on Survival of Metastatic Breast Cancer Patients: Proof of Principle
Abstract
1. Introduction
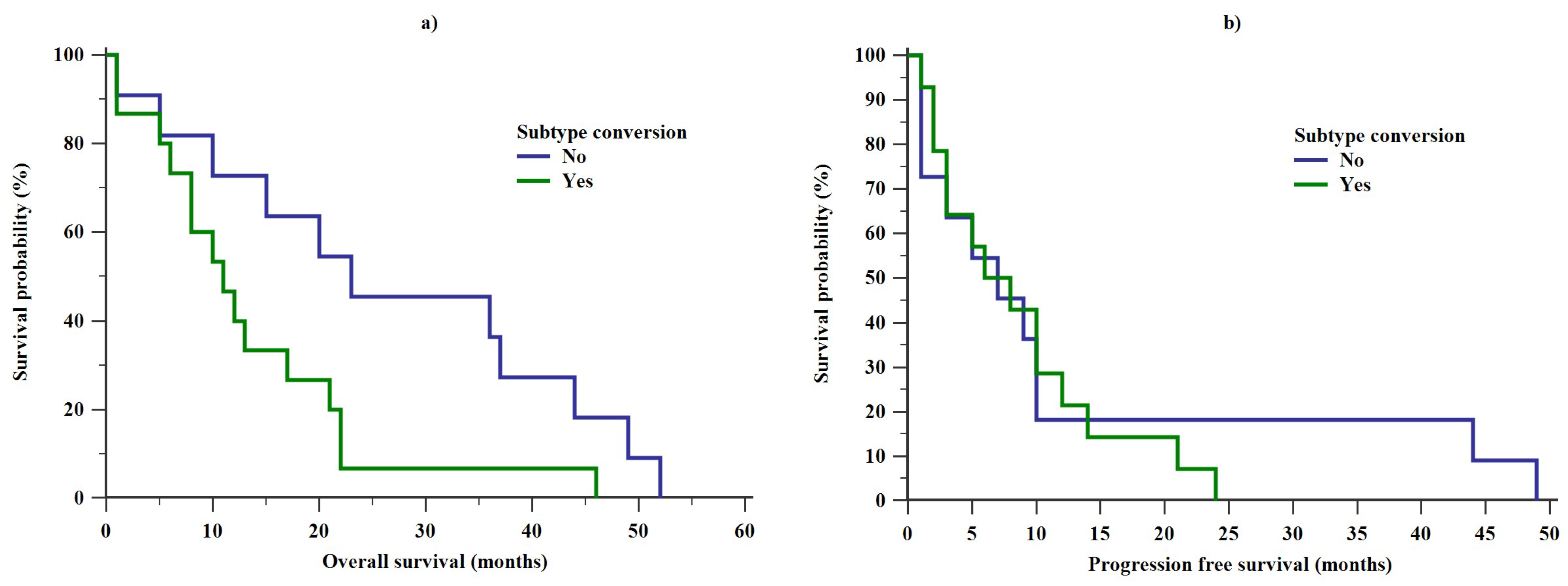
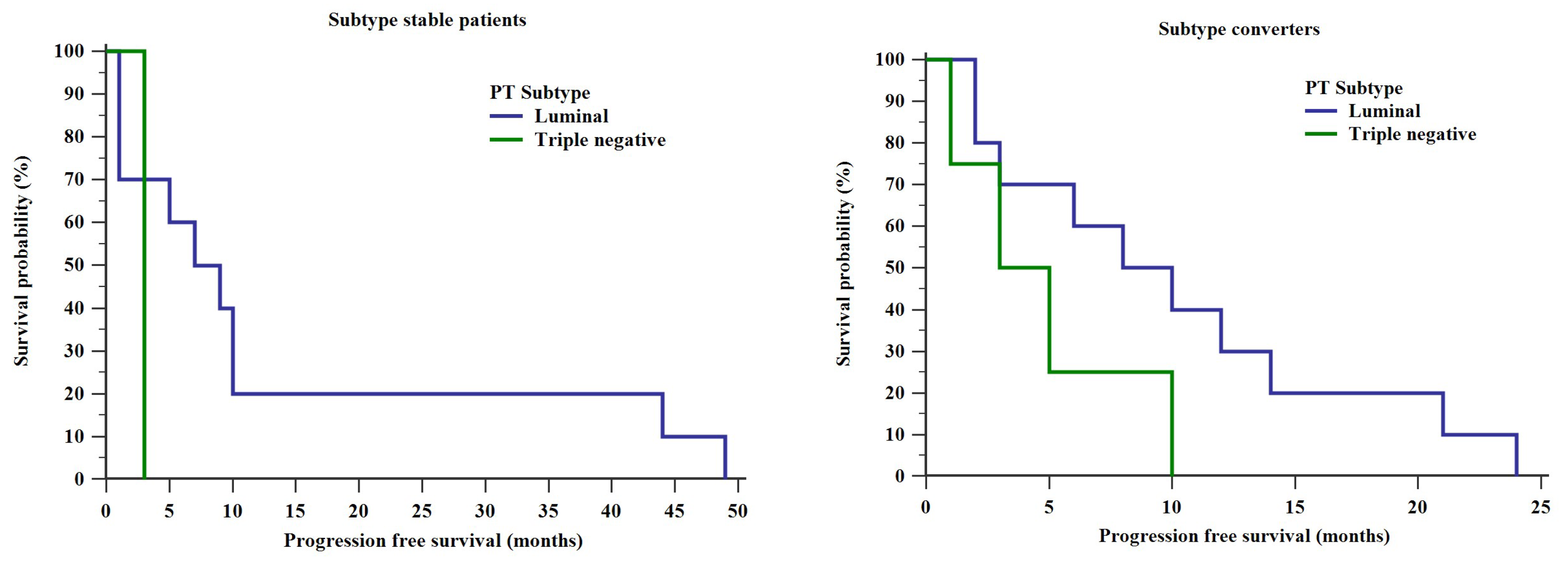
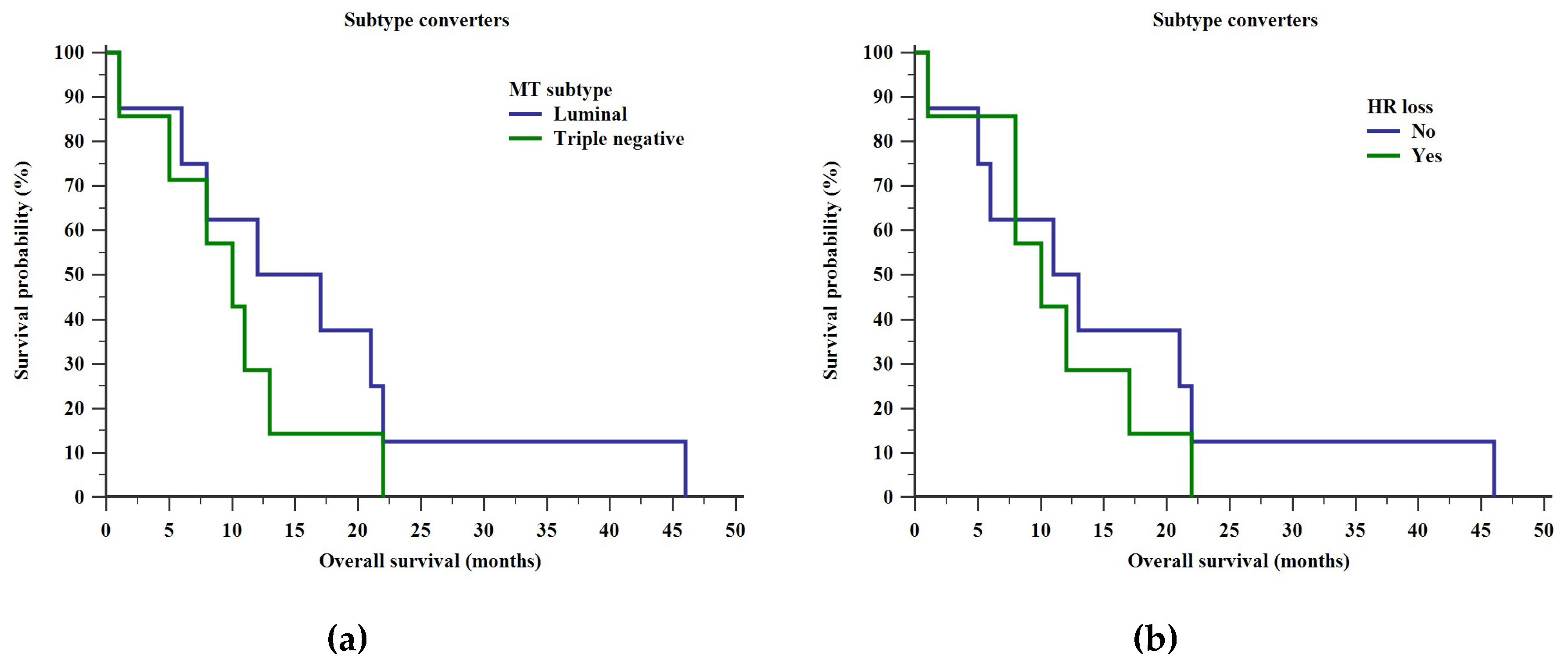
2. Results
3. Discussion
4. Materials and Methods
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Fitzmaurice, C.; Akinyemiju, T.F.; Al Lami, F.H.; Alam, T.; Alizadeh-Navaei, R.; Allen, C.; Alsharif, U.; Alvis-Guzman, N.; Amini, E.; Anderson, B.O.; et al. Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 29 cancer groups, 1990 to 2016 a systematic analysis for the global burden of disease study global burden of disease cancer collaboration. JAMA Oncol. 2018, 4, 1553–1568. [Google Scholar] [PubMed]
- Greenberg, P.A.; Hortobagyi, G.N.; Smith, T.L.; Ziegler, L.D.; Frye, D.K.; Buzdar, A.U. Long-term follow-up of patients with complete remission following combination chemotherapy for metastatic breast cancer. J. Clin. Oncol. 1996, 14, 2197–2205. [Google Scholar] [CrossRef]
- Chia, S.K.; Speers, C.H.; D’yachkova, Y.; Kang, A.; Malfair-Taylor, S.; Barnett, J.; Coldman, A.; Gelmon, K.A.; O’Reilly, S.E.; Olivotto, I.A. The impact of new chemotherapeutic and hormone agents on survival in a population-based cohort of women with metastatic breast cancer. Cancer 2007, 110, 973–979. [Google Scholar] [CrossRef]
- Güth, U.; Elfgen, C.; Montagna, G.; Schmid, S.M. Long-Term Survival and Cure in Distant Metastatic Breast Cancer. Oncology 2019, 97, 82–93. [Google Scholar] [CrossRef] [PubMed]
- Perou, C.M.; Sørlie, T.; Eisen, M.B.; van de Rijn, M.; Jeffrey, S.S.; Rees, C.A.; Pollack, J.R.; Ross, D.T.; Johnsen, H.; Akslen, L.A.; et al. Molecular portraits of human breast tumours. Nature 2000, 406, 747–752. [Google Scholar] [CrossRef] [PubMed]
- Loi, S.; Haibe-Kains, B.; Desmedt, C.; Lallemand, F.; Tutt, A.M.; Gillet, C.; Ellis, P.; Harris, A.; Bergh, J.; Foekens, J.A.; et al. Definition of clinically distinct molecular subtypes in estrogen receptor-positive breast carcinomas through genomic grade. J. Clin. Oncol. 2007, 25, 1239–1246. [Google Scholar] [CrossRef]
- Kimbung, S.; Kovács, A.; Danielsson, A.; Bendahl, P.O.; Lövgren, K.; Stolt, M.F.; Tobin, N.P.; Lindström, L.; Bergh, J.; Einbeigi, Z.; et al. Contrasting breast cancer molecular subtypes across serial tumor progression stages: Biological and prognostic implications. Oncotarget 2015, 6, 33306–33318. [Google Scholar] [CrossRef]
- Lin, N.U.; Vanderplas, A.; Hughes, M.E.; Theriault, R.L.; Edge, S.B.; Wong, Y.N.; Blayney, D.W.; Niland, J.C.; Winer, E.P.; Weeks, J.C. Clinicopathologic features, patterns of recurrence, and survival among women with triple-negative breast cancer in the National Comprehensive Cancer Network. Cancer 2012, 118, 5463–5472. [Google Scholar] [CrossRef]
- Zidan, J.; Dashkovsky, I.; Stayerman, C.; Basher, W.; Cozacov, C.; Hadary, A. Comparison of HER-2 overexpression in primary breast cancer and metastatic sites and its effect on biological targeting therapy of metastatic disease. Br. J. Cancer 2005, 93, 552–556. [Google Scholar] [CrossRef]
- Chen, S.; Huang, L.; Chen, C.M.; Shao, Z.M. Progesterone receptor loss identifies luminal-type local advanced breast cancer with poor survival in patients who fail to achieve a pathological complete response to neoadjuvant chemotherapy. Oncotarget 2015, 6, 18174–18182. [Google Scholar] [CrossRef][Green Version]
- Gong, Y.; Han, E.Y.; Guo, M.; Pusztai, L.; Sneige, N. Stability of estrogen receptor status in breast carcinoma. Cancer 2011, 117, 705–713. [Google Scholar] [CrossRef] [PubMed]
- Gong, Y.; Booser, D.J.; Sneige, N. Comparison of HER-2 status determined by fluorescence in situ hybridization in primary and metastatic breast carcinoma. Cancer 2005, 103, 1763–1769. [Google Scholar] [CrossRef] [PubMed]
- Liedtke, C.; Broglio, K.; Moulder, S.; Hsu, L.; Kau, S.W.; Symmans, W.F.; Albarracin, C.; Meric-Bernstam, F.; Woodward, W.; Theriault, R.L.; et al. Prognostic impact of discordance between triple-receptor measurements in primary and recurrent breast cancer. Ann. Oncol. 2009, 20, 1953–1958. [Google Scholar] [CrossRef] [PubMed]
- Carbognin, L.; Sperduti, I.; Ciccarese, M.; Fabi, A.; Petrucelli, L.; Vari, S.; Forcignanò, R.C.; Nortilli, R.; Vicentini, C.; Pilotto, S.; et al. Prognostic model for advanced breast carcinoma with luminal subtype and impact of hormonal maintenance: Implications for post-progression and conditional survival. Breast 2016, 29, 24–30. [Google Scholar] [CrossRef]
- Thill, M.; Jackisch, C.; Janni, W.; Müller, V.; Albert, U.-S.; Bauerfeind, I.; Blohmer, J.; Budach, W.; Dall, P.; Diel, I.; et al. AGO Recommendations for the Diagnosis and Treatment of Patients with Locally Advanced and Metastatic Breast Cancer: Update 2019. Breast Care 2019, 14, 247–255. [Google Scholar] [CrossRef]
- Emi, Y.; Kitamura, K.; Shikada, Y.; Kakeji, Y.; Takahashi, I.; Tsutsui, S. Metastatic breast cancer with HER2/neu-positive cells tends to have a morbid prognosis. Surgery 2002, 131, S217–S221. [Google Scholar] [CrossRef]
- Lindström, L.S.; Karlsson, E.; Wilking, U.M.; Johansson, U.; Hartman, J.; Lidbrink, E.K.; Hatschek, T.; Skoog, L.; Bergh, J. Clinically Used Breast Cancer Markers Such As Estrogen Receptor, Progesterone Receptor, and Human Epidermal Growth Factor Receptor 2 Are Unstable Throughout Tumor Progression. J. Clin. Oncol. 2012, 30, 2601–2608. [Google Scholar] [CrossRef]
- Hoefnagel, L.D.C.; Moelans, C.B.; Meijer, S.L.; Van Slooten, H.J.; Wesseling, P.; Wesseling, J.; Westenend, P.J.; Bart, J.; Seldenrijk, C.A.; Nagtegaal, I.D.; et al. Prognostic value of estrogen receptor α and progesterone receptor conversion in distant breast cancer metastases. Cancer 2012, 118, 4929–4935. [Google Scholar] [CrossRef]
- Stefanovic, S.; Deutsch, T.M.; Wirtz, R.; Hartkopf, A.; Sinn, P.; Schuetz, F.; Sohn, C.; Bohlmann, M.K.; Sütterlin, M.; Schneeweiss, A.; et al. Molecular Subtype Conversion between Primary and Metastatic Breast Cancer Corresponding to the Dynamics of Apoptotic and Intact Circulating Tumor Cells. Cancers 2019, 11, 342. [Google Scholar] [CrossRef]
- Cejalvo, J.M.; Pascual, T.; Fernández-Martínez, A.; Brasó-Maristany, F.; Gomis, R.R.; Perou, C.M.; Muñoz, M.; Prat, A. Clinical implications of the non-luminal intrinsic subtypes in hormone receptor-positive breast cancer. Cancer Treat. Rev. 2018, 67, 63–70. [Google Scholar] [CrossRef]
- Goldhirsch, A.; Wood, W.C.; Coates, A.S.; Gelber, R.D.; Thürlimann, B.; Senn, H.J. Strategies for subtypes-dealing with the diversity of breast cancer: Highlights of the St Gallen international expert consensus on the primary therapy of early breast cancer 2011. Ann. Oncol. 2011, 22, 1736–1747. [Google Scholar] [CrossRef] [PubMed]
- Razzak, A.R.; Lin, N.U.; Winer, E.P. Heterogeneity of breast cancer and implications of adjuvant chemotherapy. Breast Cancer 2008, 15, 31–34. [Google Scholar] [CrossRef] [PubMed]
- Hoefnagel, L.D.C.; van de Vijver, M.J.; van Slooten, H.J.; Wesseling, P.; Wesseling, J.; Westenend, P.J.; Bart, J.; Seldenrijk, C.A.; Nagtegaal, I.D.; Oudejans, J.; et al. Receptor conversion in distant breast cancer metastases. Breast Cancer Res. 2010, 12, R75. [Google Scholar] [CrossRef] [PubMed]
- Thompson, A.M.; Jordan, L.B.; Quinlan, P.; Anderson, E.; Skene, A.; Dewar, J.A.; Purdie, C.A.; Breast Recurrence in Tissues Study Group. Prospective comparison of switches in biomarker status between primary and recurrent breast cancer: The Breast Recurrence in Tissues Study (BRITS). Breast Cancer Res. 2010, 12, R92. [Google Scholar] [CrossRef]
- Cardoso, F.; van’t Veer, L.J.; Bogaerts, J.; Slaets, L.; Viale, G.; Delaloge, S.; Pierga, J.-Y.; Brain, E.; Causeret, S.; DeLorenzi, M.; et al. 70-Gene Signature as an Aid to Treatment Decisions in Early-Stage Breast Cancer. N. Engl. J. Med. 2016, 375, 717–729. [Google Scholar] [CrossRef]
- Chia, S.K.; Bramwell, V.H.; Tu, D.; Shepherd, L.E.; Jiang, S.; Vickery, T.; Mardis, E.; Leung, S.; Ung, K.; Pritchard, K.I.; et al. A 50-gene intrinsic subtype classifier for prognosis and prediction of benefit from adjuvant tamoxifen. Clin. Cancer Res. 2012, 18, 4465–4472. [Google Scholar] [CrossRef]
- Li, H.; Zhu, Y.; Burnside, E.S.; Drukker, K.; Hoadley, K.A.; Fan, C.; Conzen, S.D.; Whitman, G.J.; Sutton, E.J.; Net, J.M.; et al. MR imaging radiomics signatures for predicting the risk of breast cancer recurrence as given by research versions of MammaPrint, oncotype DX, and PAM50 gene assays. Radiology 2016, 281, 382–391. [Google Scholar] [CrossRef]
- Tong, Y.; Wu, J.; Huang, O.; He, J.; Zhu, L.; Chen, W.; Li, Y.; Chen, X.; Shen, K. 21-Gene Recurrence Score and Adjuvant Chemotherapy Decision for Breast Cancer Patients with Positive Lymph Nodes. Sci. Rep. 2019, 9, 1–10. [Google Scholar] [CrossRef]
- Riethdorf, S.; Müller, V.; Zhang, L.; Rau, T.; Loibl, S.; Komor, M.; Roller, M.; Huober, J.; Fehm, T.; Schrader, I.; et al. Detection and HER2 expression of circulating tumor cells: Prospective monitoring in breast cancer patients treated in the neoadjuvant GeparQuattro trial. Clin. Cancer Res. 2010, 16, 2634–2645. [Google Scholar] [CrossRef]
- Stefanovic, S.; Wirtz, R.; Deutsch, T.M.; Hartkopf, A.; Sinn, P.; Varga, Z.; Sobottka, B.; Sotiris, L.; Taran, F.A.; Domschke, C.; et al. Tumor biomarker conversion between primary and metastatic breast cancer: mRNA assessment and its concordance with immunohistochemistry. Oncotarget 2017, 8, 51416–51428. [Google Scholar] [CrossRef]
- Krawczyk, N.; Hartkopf, A.; Banys, M.; Meier-Stiegen, F.; Staebler, A.; Wallwiener, M.; Röhm, C.; Hoffmann, J.; Hahn, M.; Fehm, T. Prognostic relevance of induced and spontaneous apoptosis of disseminated tumor cells in primary breast cancer patients. BMC Cancer 2014, 14, 394. [Google Scholar] [CrossRef]
- Kallergi, G.; Konstantinidis, G.; Markomanolaki, H.; Papadaki, M.A.; Mavroudis, D.; Stournaras, C.; Georgoulias, V.; Agelaki, S. Apoptotic circulating tumor cells in early and metastatic breast cancer patients. Mol. Cancer Ther. 2013, 12, 1886–1895. [Google Scholar] [CrossRef] [PubMed]
- Wirtz, R.M.; Sihto, H.; Isola, J.; Heikkilä, P.; Kellokumpu-Lehtinen, P.-L.; Auvinen, P.; Turpeenniemi-Hujanen, T.; Jyrkkiö, S.; Lakis, S.; Schlombs, K.; et al. Biological subtyping of early breast cancer: A study comparing RT-qPCR with immunohistochemistry. Breast Cancer Res. Treat. 2016, 157, 437–446. [Google Scholar] [CrossRef] [PubMed]
- Loeian, M.S.; Aghaei, S.M.; Farhadi, F.; Rai, V.; Yang, H.W.; Johnson, M.D.; Aqil, F.; Mandadi, M.; Rai, S.N.; Panchapakesan, B. Liquid biopsy using the nanotube-CTC-chip: Capture of invasive CTCs with high purity using preferential adherence in breast cancer patients. Lab. Chip 2019, 19, 1899–1915. [Google Scholar] [CrossRef] [PubMed]

| Mean Age at PT Biopsy, Years, Mean ± SD | 52.3 ± 9.7 |
| Mean Age at MT Biopsy, Years, Mean ± SD | 58.1 ± 10.6 |
| Tumor Grade | Frequency (%) |
| G1 | 0 (0%) |
| G2 | 18 (53%) |
| G3 | 12 (35%) |
| GX | 4 (12%) |
| Primary Tumor Intrinsic Subtype | Frequency (%) |
| Luminal A | 13 (38.2%) |
| Luminal B | 13 (38.2%) |
| Triple-negative | 7 (20.6%) |
| NA | 1 (2.9%) |
| Metastatic Tumor Intrinsic Subtype | Frequency (%) |
| Luminal A | 5 (14.7%) |
| Luminal B | 14 (41.2%) |
| Triple-negative | 8 (23.5%) |
| NA | 7 (20.6%) |
| Subtype Dynamics | Frequency (%) |
| Subtype stable | 11 (32.4%) |
| Subtype conversion | 15 (44.1%) |
| NA | 8 (23.5%) |
| PFS, Months, Median (Range) | 6 (1–49) |
| OS, Months, Median (Range) | 17 (1–52) |
| CTC Counts | Subtype Stable | Subtype Converters | pbg | Total |
|---|---|---|---|---|
| i+aCTC1 | 10.65 (0–280) | 25 (3–350) | 0.11 | 20 (0–350) |
| i+aCTC2 | 3 (0–35) | 9 (0–1140) | 0.57 | 6 (0–1140) |
| pwg | 1 | 0.11 | / | 0.096 |
| ∆ i+aCTC | 0 (−280–19.7) | −5.5 (−263–1055) | 0.79 | −3 (−280–1055) |
| aCTC1 | 2.5 (0–200) | 4 (0–170) | 0.26 | 4.5 (0–200) |
| aCTC2 | 0 (0–23) | 1 (0–250) | 0.34 | 1 (0–250) |
| pwg | 1 | 0.73 | / | 0.8 |
| ∆ aCTC | 0 (−200–16) | −1 (−143–239) | 0.74 | 0 (−200–239) |
| iCTC1 | 8.15 (0–80) | 19 (0–180) | 0.12 | 15.5 (0–180) |
| iCTC2 | 1 (0–15) | 7 (0–890) | 0.38 | 3.5 (0–890) |
| pwg | 1 | 0.18 | / | 0.24 |
| ∆ iCTC | 0 (−80–3.7) | −7 (−120–816) | 0.74 | −4.5 (−120–816) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stefanovic, S.; Deutsch, T.M.; Wirtz, R.; Hartkopf, A.; Sinn, P.; Kohler, M.; Hofmann, J.; Bankovic, S.; Vassilev, K.; Sütterlin, M.; et al. Impact of mRNA-Assessed Molecular Subtype Conversion, Intact and Apoptotic Circulating Tumor Cells on Survival of Metastatic Breast Cancer Patients: Proof of Principle. Diagnostics 2020, 10, 369. https://doi.org/10.3390/diagnostics10060369
Stefanovic S, Deutsch TM, Wirtz R, Hartkopf A, Sinn P, Kohler M, Hofmann J, Bankovic S, Vassilev K, Sütterlin M, et al. Impact of mRNA-Assessed Molecular Subtype Conversion, Intact and Apoptotic Circulating Tumor Cells on Survival of Metastatic Breast Cancer Patients: Proof of Principle. Diagnostics. 2020; 10(6):369. https://doi.org/10.3390/diagnostics10060369
Chicago/Turabian StyleStefanovic, Stefan, Thomas M. Deutsch, Ralph Wirtz, Andreas Hartkopf, Peter Sinn, Maximilian Kohler, Jan Hofmann, Sanja Bankovic, Katja Vassilev, Marc Sütterlin, and et al. 2020. "Impact of mRNA-Assessed Molecular Subtype Conversion, Intact and Apoptotic Circulating Tumor Cells on Survival of Metastatic Breast Cancer Patients: Proof of Principle" Diagnostics 10, no. 6: 369. https://doi.org/10.3390/diagnostics10060369
APA StyleStefanovic, S., Deutsch, T. M., Wirtz, R., Hartkopf, A., Sinn, P., Kohler, M., Hofmann, J., Bankovic, S., Vassilev, K., Sütterlin, M., Schneeweiss, A., & Wallwiener, M. (2020). Impact of mRNA-Assessed Molecular Subtype Conversion, Intact and Apoptotic Circulating Tumor Cells on Survival of Metastatic Breast Cancer Patients: Proof of Principle. Diagnostics, 10(6), 369. https://doi.org/10.3390/diagnostics10060369






