Biochemical Biomarkers of Mucosal Healing for Inflammatory Bowel Disease in Adults
Abstract
1. Introduction
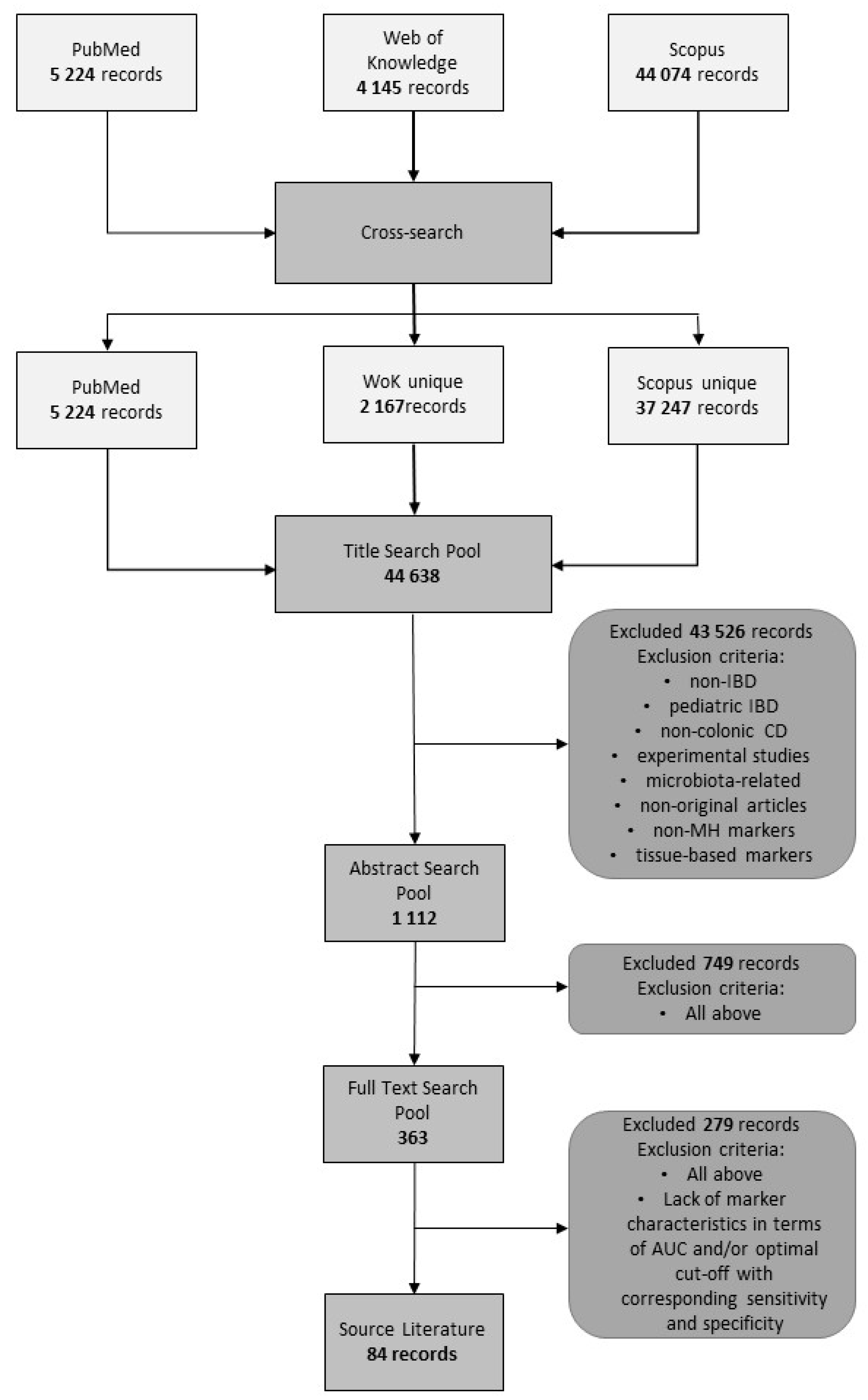
2. Methods
3. Results
3.1. Interpretative Synthesis of Data: Fecal Markers
3.1.1. Calprotectin
3.1.2. Fecal Calprotectin as a Marker of Mucosal Healing
3.1.3. Limitations of Fecal Calprotectin
3.1.4. Technical Considerations
3.1.5. Other Fecal Markers
3.2. Interpretative Synthesis of Data: Serum-Based Markers
3.2.1. C-Reactive Protein (CRP)
3.2.2. Other Acute Phase Reactants (APRs)
3.2.3. Cytokines, Their Receptors, and Growth Factors
3.2.4. Other Serum-Based Markers
3.3. Interpretative Synthesis of Data: Urine-Based Markers
4. Review Limitations
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Colombel, J.F.; Mahadevan, U. Inflammatory bowel disease 2017: Innovations and changing paradigms. Gastroenterology 2017, 152, 309–312. [Google Scholar] [CrossRef]
- deSouza, H.; Fiocchi, C. Network medicine: A mandatory next step for inflammatory bowel disease. Inflam. Bowel Dis. 2018, 4, 671–679. [Google Scholar] [CrossRef] [PubMed]
- Annese, V.; Daperno, M.; Rutter, M.D.; Amiot, A.; Bossuyt, P.; East, J.; Ferrante, M.; Götz, M.; Katsanos, K.H.; Kießlich, R.; et al. European evidence based consensus for endoscopy in inflammatory bowel disease. J. Crohn's Colitis 2013, 7, 982–1018. [Google Scholar] [CrossRef] [PubMed]
- Frøslie, K.F.; Jahnsen, J.; Moum, B.A.; Vatn, M.H.; IBSEN Group. Mucosal healing in inflammatory bowel disease: Results from a Norwegian population-based cohort. Gastroenterology 2007, 133, 412–422. [Google Scholar] [CrossRef]
- Ardizzone, S.; Cassinotti, A.; Duca, P.; Mazzali, C.; Penati, C.; Manes, G.; Marmo, R.; Massari, A.; Molteni, P.; Maconi, G.; et al. Mucosal healing predicts late outcomes after the first course of corticosteroids for newly diagnosed ulcerative colitis. Clin. Gastroenterol. Hepatol. 2011, 9, 483–489. [Google Scholar] [CrossRef] [PubMed]
- Colombel, J.F.; Rutgeerts, P.; Reinisch, W.; Esser, D.; Wang, Y.; Lang, Y.; Marano, C.W.; Strauss, R.; Oddens, B.J.; Feagan, B.G.; et al. Early mucosal healing with infliximab is associated with improved long-term clinical outcomes in ulcerative colitis. Gastroenterology 2011, 141, 1194–1201. [Google Scholar] [CrossRef] [PubMed]
- Rutter, M.; Saunders, B.; Wilkinson, K.; Rumbles, S.; Schofield, G.; Kamm, M.; Williams, C.; Price, A.; Talbot, I.; Forbes, A. Severity of inflammation is a risk factor for colorectal neoplasia in ulcerative colitis. Gastroenterology 2004, 126, 451–459. [Google Scholar] [CrossRef] [PubMed]
- Rutter, M.; Saunders, B.P.; Wilkinson, K.H.; Rumbles, S.; Schofield, G.; Kamm, M.A.; Williams, C.B.; Price, A.B.; Talbot, I.C.; Forbes, A. Cancer surveillance in longstanding ulcerative colitis: Endoscopic appearances help predict cancer risk. Gut 2004, 53, 1813–1816. [Google Scholar] [CrossRef]
- Peyrin-Biroulet, L.; Sandborn, W.; Sands, B.E.; Reinisch, W.; Bemelman, W.; Bryant, R.V.; D’Haens, G.; Dotan, I.; Dubinsky, M.; Feagan, B.; et al. Selecting therapeutic targets in inflammatory bowel disease (STRIDE): Determining therapeutic goals for treat-to-target. Am. J. Gastroenterol. 2015, 110, 1324–1338. [Google Scholar] [CrossRef]
- Shah, S.C.; Colombel, J.F.; Sands, B.E.; Narula, N. Systematic review with meta-analysis: Mucosal healing is associated with improved long-term outcomes in Crohn’s disease. Aliment. Pharmacol. Ther. 2016, 43, 317–333. [Google Scholar] [CrossRef]
- Reinink, A.R.; Lee, T.C.; Higgins, P.D. Endoscopic mucosal healing predicts favorable clinical outcomes in inflammatory bowel disease: A meta-analysis. Inflamm. Bowel Dis. 2016, 22, 1859–1869. [Google Scholar] [CrossRef]
- Hanauer, S.B.; Feagan, B.G.; Lichtenstein, G.R.; Mayer, L.F.; Schreiber, S.; Colombel, J.F.; Rachmilewitz, D.; Wolf, D.C.; Olson, A.; Bao, W.; et al. Maintenance infliximab for Crohn’s disease: The ACCENT I randomised trial. Lancet 2002, 359, 1541–1549. [Google Scholar] [CrossRef]
- Colombel, J.F.; Sandborn, W.J.; Reinisch, W.; Mantzaris, G.J.; Kornbluth, A.; Rachmilewitz, D.; Lichtiger, S.; D’Haens, G.; Diamond, R.H.; Broussard, D.L.; et al. Infliximab, azathioprine, or combination therapy for Crohn’s disease. N. Engl. J. Med. 2010, 362, 1383–1395. [Google Scholar] [CrossRef] [PubMed]
- Sanborn, W.; Colombel, J.F.; Rutgeerts, P.; Yang, M.; Lomax, K.; Pollack, P.; Thakkar, R.; Camez, A.; Chen, N.; Chao, J.; et al. Achievement of early deep remission predicts better long-term outcomes for adalimumab-treated patients with Crohn’s disease: Data from EXTEND. Am. J. Gastroenterol. 2010, 105, S434–S435. [Google Scholar]
- Neurath, M.F.; Travis, S.P.L. Mucosal healing in inflammatory bowel diseases: A systemic review. Gut 2012, 61, 1619–1635. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, K.W.; Tremaine, W.J.; Ilstrup, D.M. Coated oral 5-aminosalicylic acid therapy for mildly to moderately active ulcerative colitis. A randomized study. N. Engl. J. Med. 1987, 317, 1625–1629. [Google Scholar] [CrossRef] [PubMed]
- Rutgeerts, P.; Sandborn, W.J.; Feagan, B.G.; Reinisch, W.; Olson, A.; Johanns, J. Infliximab for induction and maintenance therapy for ulcerative colitis. N. Engl. J. Med. 2005, 353, 2462–2476. [Google Scholar] [CrossRef]
- Maaser, C.; Sturm, A.; Vavricka, S.R.; Kucharzik, T.; Fiorino, G.; Annese, V.; Calabrese, E.; Baumgart, D.C.; Bettenworth, D.; Borralho Nunes, P.; et al. ECCO-ESGAR guideline for diagnostic assessment in IBD part 1: Initial diagnosis, monitoring of known IBD, detection of complications. J. Crohn's Colitis 2019, 13, 144–164. [Google Scholar] [CrossRef]
- Sturm, A.; Maaser, C.; Calabrese, E.; Annese, V.; Fiorino, G.; Kucharzik, T.; Vavricka, S.R.; Verstockt, B.; van Rheenen, P.; Tolan, D.; et al. ECCO-ESGAR Guideline for Diagnostic Assessment in IBD Part 2: IBD scores and general principles and technical aspects. J. Crohn's Colitis 2019, 13, 273–284. [Google Scholar] [CrossRef]
- Matsuoka, K.; Kobayashi, T.; Ueno, F.; Matsui, T.; Hirai, F.; Inoue, N.; Kato, J.; Kobayashi, K.; Koganei, K.; Kunisaki, R.; et al. Evidence-based clinical practice guidelines for inflammatory bowel disease. J. Gastroenterol. 2018, 53, 305–353. [Google Scholar] [CrossRef]
- Travis, S.P.; Schnell, D.; Krzeski, P.; Abreu, M.T.; Altman, D.G.; Colombel, J.F.; Feagan, B.G.; Hanauer, S.B.; Lichtenstein, G.R.; Marteau, P.R.; et al. Reliability and initial validation of the Ulcerative Colitis Endoscopic Index of Severity. Gastroenterology 2013, 145, 987–995. [Google Scholar] [CrossRef] [PubMed]
- Samuel, S.; Bruining, D.H.; Loftus, E.V., Jr.; Thia, K.T.; Schroeder, K.W.; Tremaine, W.J.; Faubion, W.A.; Kane, S.V.; Pardi, D.S.; de Groen, P.C.; et al. Validation of the ulcerative colitis colonoscopic index of severity and its correlation with disease activity measures. Clin. Gastroenterol. Hepatol. 2013, 11, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Mary, J.Y.; Modigliani, R. Development and validation of an endoscopic index of the severity for Crohn’s disease: A prospective multicentre study. Gut 1989, 30, 983–989. [Google Scholar] [CrossRef] [PubMed]
- Daperno, M.; D’Haens, G.; Van Assche, G.; Baert, F.; Bulois, P.; Maunoury, V.; Sostegni, R.; Rocca, R.; Pera, A.; Gevers, A.; et al. Development and validation of a new, simplified endoscopic activity score for Crohn’s disease: The SES-CD. Gastrointest. Endosc. 2004, 60, 505–512. [Google Scholar] [CrossRef]
- Sipponen, T.; Nuutinen, H.; Turunen, U.; Färkkilä, M. Endoscopic evaluation of Crohn’s disease activity: Comparison of the CDEIS and the SES-CD. Inflamm. Bowel Dis. 2010, 16, 2131–2136. [Google Scholar] [CrossRef] [PubMed]
- af Björkesten, C.G.; Nieminen, U.; Turunen, U.; Arkkila, P.; Sipponen, T.; Färkkilä, M. Surrogate markers and clinical indices, alone or combined, as indicators for endoscopic remission in anti-TNF-treated luminal Crohn’s disease. Scand. J. Gastroenterol. 2012, 47, 528–537. [Google Scholar] [CrossRef]
- Ayling, R.M.; Kok, K. Fecal calprotectin. Adv. Clin. Chem. 2018, 87, 161–190. [Google Scholar] [CrossRef]
- D’Haens, G.; Ferrante, M.; Vermeire, S.; Baert, F.; Noman, M.; Moortgat, L.; Geens, P.; Iwens, D.; Aerden, I.; Van Assche, G.; et al. Fecal calprotectin is a surrogate marker for endoscopic lesions in inflammatory bowel disease. Inflamm. Bowel Dis. 2012, 18, 2218–2224. [Google Scholar] [CrossRef]
- Ricanek, P.; Brackmann, S.; Perminow, G.; Lyckander, L.G.; Sponheim, J.; Holme, O.; Høie, O.; Rydning, A.; Vatn, M.H.; IBSEN II Study Group. Evaluation of disease activity in IBD at the time of diagnosis by the use of clinical, biochemical, and fecal markers. Scand. J. Gastroenterol. 2011, 46, 1081–1091. [Google Scholar] [CrossRef]
- Schoepfer, A.M.; Beglinger, C.; Straumann, A.; Trummler, M.; Vavricka, S.R.; Bruegger, L.E.; Seibold, F. Fecal calprotectin correlates more closely with the Simple Endoscopic Score for Crohn’s Disease (SES-CD) than CRP, blood leukocytes, and the CDAI. Am. J. Gastroenterol. 2010, 105, 162–169. [Google Scholar] [CrossRef]
- Schoepfer, A.M.; Beglinger, C.; Straumann, A.; Trummler, M.; Renzulli, P.; Seibold, F. Ulcerative colitis: Correlation of the Rachmilewitz Endoscopic Activity Index with fecal calprotectin, clinical activity, C-reactive protein, and blood leukocytes. Inflamm. Bowel Dis. 2009, 15, 1851–1858. [Google Scholar] [CrossRef] [PubMed]
- Jusué, V.; Chaparro, M.; Gisbert, J.P. Accuracy of fecal calprotectin for the prediction of endoscopic activity in patients with inflammatory bowel disease. Dig. Liv. Dis. 2018, 50, 353–359. [Google Scholar] [CrossRef] [PubMed]
- Sipponen, T.; Björkesten, C.G.; Färkkilä, M.; Nuutinen, H.; Savilahti, E.; Kolho, K.L. Faecal calprotectin and lactoferrin are reliable surrogate markers of endoscopic response during Crohn’s disease treatment. Scand. J. Gastroenterol. 2010, 45, 325–331. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, S.; Dias de Castro, F.; Leite, S.; Moreir, M.H.; Cotter, J. Low fecal calprotectin predicts clinical remission in Crohn’s disease patients: The simple answer to a challenging question. Scand. J. Gastroenterol. 2019, 54, 49–54. [Google Scholar] [CrossRef]
- Shimoyama, T.; Yamamoto, T.; Umegae, S.; Shimoyama, M. Faecal calprotectin level for assessing endoscopic activity and predicting future clinical course in patients with moderately active ulcerative colitis undergoing granulomonocytapheresis: A prospective cohort study. BMC Gastroenterol. 2018, 18, 120. [Google Scholar] [CrossRef]
- De Vos, M.; Dewit, O.; D’Haens, G.; Baert, F.; Fontaine, F.; Vermeire, S.; Franchimont, D.; Moreels, T.; Staessen, D.; Terriere, L.; et al. Fast and sharp decrease in calprotectin predicts remission by infliximab in anti-TNF naïve patients with ulcerative colitis. J. Crohn's Colitis 2012, 6, 557–562. [Google Scholar] [CrossRef]
- Wei, S.C.; Tung, C.C.; Weng, M.T.; Wong, J.M. Experience of patients with inflammatory bowel disease in using a home fecal calprotectin test as an objective reported outcome for self-monitoring. Intest. Res. 2018, 16, 546–553. [Google Scholar] [CrossRef]
- Vinding, K.K.; Elsberg, H.; Thorkilgaard, T.; Belard, E.; Pedersen, N.; Elkjaer, M.; Marker, D.; Carlsen, K.; Burisch, J.; Munkholm, P. Fecal calprotectin measured by patients at home using smartphones—a new clinical tool in monitoring patients with inflammatory bowel disease. Inflamm. Bowel Dis. 2016, 22, 336–344. [Google Scholar] [CrossRef]
- Stawczyk-Eder, K.; Eder, P.; Lykowska-Szuber, L.; Krela-Kazmierczak, I.; Klimczak, K.; Szymczak, A.; Szachta, P.; Katulska, K.; Linke, K. Is faecal calprotectin equally useful in all Crohn’s disease locations? A prospective, comparative study. Arch. Med. Sci. 2015, 11, 353–361. [Google Scholar] [CrossRef]
- Gecse, K.B.; Brandse, J.F.; van Wilpe, S.; Löwenberg, M.; Ponsioen, C.; van den Brink, G.; D’Haens, G. Impact of disease location on fecal calprotectin levels in Crohn’s disease. Scand. J. Gastroenterol. 2015, 50, 841–847. [Google Scholar] [CrossRef]
- Goutorbe, F.; Goutte, M.; Minet-Quinard, R.; Boucher, A.-L.; Pereira, B.; Bommelaer, G.; Buissona, A. Endoscopic factors influencing fecal calprotectin value in Crohn’s disease. J. Crohn’s Colitis 2015, 9, 1113–1119. [Google Scholar] [CrossRef] [PubMed]
- Carlsen, K.; Buhl, L.; Elsberg, H.; Maagaard, L.; Thorkilgaard, T.; Wergeland, S.; Jakobsen, C.; Wewer, V.; Florholmen, J.; Goll, R.; et al. The sensitivity of fecal calprotectin in predicting deep remission in ulcerative colitis. Scand. J. Gastroenterol. 2018, 53, 825–830. [Google Scholar] [CrossRef]
- Jha, A.K.; Chaudhary, M.; Dayal, V.M.; Kumar, A.; Jha, S.K.; Jha, P.; Purkayastha, S.; Ranjan, R. Optimal cut-off value of fecal calprotectin for the evaluation of ulcerative colitis: An unsolved issue? JGH Open 2018, 2, 207–213. [Google Scholar] [CrossRef]
- Mak, W.Y.; Buisson, A.; Andersen, M.J.; Lei, D.; Pekow, J.; Cohen, R.D.; Kahn, S.A.; Pereira, B.; Rubin, D.T. Fecal calprotectin in assessing endoscopic and histological remission in patients with ulcerative colitis. Dig. Dis. Sci. 2018, 63, 1294–1301. [Google Scholar] [CrossRef] [PubMed]
- Mine, S.; Takesshima, F.; Akazawa, Y.; Matsushima, K.; Minami, H.; Yamaguchi, N.; Ohnita, K.; Isomoto, H.; Nakao, K. Correlation of fecal markers with magnifying endoscopic stratification in patients with ulcerative colitis who are in clinical remission. Digestion 2018, 97, 82–89. [Google Scholar] [CrossRef]
- Walsh, A.; Kormilitzin, A.; Hinds, C.; Sexton, V.; Brain, O.; Keshav, S.; Uhlig, H.; Geddes, J.; Goodwin, G.; Peters, M.; et al. Defining faecal calprotectin thresholds as a surrogate for endoscopic and histological disease activity in ulcerative colitis–a prospective analysis. J. Crohn’s Colitis 2018, 13, 424–430. [Google Scholar] [CrossRef]
- Chen, J.M.; Liu, T.; Gao, S.; Tong, X.D.; Deng, F.H.; Nie, B. Efficacy of noninvasive evaluations in monitoring inflammatory bowel disease activity: A prospective study in China. World J. Gastroenterol. 2017, 23, 8235–8247. [Google Scholar] [CrossRef]
- Kostas, A.; Siakavellas, S.I.; Kosmidis, C.; Takou, A.; Nikou, J.; Maropoulos, G.; Vlachogiannakos, J.; Papatheodoridis, G.V.; Papaconstantinou, I.; Bamias, G. Fecal calprotectin measurement is a marker of short term clinical outcome and presence of mucosal healing in patients with inflammatory bowel disease. World J. Gastroenterol. 2017, 23, 7387–7396. [Google Scholar] [CrossRef]
- Lee, S.H.; Kim, M.J.; Chang, K.; Song, E.M.; Hwang, S.W.; Park, S.H.; Yang, D.H.; Kim, K.J.; Byeon, J.S.; Myung, S.J.; et al. Fecal calprotectin predicts complete mucosal healing and better correlates with the ulcerative colitis endoscopic index of severity than with the Mayo endoscopic subscore in patients with ulcerative colitis. BMC Gastroenterol. 2017, 17, 110. [Google Scholar] [CrossRef]
- Patel, A.; Panchal, H.; Dubinsky, M.C. Fecal Calprotectin Levels Predict Histological Healing in Ulcerative Colitis. Inflamm. Bowel Dis. 2017, 23, 1600–1604. [Google Scholar] [CrossRef]
- Hiraoka, S.; Takashima, S.; Inokuchi, T.; Nakarai, A.; Takahara, M.; Harada, K.; Seki, Y.; Watanabe, K.; Kato, J.; Okada, H. The novel latex agglutination turbidimetric immunoassay system for simultaneous measurements of calprotectin and hemoglobin in feces. Intest. Res. 2018. [CrossRef] [PubMed]
- Zittan, E.; Kelly, O.B.; Kirsch, R.; Milgrom, R.; Burns, J.; Nguyen, G.C.; Croitoru, K.; Van Assche, G.; Silverberg, M.S.; Steinhart, A.H. Low fecal calprotectin correlates with histological remission and mucosal healing in ulcerative colitis and colonic Crohn’s disease. Inflamm. Bowel Dis. 2016, 22, 623–630. [Google Scholar] [CrossRef] [PubMed]
- Langhorst, J.; Boone, J.; Lauche, R.; Rueffer, A.; Dobos, G. Faecal lactoferrin, calprotectin, PMN-elastase, CRP, and white blood cell count as indicators for mucosal healing and clinical course of disease in patients with mild to moderate ulcerative colitis: Post hoc analysis of a prospective clinical trial. J. Crohn’s Colitis 2016, 10, 786–794. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.C.; Wong, J.M.; Tung, C.C.; Lin, C.P.; Chou, J.W.; Wang, H.Y.; Shieh, M.J.; Chang, C.H.; Liu, H.H.; Wei, S.C. Taiwan Society of Inflammatory Bowel Disease Multicenter Study. Fecal calprotectin correlated with endoscopic remission for Asian inflammatory bowel disease patients. World J. Gastroenterol. 2015, 21, 13566–13573. [Google Scholar] [CrossRef]
- Lobatón, T.; Rodríguez-Moranta, F.; Lopez, A.; Sánchez, E.; Rodríguez-Alonso, L.; Guardiola, J. A new rapid quantitative test for fecal calprotectin predicts endoscopic activity in ulcerative colitis. Inflam. Bowel Dis. 2013, 19, 1034–1042. [Google Scholar] [CrossRef]
- Nancey, S.; Boschetti, G.; Moussata, D.; Cotte, E.; Peyras, J.; Cuerq, C.; Haybrard, J.; Charlois, A.-L.; Mialon, A.; Chauvenet, M.; et al. Neopterin is a novel reliable fecal marker as accurate as calprotectin for predicting endoscopic disease activity in patients with inflammatory bowel diseases. Inflamm. Bowel Dis. 2013, 19, 1043–1052. [Google Scholar] [CrossRef]
- Önal, İ.K.; Beyazit, Y.; Şener, B.; Savuk, B.; Özer Etık, D.; Sayilir, A.; Öztaş, E.; Torun, S.; Özderın Özın, Y.; Tunç Demırel, B.; et al. The value of fecal calprotectin as a marker of intestinal inflammation in patients with ulcerative colitis. Turk. J. Gastroenterol. 2012, 23, 509–514. [Google Scholar] [CrossRef]
- Schoepfer, A.M.; Beglinger, C.; Straumann, A.; Safroneeva, E.; Romero, Y.; Armstrong, D.; Schmidt, C.; Trummler, M.; Pittet, V.; Vavricka, S.R. Fecal calprotectin more accurately reflects endoscopic activity of ulcerative colitis than the Lichtiger Index, C-reactive protein, platelets, hemoglobin, and blood leukocytes. Inflamm. Bowel Dis. 2013, 19, 332–341. [Google Scholar] [CrossRef]
- Falvey, J.D.; Hoskin, T.; Berrie Meijer, B.; Ashcroft, A.; Walmsley, R.; Day, A.S.; Gearry, R.B. Disease activity assessment in IBD: Clinical indices and biomarkers fail to predict endoscopic remission. Inflamm. Bowel Dis. 2015, 21, 824–831. [Google Scholar] [CrossRef]
- Kristensen, V.; Klepp, P.; Cvancarova, M.; Røseth, A.; Skar, V.; Moum, B. Prediction of endoscopic disease activity in ulcerative colitis by two different assays for fecal calprotectin. J. Crohn’s Colitis 2015, 9, 164–169. [Google Scholar] [CrossRef]
- Scaioli, E.; Scagliarinib, M.; Cardamonea, C.; Liverania, E.; Ugolinia, G.; Festia, D.; Bazzolia, F.; Belluzzia, A. Clinical application of faecal calprotectin in ulcerative colitis patients. Eur. J. Gastroenterol. Hepatol. 2015, 27, 1418–1424. [Google Scholar] [CrossRef]
- Takashima, S.; Kato, J.; Hiraoka, S.; Nak, A.; Takei, D.; Inokuchi, T.; Sugihara, Y.; Takahara, M.; Harada, K.; Okada, H.; et al. Evaluation of mucosal healing in ulcerative colitis by fecal calprotectin vs. fecal immunochemical test. Am. J. Gastroenterol. 2015, 110, 873–880. [Google Scholar] [CrossRef] [PubMed]
- Theede, K.; Holck, S.; Ibsen, P.; Ladelund, S.; Nordgaard-Lassen, I.; Nielsen, A.M. Level of fecal calprotectin correlates with endoscopic and histologic inflammation and identifies patients with mucosal healing of ulcerative colitis. Clin. Gastroenterol. Hepatol. 2015, 13, 1929–1936. [Google Scholar] [CrossRef] [PubMed]
- Voiosu, T.; Benguş, A.; Dinu, R.; Voiosu, A.M.; Bălănescu, P.; Băicuş, C.; Diculescu, M.; Radu Voiosu, R.; Mateescu, B. Rapid fecal calprotectin level assessment and the SIBDQ score can accurately detect active mucosal inflammation in IBD patients in clinical remission: A prospective study. J. Gastrointest. Liver Dis. 2014, 23, 273–278. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, S.; Takeuchi, Y.; Arai, K.; Fukuda, K.; Kuroki, Y.; Asonuma, K.; Takahashi, H.; Saruta, M.; Yoshida, H. Fecal calprotectin is a clinically relevant biomarker of mucosal healing in patients with quiescent ulcerative colitis. J. Gastroenterol. Hepatol. 2016, 31, 93–98. [Google Scholar] [CrossRef]
- Nakov, R.; Velikova, T.; Nakov, V.; Gerova, V.; Tankova, L. Serum trefoil factor 3 predicts disease activity in patients with ulcerative colitis. J. Gastrointest. Liver Dis. 2019, 28, 169–174. [Google Scholar] [CrossRef]
- Hart, L.; Chavannes, M.; Kherad, O.; Maedler, C.; Mourad, N.; Marcus, V.; Afif, W.; Bitton, A.; Lakatos, P.L.; Brassard, P.; et al. Faecal calprotectin predicts endoscopic and histological activity in clinically quiescent ulcerative colitis. J. Crohn’s Colitis 2020, 14, 46–52. [Google Scholar] [CrossRef]
- Karling, P.; Lundgren, D.; Eklöf, V.; Palmqvist, R.; Hultdin, J. Improved monitoring of inflammatory activity in patients with ulcerative colitis by combination of faecal tests for haemoglobin and calprotectin. Scand. J. Clin. Lab. Investig. 2019, 79, 341–346. [Google Scholar] [CrossRef]
- Lee, Y.W.; Lee, K.M.; Lee, J.M.; Chung, Y.Y.; Kim, D.B.; Kim, Y.J.; Chung, W.C.; Paik, C.N. The usefulness of fecal calprotectin in assessing inflammatory bowel disease activity. Korean J. Intern. Med. 2019, 34, 72–80. [Google Scholar] [CrossRef]
- Ryu, D.G.; Kim, H.W.; Park, S.B.; Kang, D.H.; Choi, C.W.; Kim, S.J.; Nam, H.S. Clinical implications of fecal calprotectin and fecal immunochemical test on mucosal status in patients with ulcerative colitis. Medicine 2019, 98, e17080. [Google Scholar] [CrossRef]
- Iwamoto, F.; Matsuoka, K.; Motobayashi, M.; Takenaka, K.; Kuno, T.; Tanaka, K.; Tsukui, Y.; Kobayashi, S.; Yoshida, T.; Fujii, T.; et al. Prediction of disease activity of Crohn’s disease through fecal calprotectin evaluated by balloon-assisted endoscopy. J. Gastroenterol. Hepatol. 2018, 33, 1984–1989. [Google Scholar] [CrossRef] [PubMed]
- Lopes, S.; Andrade, P.; Afonso, J.; Cunha, R.; Rodrigues-Pinto, E.; Ramos, I.; Macedo, G.; Magro, F. Monitoring Crohn’s disease activity: Endoscopy, fecal markers and computed tomography enterography. Ther. Adv. Gastroenterol. 2018, 11, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Vázquez-Morón, J.M.; Pallarés-Manrique, H.; Machancoses, F.H.; Ramos-Lora, M.; Ruiz-Frutos, C. Accurate cut-offs for predicting endoscopic activity and mucosal healing in Crohn’s disease with fecal calprotectin. Rev. Esp. Enferm. Dig. 2016, 109, 130–136. [Google Scholar] [CrossRef][Green Version]
- Arai, T.; Takeuchi, K.; Miyamura, M.; Ishikawa, R.; Yamada, A.; Katsumata, M.; Igarashi, Y.; Suzuki, Y. Level of fecal calprotectin correlates with severity of small bowel Crohn’s disease, measured by balloon-assisted enteroscopy and computed tomography enterography. Clin. Gastroenterol. Hepatol. 2017, 15, 56–62. [Google Scholar] [CrossRef] [PubMed]
- Inokuchi, T.; Kato, J.; Hiraoka, S.; Takashima, S.; Nakarai, A.; Takei, D.; Sugihara, Y.; Takahara, M.; Kawano, S.; Harada, K.; et al. Fecal immunochemical test versus fecal calprotectin for prediction of mucosal healing in Crohn’s disease. Inflamm. Bowel Dis. 2016, 22, 1078–1085. [Google Scholar] [CrossRef]
- Lobatón, T.; López-García, A.; Rodríguez-Moranta, F.; Ruiz, A.; Rodríguez, L.; Guardiola, J. A new rapid test for fecal calprotectin predicts endoscopic remission and postoperative recurrence in Crohn’s disease. J. Crohn’s Colitis 2013, 7, e641–e651. [Google Scholar] [CrossRef]
- Schaffer, T.; Schoepfer, A.M.; Seiboldb, F.; on behalf of the Swiss IBD Cohort Study Group. Serum ficolin-2 correlates worse than fecal calprotectin and CRP with endoscopic Crohn’ disease activity. J. Crohn’s Colitis 2014, 8, 1125–1132. [Google Scholar] [CrossRef]
- Karczewski, J.; Swora-Cwynar, E.; Rzymski, P.; Poniedziałek, B.; Adamski, Z. Selected biologic markers of inflammation and activity of Crohn’s disease. Autoimmunity 2015, 48, 318–327. [Google Scholar] [CrossRef]
- Ma, C.; Lumb, R.; Walker, E.V.; Foshaug, R.R.; Dang, T.T.; Verma, S.; Huang, V.W.; Kroeker, K.I.; M Wong, K.; Dieleman, L.A.; et al. Noninvasive fecal immunochemical testing and fecal calprotectin predict mucosal healing in inflammatory bowel disease: A prospective cohort study. Inflamm. Bowel Dis. 2017, 23, 1643–1649. [Google Scholar] [CrossRef]
- Theede, K.; Holck, S.; Ibsen, P.; Kallemose, T.; Nordgaard-Lassen, I.; Mertz Nielsen, A. Fecal calprotectin predicts relapse and histological mucosal healing in ulcerative colitis. Inflamm. Bowel Dis. 2016, 22, 1042–1048. [Google Scholar] [CrossRef]
- Bodelier, A.G.L.; Jonkers, D.; van den Heuvel, T.; de Boer1, E.; Hameeteman, W.; Masclee, A.; Pierik, M.J. High Percentage of IBD patients with indefinite fecal calprotectin levels: Additional value of a combination score. Dig. Dis. Sci. 2017, 62, 465–472. [Google Scholar] [CrossRef] [PubMed]
- Laserna-Mendieta, E.J.; Lucendo, A.J. Faecal calprotectin in inflammatory bowel diseases: A review focused on meta-analyses and routine usage limitations. Clin. Chem. Lab. Med. 2019, 57, 1295–1307. [Google Scholar] [CrossRef] [PubMed]
- Meucci, G.; D’Incà, R.; Maieron, R.; Orzes, N.; Vecchi, M.; Visentini, D.; Minoli, G.; Dal Pont, E.; Zilli, M.; Benedetti, E.; et al. Diagnostic value of faecal calprotectin in unselected outpatients referred for colonoscopy: A multicenter prospective study. Dig. Liver Dis. 2010, 42, 191–195. [Google Scholar] [CrossRef] [PubMed]
- Julsgaard, M.; Hvas, C.L.; Gearry, R.B.; Vestergaard, T.; Fallingborg, J.; Svenningsen, L.; Kjeldsen, J.; Sparrow, M.P.; Wildt, S.; Kelsen, J.; et al. Fecal calprotectin is not affected by pregnancy: Clinical implications for the management of pregnant patients with inflammatory bowel disease. Inflamm. Bowel Dis. 2017, 23, 1240–1246. [Google Scholar] [CrossRef] [PubMed]
- Lundgren, D.; Eklöf, V.; Palmqvist, R.; Hultdin, J.; Karling, P. Proton pump inhibitor use is associated with elevated faecal calprotectin levels. A cross-sectional study on subjects referred for colonoscopy. Scand. J. Gastroenterol. 2019, 4, 152–157. [Google Scholar] [CrossRef]
- Lasson, A.; Stotzer, P.O.; Öhman, L.; Isaksson, S.; Sapnara, M.; Strid, H. The intra-individual variability of faecal calprotectin: A prospective study in patients with active ulcerative colitis. J. Crohn’s Colitis 2015, 9, 26–32. [Google Scholar] [CrossRef]
- Toyonaga, T.; Kobayashi, T.; Nakano, M.; Saito, E.; Umeda, S.; Okabayashi, S.; Ozaki, R.; Hibi, T. Usefulness of fecal calprotectin for the early prediction of short-term outcomes of remission induction treatments in ulcerative colitis in comparison with two-item patient-reported outcome. PLoS ONE 2017, 12, e0185131. [Google Scholar] [CrossRef]
- Acevedoa, D.; Salvadora, M.P.; Girbesa, J.; Estana, N. Fecal Calprotectin: A comparison of two commercial enzymoimmunoassays and study of fecal extract stability at room temperature. J. Clin. Med. Res. 2018, 10, 396–404. [Google Scholar] [CrossRef][Green Version]
- Amcoff, K.; Stridsberg, M.; Lampinen, M.; Magnuson, A.; Carlson, M.; Halfvarson, J. Clinical implications of assay-specific differences in f-calprotectin when monitoring inflammatory bowel disease activity over time. Scand. J. Gastroenterol. 2016, 52, 344–350. [Google Scholar] [CrossRef]
- Moniuszko, A.; Głuszek, G.; Rydzewska, G. Rapid fecal calprotectin test for prediction of mucosal inflammation in ulcerative colitis and Crohn disease: A prospective cohort study. Pol. Arch. Intern. Med. 2017, 127, 312–318. [Google Scholar] [CrossRef]
- Annaházi, A.; Molnár, T.; Farkas, K.; Rosztóczy, A.; Izbéki, F.; Gecse, K.; Inczefi, O.; Nagy, F.; Földesi, I.; Szűcs, M.; et al. Fecal MMP-9: A new noninvasive differential diagnostic and activity marker in ulcerative colitis. Inflamm. Bowel Dis. 2013, 19, 316–320. [Google Scholar] [CrossRef] [PubMed]
- Buisson, A.; Vazeille, E.; Minet-Quinard, R.; Goutte, M.; Bouvier, D.; Goutorbe, F.; Pereira, B.; Barnich, N.; Bommelaer, G. Fecal matrix metalloprotease-9 and lipocalin-2 as biomarkers in detecting endoscopic activity in patients with inflammatory bowel diseases. J. Clin. Gastroenterol. 2018, 52, e53–e62. [Google Scholar] [CrossRef] [PubMed]
- Farkas, K.; Saródi, Z.; Bálint, A.; Földesi, I.; Tiszlavicz, L.; Szűcs, M.; Nyári, T.; Tajti, J.; Nagy, F.; Szepes, Z.; et al. The diagnostic value of a new fecal marker, matrix metalloprotease-9, in different types of inflammatory bowel diseases. J. Crohn’s Colitis 2015, 9, 231–237. [Google Scholar] [CrossRef] [PubMed]
- Sagawa, T.; Kakizaki, S.; Tomizawa, T.; Nakayama, T.; Tanaka, H.; Tojima, H.; Sato, K.; Kusano, M.; Okamura, S.; Yamada, M. Faecal lactoferrin is a useful biomarker for mucosal healing in patients with ulcerative colitis during granulocyte and monocyte adsorptive apheresis therapy. Colorectal Dis. 2016, 18, 696–702. [Google Scholar] [CrossRef]
- Magro, F.; Lopes, S.; Coelho, R.; Cotter, J.; Dias de Castro, F.; Tavares de Sousa, H.; Salgado, M.; Andrade, P.; Vieira, A.; Figueiredo, P.; et al. Portuguese IBD Study Group [GEDII. Accuracy of faecal calprotectin and neutrophil gelatinase B-associated lipocalin in evaluating subclinical inflammation in UlceRaTIVE colitis-the ACERTIVE study. J. Crohn’s Colitis 2017, 11, 435–444. [Google Scholar] [CrossRef][Green Version]
- Thorsvik, S.; Damås, J.K.; Granlund, A.V.; Flo, T.H.; Bergh, K.; Østvik, A.E.; Sandvik, A.K. Fecal neutrophil gelatinase-associated lipocalin as a biomarker for inflammatory bowel disease. J. Gastroenterol. Hepatol. 2017, 32, 128–135. [Google Scholar] [CrossRef]
- Kanmura, S.; Hamamoto, H.; Morinaga, Y.; Oda, K.; Fujita, T.; Arima, S.; Nasu, Y.; Sasaki, F.; Hashimoto, S.; Taguchi, H.; et al. Fecal human neutrophil peptide levels correlate with intestinal inflammation in ulcerative colitis. Digestion 2016, 93, 300–308. [Google Scholar] [CrossRef]
- Klimczak, K.; Lykowska-Szuber, L.; Eder, P.; Krela-Kazmierczak, I.; Stawczyk-Eder, K.; Szymczak, A.; Michalak, M.; Studniarek, A.; Linke, K. The diagnostic usefulness of fecal lactoferrin in the assessment of Crohn’s disease activity. Eur. J. Intern. Med. 2015, 26, 623–627. [Google Scholar] [CrossRef]
- Beyazit, Y.; Koklu, S.; Tas, A.; Purnak, T.; Sayilir, A.; Kurt, M.; Turhan, T.; Celik, T.; Suvak, B.; Torun, S.; et al. Serum adenosine deaminase activity as a predictor of disease severity in ulcerative colitis. J. Crohn’s Colitis 2012, 6, 102–107. [Google Scholar] [CrossRef]
- de Bruyn, M.; Arijs, I.; Wollants, W.J.; Machiels, K.; Van Steen, K.; Van Assche, G.; Ferrante, M.; Rutgeerts, P.; Vermeire, S.; Opdenakker, G. Neutrophil gelatinase B-associated lipocalin and matrix metalloproteinase-9 complex as a surrogate serum marker of mucosal healing in ulcerative colitis. Inflamm. Bowel Dis. 2014, 20, 1198–1207. [Google Scholar] [CrossRef]
- Dranga, M.; Mihai, C.; Drug, V.; Dumitrescu, G.; Prelipcean, C.C. A rapid test for assessing disease activity in ulcerative colitis. Turk. J. Gastroenterol. 2016, 27, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Hassan, E.A.; Ramadan, H.K.; Ismael, A.A.; Mohamed, K.F.; El-Attar, M.M.; Alhelali, I. Noninvasive biomarkers as surrogate predictors of clinical and endoscopic remission after infliximab induction in patients with refractory ulcerative colitis. Saud. J. Gastroenterol. 2017, 23, 238–245. [Google Scholar] [CrossRef]
- Jung, Y.S.; Park, J.J.; Kim, S.W.; Hong, S.P.; Kim, T.I.; Kim, W.H.; Cheon, J.H. Correlation between soluble triggering receptor expressed on myeloid cells-1 (sTREM-1) expression and endoscopic activity in inflammatory bowel diseases. Dig. Liver Dis. 2012, 44, 897–903. [Google Scholar] [CrossRef] [PubMed]
- Neubauer, K.; Matusiewicz, M.; Bednarz-Misa, I.; Gorska, S.; Gamian, A.; Krzystek-Korpacka, M. Diagnostic potential of systemic eosinophil-associated cytokines and growth factors in IBD. Gastroenterol. Res. Pract. 2018, 2018, 7265812. [Google Scholar] [CrossRef]
- Rosenberg, L.; Lawlor, G.O.; Zenlea, T.; Goldsmith, J.D.; Gifford, A.; Falchuk, K.R.; Wolf, J.L.; Cheifetz, A.S.; Robson, S.C.; Moss, A.C. Predictors of endoscopic inflammation in patients with ulcerative colitis in clinical remission. Inflamm. Bowel Dis. 2013, 19, 779–784. [Google Scholar] [CrossRef]
- Samant, H.; Desai, D.; Abraham, P.; Joshi, A.; Gupta, T.; Dherai, A.; Ashavaid, T. Fecal calprotectin and its correlation with inflammatory markers and endoscopy in patients from India with inflammatory bowel disease. Indian J. Gastroenterol. 2015, 34, 431–435. [Google Scholar] [CrossRef]
- Shinzaki, S.; Matsuoka, K.; Iijima, H.; Mizuno, S.; Serada, S.; Fujimoto, M.; Arai, N.; Koyama, N.; Morii, E.; Watanabe, M.; et al. Leucine-rich Alpha-2 glycoprotein is a serum biomarker of mucosal healing in ulcerative colitis. J. Crohn’s Colitis 2017, 11, 84–91. [Google Scholar] [CrossRef]
- Tran, D.H.; Wang, J.; Ha, C.; Ho, W.; Mattai, S.A.; Oikonomopoulos, A.; Weiss, G.; Lacey, P.; Cheng, M.; Shieh, C.; et al. Circulating cathelicidin levels correlate with mucosal disease activity in ulcerative colitis, risk of intestinal stricture in Crohn’s disease, and clinical prognosis in inflammatory bowel disease. BMC Gastroenterol. 2017, 17, 63. [Google Scholar] [CrossRef]
- Uchihara, M.; Kato, J.; Tsuda, S.; Yoshida, T.; Maekita, T.; Iguchi, M.; Kitano, M. Blood biomarkers reflect integration of severity and extent of endoscopic inflammation in ulcerative colitis. JGH Open 2017, 1, 98–104. [Google Scholar] [CrossRef]
- Yoon, J.Y.; Park, S.J.; Hong, S.P.; Kim, T.I.; Kim, W.H.; Cheon, J.H. Correlations of C-reactive protein levels and erythrocyte sedimentation rates with endoscopic activity indices in patients with ulcerative colitis. Dig. Dis. Sci. 2014, 59, 829–837. [Google Scholar] [CrossRef]
- de Bruyn, M.; Arijs, I.; De Hertogh, G.; Ferrante, M.; Van Assche, G.; Rutgeerts, P.; Vermeire, S.; Opdenakker, G. Serum neutrophil gelatinase B-associated lipocalin and matrix metalloproteinase-9 complex as a surrogate marker for mucosal healing in patients with Crohn’s disease. J. Crohn’s Colitis 2015, 9, 1079–1087. [Google Scholar] [CrossRef] [PubMed]
- Hosomi, S.; Yamagami, H.; Itani, S.; Yukawa, T.; Otani, K.; Nagami, Y.; Tanaka, F.; Taira, K.; Kamata, N.; Tanigawa, T.; et al. Sepsis Markers Soluble IL-2 Receptor and soluble CD14 subtype as potential biomarkers for complete mucosal healing in patients with inflammatory bowel disease. J. Crohn’s Colitis 2018, 12, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Ishihara, S.; Tada, Y.; Kawashima, K.; Kataoka, M.; Sonoyama, H.; Yamashita, N.; Oka, A.; Kusunoki, R.; Fukuba, N.; Mishima, Y.; et al. Serum amyloid A level correlated with endoscopic findings in patients with Crohn’s disease-Possible biomarker for evaluating mucosal healing. Dig. Liver Dis. 2018, 50, 553–558. [Google Scholar] [CrossRef] [PubMed]
- Miranda-García, P.; Chaparro, M.; Gisbert, J.P. Correlation between serological biomarkers and endoscopic activity in patients with inflammatory bowel disease. Gastroenterol. Hepatol. 2016, 39, 508–515. [Google Scholar] [CrossRef]
- Yarur, A.J.; Quintero, M.A.; Jain, A.; Czul, F.; Barkin, J.S.; Abreu, M.T. Serum Amyloid A as a surrogate marker for mucosal and histologic inflammation in patients with Crohn’s disease. Inflamm. Bowel Dis. 2017, 23, 158–164. [Google Scholar] [CrossRef]
- Mańkowska-Wierzbicka, D.; Swora-Cwynar, E.; Poniedziałek, B.; Adamski, Z.; Dobrowolska, A.; Karczewski, J. Usefulness of selected laboratory markers in ulcerative colitis. Eur. Cytokine Netw. 2015, 26, 26–37. [Google Scholar] [CrossRef]
- Moein, S.; Qujeq, D.; Vaghari Tabari, M.; Kashifard, M.; Hajian-Tilaki, K. Diagnostic accuracy of fecal calprotectin in assessing the severity of inflammatory bowel disease: From laboratory to clinic. Casp. J. Intern. Med. 2017, 8, 178–182. [Google Scholar] [CrossRef]
- Meuwis, M.A.; Vernier-Massouille, G.; Grimaud, J.C.; Bouhnik, Y.; Laharie, D.; Piver, E.; Seidel, L.; Colombel, J.F.; Louis, E.; GETAID (Groupe d’Étude Thérapeutique Des Affections Inflammatoires Digestives). Serum calprotectin as a biomarker for Crohn’s disease. J. Crohn’s Colitis 2013, 7, e678–e683. [Google Scholar] [CrossRef]
- Matusiewicz, M.; Neubauer, K.; Bednarz-Misa, I.; Gorska, S.; Krzystek-Korpacka, M. Systemic interleukin-9 in inflammatory bowel disease: Association with mucosal healing in ulcerative colitis. World J. Gastroenterol. 2017, 23, 4039–4046. [Google Scholar] [CrossRef]
- Wu, H.M.; Wei, J.; Li, J.; Wang, K.; Ye, L.; Qi, Y.; Yuan, B.S.; Yang, Y.L.; Zhao, L.; Yang, Z.; et al. Serum procalcitonin as a potential early predictor of short-term outcomes in acute severe ulcerative colitis. Dig. Dis. Sci. 2019, 64, 3263–3273. [Google Scholar] [CrossRef]
- Koido, S.; Ohkusa, T.; Takakura, K.; Odahara, S.; Tsukinaga, S.; Yukawa, T.; Mitobe, J.; Kajihara, M.; Uchiyama, K.; Arakawa, H.; et al. Clinical significance of serum procalcitonin in patients with ulcerative colitis. World J. Gastroenterol. 2013, 19, 8335–8341. [Google Scholar] [CrossRef] [PubMed]
- Oussalah, A.; Laurent, V.; Bruot, O.; Guéant, J.L.; Régent, D.; Bigard, M.A.; Peyrin-Biroulet, L. Additional benefit of procalcitonin to C-reactive protein to assess disease activity and severity in Crohn’s disease. Aliment. Pharmacol. Ther. 2010, 32, 1135–1144. [Google Scholar] [CrossRef] [PubMed]
- Zezos, P.; Papaioannou, G.; Nikolaidis, N.; Patsiaoura, K.; Vassiliadis, T.; Mpoumponaris, A.; Giouleme, O.; Evgenidis, N. Elevated markers of thrombin generation and fibrinolysis in patients with active and quiescent ulcerative colitis. Med. Sci. Monit. 2009, 15, CR563–CR572. [Google Scholar] [PubMed]
- Eder, P.; Stawczyk-Eder, K.; Korybalska, K.; Czepulis, N.; Luczak, J.; Lykowska-Szuber, L.; Krela-Kazmierczak, I.; Linke, K.; Witowski, J. Trefoil factor-3 is not a useful marker of mucosal healing in Crohn’s disease treated with anti-TNF-α antibodies. World J. Gastroenterol. 2017, 23, 135–140. [Google Scholar] [CrossRef] [PubMed]
- Matusiewicz, M.; Neubauer, K.; Lewandowska, P.; Gamian, A.; Krzystek-Korpacka, M. Reduced transferrin levels in active inflammatory bowel disease. Biomed. Res. Int. 2017, 2017, 9541370. [Google Scholar] [CrossRef] [PubMed]
- Algaba, A.; Linares, P.M.; Fernández-Contreras, M.E.; Ordoñez, A.; Trápaga, J.; Guerra, I.; Chaparro, M.; de la Poza, G.; Gisbert, J.P.; Bermejo, F. Relationship between levels of angiogenic and lymphangiogenic factors and the endoscopic, histological and clinical activity, and acute-phase reactants in patients with inflammatory bowel disease. J. Crohn’s Colitis 2013, 7, e569–e579. [Google Scholar] [CrossRef]
- Boga, S.; Alkim, H.; Koksal, A.R.; Ozagari, A.A.; Bayram, M.; Tekin Neijmann, S.; Sen, I.; Alkim, C. Serum ST2 in inflammatory bowel disease: A potential biomarker for disease activity. J. Investig. Med. 2016, 64, 1016–1024. [Google Scholar] [CrossRef]
- Díaz-Jiménez, D.; Núñez, L.E.; Beltrán, C.J.; Candia, E.; Suazo, C.; Alvarez-Lobos, M.; González, M.J.; Hermoso, M.A.; Quera, R. Soluble ST2: A new and promising activity marker in ulcerative colitis. World J. Gastroenterol. 2011, 17, 2181–2190. [Google Scholar] [CrossRef]
- Díaz-Jiménez, D.; De la Fuente, M.; Dubois-Camacho, K.; Landskron, G.; Fuentes, J.; Pérez, T.; González, M.J.; Simian, D.; Hermoso, M.A.; Quera, R. Soluble ST2 is a sensitive clinical marker of ulcerative colitis evolution. BMC Gastroenterol. 2016, 16, 103. [Google Scholar] [CrossRef]
- Budzynska, A.; Gawron-Kiszka, M.; Nowakowska-Dulawa, E.; Spiewak, J.; Lesinska, M.; Kukla, M.; Waluga, M.; Hartleb, M. Serum neutrophil gelatinase-associated lipocalin (NGAL) correlates with clinical and endoscopic activity in ulcerative colitis but fails to predict activity in Crohn’s disease. J. Physiol. Pharmacol. 2017, 68, 859–865. [Google Scholar]
- Neubauer, K.; Bednarz-Misa, I.; Walecka-Zacharska, E.; Wierzbicki, J.; Agrawal, A.; Gamian, A.; Krzystek-Korpacka, M. Oversecretion and overexpression of nicotinamide phosphoribosyltransferase/Pre-B Colony-Enhancing Factor/Visfatin in inflammatory bowel disease reflects the disease activity, severity of inflammatory response and hypoxia. Int. J. Mol. Sci. 2019, 20, 166. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Perálvarez, M.L.; García-Sánchez, V.; Villar-Pastor, C.M.; González, R.; Iglesias-Flores, E.; Muntane, J.; Gómez-Camacho, F. Role of serum cytokine profile in ulcerative colitis assessment. Inflamm. Bowel Dis. 2012, 18, 1864–1871. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, S.; Kedia, S.; Kumar, S.; Pratap Mouli, V.; Dhingra, R.; Sachdev, V.; Tiwari, V.; Kurrey, L.; Pradhan, R.; Ahuja, V. Serum human trefoil factor 3 is a biomarker for mucosal healing in ulcerative colitis patients with minimal disease activity. J. Crohn’s Colitis 2015, 9, 575–579. [Google Scholar] [CrossRef] [PubMed]
- Trejo-Vazquez, F.; Garza-Veloz, I.; Villela-Ramirez, G.A.; Ortiz-Castro, Y.; Mauricio-Saucedo, P.; Cardenas-Vargas, E.; Diaz-Baez, M.; Cid-Baez, M.A.; Castañeda-Miranda, R.; Ortiz-Rodriguez, J.M.; et al. Positive association between leptin serum levels and disease activity on endoscopy in inflammatory bowel disease: A case-control study. Exp. Ther. Med. 2018, 15, 3336–3344. [Google Scholar] [CrossRef]
- Markanday, A. Acute Phase Reactants in Infections: Evidence-based review and a guide for clinicians. Open Forum Infect. Dis. 2015, 2, ofv098. [Google Scholar] [CrossRef]
- Soufli, I.; Toumi, R.; Touil-Boukoffa, C. Overview of cytokines and nitric oxide involvement in immuno-pathogenesis of inflammatory bowel diseases. World J. Gastrointest. Pharmacol. Ther. 2016, 7, 353–360. [Google Scholar] [CrossRef]
- Ljuca, F.; Gegic, A.; Salkic, N.N.; Pavlovic-Calic, N. Circulating cytokines reflect mucosal inflammatory status in patients with Crohn’s disease. Dig. Dis. Sci. 2010, 55, 2316–2326. [Google Scholar] [CrossRef]
- Feng, T.; Chen, B.; Li, L.; Huang, S.; Ben-Horin, S.; Qiu, Y.; Feng, R.; Li, M.; Mao, R.; He, Y.; et al. Serum interleukin 9 levels predict disease severity and the clinical efficacy of infliximab in patients with Crohn’s disease. Inflamm. Bowel Dis. 2017, 23, 1817–1824. [Google Scholar] [CrossRef]
- Linares, P.M.; Algaba, A.; Urzainqui, A.; Guijarro-Rojas, M.; González-Tajuelo, R.; Garrido, J.; Chaparro, M.; Gisbert, J.P.; Bermejo, F.; Guerra, I.; et al. Ratio of circulating estrogen receptors beta and alpha (ERβ/ERα) indicates endoscopic activity in patients with Crohn’s disease. Dig. Dis. Sci. 2017, 62, 2744–2754. [Google Scholar] [CrossRef]
- Yarur, A.J.; Jain, A.; Quintero, M.A.; Czul, F.; Deshpande, A.R.; Kerman, D.H.; Abreu, M.T. Inflammatory cytokine profile in Crohn’s disease nonresponders to optimal antitumor necrosis factor therapy. J. Clin. Gastroenterol. 2019, 53, 210–215. [Google Scholar] [CrossRef]
- Nakov, R.; Velikova, T.; Nakov, V.; Ianiro, G.; Gerova, V.; Tankova, L. Serum trefoil factor 3 predicts disease activity in patients with ulcerative colitis. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 788–794. [Google Scholar] [CrossRef]
- Arai, Y.; Arihiro, S.; Matsuura, T.; Kato, T.; Matsuoka, M.; Saruta, M.; Mitsunaga, M.; Matsuura, M.; Fujiwara, M.; Okayasu, I.; et al. Prostaglandin E-major urinary metabolite as a reliable surrogate marker for mucosal inflammation in ulcerative colitis. Inflamm Bowel Dis 2014, 20, 1208–1216. [Google Scholar] [CrossRef] [PubMed]
- Ng, S.C.; Shi, H.Y.; Hamidi, N.; Tang, W.; Benchimol, E.I.; Panaccione, R.; Ghosh, S.; Wu, J.C.Y.; Chan, F.K.L.; Sung, J.J.Y.; et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: A systematic review of population-based studies. Lancet 2018, 390, 2769–2778. [Google Scholar] [CrossRef]
- Boyapati, R.K.; Kalla, R.; Satsangi, J.; Ho, G.T. Biomarkers in Search of Precision Medicine in IBD. Am. J. Gastroenterol. 2016, 111, 1682–1690. [Google Scholar] [CrossRef] [PubMed]
- Torres, J.; Petralia, F.; Sato, T.; Wang, P.; Telesco, S.E.; Choung, R.S.; Strauss, R.; Li, X.-J.; Laird, R.M.; Gutierrez, R.L.; et al. Serum biomarkers identify patients who will develop inflammatory bowel diseases up to 5 y before diagnosis. Gastroenterology 2020. [Google Scholar] [CrossRef]
| Endoscopic Activity Indices | |||
|---|---|---|---|
| Parameter | MES [16] | UCEIS [21] | UCCIS [22] |
| Erythema | + | ||
| Granularity | + | ||
| Vascular pattern | + | + | + |
| Friability | + | + | + |
| Erosions | + | + | + |
| Ulceration | + | + | + |
| Exudate | |||
| MH definition | 0–1 | nd | nd |
| Validation | + | + | |
| Authors | N MH/Total | MH Definition | Marker Performance | Assay * | ||
|---|---|---|---|---|---|---|
| AUC | Cutoff (µg/g) | Sens. and Spec. | Type, Manufacturer | |||
| Carlsen et al. [42] | 68/106 | MES = 0 and GS ≤ 1 | 0.87 | ≤25 | 58% and 90% | ELISA, Calpro Ltd. |
| Jha et al. [43] | 5/81 | MES ≤ 1 | 0.94 | 158 | 90% and 85% | ELiA, Phadia 100 Calprotectin |
| Jusué et al. [32] | 30/48 | MES = 0 | 0.91 0.92 | 501 1022 | 79% and 85%1 79% and 85%2 | rapid kit, Quantum Blue®, Bühlmann 1low-range test; 2high-range test |
| Mak et al. [44] | 23/61 | MES ≤ 1 | 0.78 | <250 | 77% and 67% | ELISA, Genova Diagnostics |
| Mine et al. [45] | 45/60 | MES = 0 | 0.77 | 201 | 71% and 78% | EliA, Immunodiahnostik AG |
| Walsh et al. [46] | 21/66 | UCEIS ≤ 1 | 0.92 | 187 | 100% and 67% | IBDoc® FCal test, Bühlmann |
| Chen et al. [47] | 12/44 | MES ≤ 2 | 0.96 | ≤250 | 85% and 100% | ELISA, Bühlmann Calprotectin |
| Kostas et al. [48] | 39/149 (mixed UC and CD) | MES = 0 ML absence (CD) | 0.96 | 174 | 92% and 87% | ELISA, EK CAL, Bühlmann |
| Lee et al. [49] | na/181 | MES = 0 UCEIS = 0 | 0.88 | 187 | 86% and 89% | rapid test, Quantum Blue®, Bühlmann |
| Patel et al. [50] | 31/60 | MES ≤ 1 | 0.92 | 60 | 86% and 87% | EliA, na |
| Hiraoka et al. [51] | 75/152 | MES = 0 | 0.81 0.822 | <1841 <2242 | 78% and 69%1 79% and 78%2 | 1ELISA, Phical Calprotectin; 2LATIA |
| Zittan et al. [52] | 44/58 (mixed UC and CD) | MES = 0 SES-CD ≤ 3 | 0.91 | <100 | 71% and 91% | Buhlmann Quantum Blue Calprotectin High Range Immunoassay |
| Langhorst et al. [53] | 72/179 colonoscopies in 91patients | RI ≤ 1 | 0.7 | 13.9 | 11% and 99% | IDK® Calprotectin ELISA |
| Lin et al. [54] | na/52 | UCEIS < 3 | 0.97 | 918 | 88% and 75% | ELISA, Quantum Blue Calprotectin High Range Rapid Test |
| Lobatón et al. [55] | 35/146 colonoscopies in 123 patients | MES = 0 | 0.921 0.862 | 160 | 67% and 85%1 65% and 84%2 | 1Bühlmann ELISA 2QPOCT- Quantum Blue |
| Nancey et al. [56] | 20/55 | RI ≤ 2 | 0.96 | 250 | 87% and 91% | ELISA, Bühlmann |
| Önal et al. [57] | 30/60 | RI < 4 | 0.81 | 99.5 mg/L | 77% and 79% | ELISA Phi Cal |
| Schoepfer et al. [58] | 54/228 | mBS ≤ 1 | 0.94 | 57 | 90% and 91% | ELISA Phi Cal |
| Falvey et al. [59] | na/65 | mBS = 0 | 0.81 | 125 | 74% and 80% | ELISA CalPro |
| Kristensen et al. [60] | 18/62 | MES = 0 | 0.88 | 61 | 84% and 83% | Calpro ELISA |
| Kristensen et al. [60] | 18/62 | MES = 0 | 0.93 | 96 | 91% and 83% | ELISA, Bühlmann |
| Scaioli et al. [61] | 45/121 | MES = 0 | 0.98 | 110 | 98% and 90% | ELISA, Calprest |
| Takashima et al. [62] | 77/105 colonoscopies in 92 patients | MES ≤ 1 | 0.82 | 200 | 77% and 72% | ELISA PhiCal |
| Theede et al. [63] | na/120 | MES = 01 UCEIS = 02 | 0.88 | 192 | 75% and 88%1 79% and 87%2 | ELISA, Bülhmann |
| Voiosu et al. [64] | 16/48 (mixed UC and CD) | MES = 0 SES-CD ≤ 3 | 0.77 | 30 | 94% and 50% | Buhlmann Quantum Blue Reader® |
| Yamaguchi et al. [65] | 94/105 (CR) | MES = 01 MES ≤ 12 | 0.641 0.872 | 1941 2002 | 71% and 53%1 67% and 91%2 | EliA Calprotectin 2, Thermo Fisher Scientific |
| Nakov et al. [66] | 50/116 | UCEIS = 0 and MES = 0 | 0.988 | 99 | 97% and 98% | Quantum Blue® Calprotectin, Bühlmann Laboratories AG |
| Hart et al. [67] | 159/185 | MES ≤ 1 | 0.722 | 170 | 65% and 69% | ELISA, Buhlmann |
| Karling et al. [68] | 34/88 | MES = 0 | 0.707 | 63 | 67% and 68% | CALPRO, Calprotectin ELISA Test |
| Yang Woon et al. [69] | 7/29 | MES = 0 | 0.88 | 201 | 82% and 100% | ELISA, Bühlmann Laboratories AG |
| Ryu et al. [70] | 51/174 | MES = 0 | 0.863 | 170 | 78.4% and 74.8% | ELISA, Thermo Fisher Scientific |
| Ryu et al. [70] | 59/174 | UCEIS ≤ 1 | 0.847 | 170 | 74.6% and 76.5% | ELISA, Thermo Fisher Scientific |
| Authors | N MH/Total | MH Definition | Marker Performance | Assay * | ||
|---|---|---|---|---|---|---|
| AUC | Cutoff (µg/g) | Sens. and Spec. | Type, Manufacturer | |||
| Iwamoto et al. [71] | 39/69 | eSES-CD = 0 | 0.91 | 92 | 94% and 88% | EliA, Calprotectin 2 |
| Lopes et al. [72] | 19/29 | SES-CD = 0 | 0.88 | 100 | 92% and 65% | EliA, Calprotectin |
| Chen et al. [47] | 56/92 | SES-CD | 0.91 | 250 | 93% and 70% | ELISA, Bühlmann Calprotectin |
| Vazquez-Maron et al. [73] | 22/71 | SES-CD ≤ 2 | na | 71 | 96% and 52% | ELISA, Calprest® |
| Arai et al. [74] | 123 colonoscopies in 89 patients | SES-CD = 0 | 0.81 | 215 | 83% and 71% | ELISA, PhiCal Calprotectin |
| Inokuchi et al. [75] | 23/71 | SES-CD = 0 | 0.82 | 180 | 87% and 71% | ELISA, Phical Calprotectin |
| Jusué et al. [32] | 24/52 | SES-CD = 0 | 0.71 0.72 | 541 1222 | 63% and 71%1 75% and 71%2 | Rapid Kit, Quantum Blue® Bühlmann 1low-range test; 2high-range test |
| Lin et al. [54] | na/36 | CDEIS < 6 | 0.74 | 918 | 50% and 100% | ELISA, Quantum Blue Calprotectin High Range Rapid Test |
| Lobatón et al. [76] | 40/115 | CDEIS ≤ 3 | 0.941 0.932 | 2741 2722 | 76% and 97% | 1Bühlmann ELISA, 2QPOCT-Quantum Blue |
| Nancey et al. [56] | 40/78 | SES-CD ≤ 2 | 0.77 | 250 | 78% and 71% | ELISA, Bühlmann |
| Schaffer et al. [77] | 51/136 | SES-CD ≤ 3 | 0.83 | 250 | 76% and 75% | ELISA assay RIDASCREEN® CALPROTECTIN, R-Biopharm AG |
| Falvey et al. [59] | na/108 | SES-CD ≤ 2 | 0.74 | 125 | 71% and 71% | ELISA CalPro |
| Karczewski et al. [78] | 5/55 | CDEIS < 3 | na | 76 | 96% and 80% | CalproLabTM ELISA kit |
| Björkesten et al. [26] | 23/126 colonoscopies in 64 patients | SES-CD ≤ 2 | 0.85 | 100 | 84% and 74% | ELISA, PhiCal |
| Marker | N MH/Total | MH Definition | ↑/↓ in Active Disease | Correlation with Endoscopic Score | Marker Performance | Authors | ||
|---|---|---|---|---|---|---|---|---|
| AUC | Cutoff | Sens. and Spec. | ||||||
| MMP-9 | na/47 | MES < 1 | ↑ | r = 0.653, p < 0.002 | 0.9 | 2.38 ng/mL | 97% and 83% | Annahazi et al. [91] |
| MMP-9 | 9/32 | MES ≤ 1 | ↑ | r = 0.58, p < 0.001 | 0.87 | 900 ng/g | 80% and 91% | Buisson et al. [92] |
| MMP-9 | na/54 | MES ≤ 1 | ↑ | r = 0.381, p = 0.021 | 0.81 | 0.20 ng/mL | 75% and 96% | Farkas et al. [93] |
| Lactoferrin | 72/179 colonoscopies in 91patients | RI ≤ 1 | ↑ | r = 0.4, p < 0.001 | 0.73 | 11.9 μg/g | 75% and 63% | Langhorst et al. [53] |
| Lactoferrin | 18/60 | UCEIS = 0 | ↑ | r = 0.56, p < 0.0001 | 0.71 | 78.3 ng/mL | 57% and 88% | Mine et al. [45] |
| Lactoferrin | na/20 | MES = 0 | ↑ | r = 0.792, p < 0.01 | 0.88 | 288.8 ng/mL | 69% and 100% | Sagawa et al. [94] |
| Lipocalin-2 | 9/32 | MES ≤ 1 | ↑ | ns | 0.68 | 6700 ng/g | 80% and 82% | Buisson et al. [92] |
| Lipocalin-2 | 265/370 | MES = 0 | ↑ | na | 0.65 | 7 μg/g1 10 μg/g2 | 40% and 77%1 62% and 62%2 | Magro et al. [95] |
| Lipocalin-2 | UC: 15/431 | MES ≤ 11 | ↑ | r = 0.82, p < 0.011 | 0.86 | 2.2 mg/kg | 78% and 87% | Thorsvik et al. [96] |
| CD: 7/302 | SES-CD ≤ 22 | r = 0.58, p < 0.012 | ||||||
| Neopterin | 20/55 | RI ≤ 2 | ↑ | r = 0.72, p < 0.001 | 0.98 | 200 pmol/g | 100% and 74% | Nancey et al. [56] |
| F-HNP | 26/45 | MES ≤ 1 | ↑ | r = 0.659, p < 0.001 | 0.86 | 32 ng/mL | 96% and 74% | Kanmura et al. [97] |
| PMNE | 72/179 colonoscopies in 91 patients | RI ≤ 1 | ↑ | r = 0.38, p < 0.001 | 0.7 | 0.035 μg/mL | 39% and 87% | Langhorst et al. [53] |
| Marker | N MH/Total | MH Definition | ↑/↓ in Active Disease | Correlation with Endoscopic Score | Marker Performance | Authors | ||
|---|---|---|---|---|---|---|---|---|
| AUC | Cutoff | Sens. and Spec. | ||||||
| MMP-9 | 31/54 | CDEIS no ulceration | ↑ | r = 0.55, p < 0.001 | 0.72 | 350 ng/g | 64% and 90% | Buisson et al. [92] |
| MMP-9 | na/50 | SES-CD ≤ 4 | ↑ | na | ns | Farkas et al. [93] | ||
| Lactoferrin | na/101 | SES-CD ≤ 3 | ↑ | r = 0.5, p < 0.0001 | 0.68 | 145.82 μg/mL | 85% and 61% | Klimczak et al. [98] |
| Lipocalin-2 | 31/54 | CDEIS no ulceration | ↑ | r = 0.49, p < 0.001 | 0.68 | 67000 ng/g | 46% and 86% | Buisson et al. [92] |
| Neopterin | 40/78 | SES-CD ≤ 2 | ↑ | r = 0.47, p < 0.001 | 0.75 | 200 pmol/g | 73% and 74% | Nancy et al. [56] |
| N MH/Total | MH Definition | Correlation with Endoscopic Score | Marker Performance | Authors | ||
|---|---|---|---|---|---|---|
| AUC | Cutoff | Sens. and Spec. | ||||
| 47/791 66/792 | MES = 01 MES ≤ 12 | r = 0.61, p < 0.05 | 0.771 0.962 | 0.05 mg/dL1 0.39 mg/dL2 | 71% and 71%1 92% and 92%2 | Arai et al. [74] |
| 20/43 | EAI ≤ 3 | na | 0.80 | 3.8 mg/L | 90% and 67% | Beyazit et al. [99] |
| 6/34 | MES = 0 | r = 0.35, p = 0.07 | na | 5 mg/L | 65% and 50% | Bodelier et al. [81] |
| 12/19 (CR)1 12/45 (all)2 | MES ≤ 2 | r = 0.634, p < 0.001 | 0.861 0.812 | 5 mg/L | 100% and 23%1 100% and 42%2 | Chen et al. [47] |
| 28/66 (NT)1 88/132 (T)2 pooled3 | MES ≤ 1 | r = 0.317, p < 0.011 r = 0.408, p < 0.012 r = 0.372, p < 0.013 | 0.701 0.802 0.773 | 5 mg/L1 7.2 mg/L2 7.3 mg/L3 | na and 82%1 44% and 93%2 39% and 91%3 | de Bruyn et al. [100] |
| 48/103 | MES ≤ 1 | r = 0.326, p = 0.001 | 0.60 | 0.7 mg/L | 52% and 69% | Dranga et al. [101] |
| 29/44 | MES ≤ 1 | na | 0.77 | 28 mg/L | 71% and 85% | Hassan et al. [102] |
| 10/85 | MES = 0 | r = 0.386, p < 0.001 | na | 0.5 mg/dL | 33% and 100% | Jung et al. [103] |
| 30/48 | MES = 0 | ns | 0.7 | 0.25 mg/dL | 81% and 53% | Jusué et al. [32] |
| 72/179 colonoscopies in 91 patients | RI ≤ 1 | r = 0.29, p < 0.001 | 0.65 | 0.25 mg/dL | 46% and 82% | Langhorst et al. [53] |
| 16/53 | MES = 0 | r = 0.46, p < 0.001 | 0.673 | 0.5 mg/L | 54% and 83% | Neubauer et al. [104] |
| 82/149 | MES = 01 MES ≤ 12 | na | 0.621 0.762 | 1.6 mg/L1 2.2 mg/L2 | 58% and 66%1 65% and 82%2 | Rosenberg et al. [105] |
| 7/32 | MES ≤ 1 | r = 0.387, p < 0.05 | 0.77 | na | na | Samant et al. [106] |
| 34/134 | RI ≤ 3 | r = 0.503, p < 0.001 | na | 5 mg/L | 67% and 60% | Schoepfer et al. [30] |
| 54/228 | mBS ≤ 1 | r = 0.556, p < 0.001 | 0.78 | 6 mg/L | 72% and 68% | Schoepfer et al. [58] |
| 16/1291 50/1292 | Matts’ = 11 Matts’ ≤ 22 | na | 0.671 0.812 | na | na | Shinzaki et al. [107] |
| 9/34 (na)* | MES = 0 | na | 0.71 | 0.4 mg/L | 71% and 30% | Tran et al. [108] |
| 52/207 | mMES ≤ 11 cMES ≤ 82 | r = 0.38, p < 0.051 r = 0.52, p < 0.052 | 0.751 0.902 | 0.23 mg/dL | 81% and 57%1 81% and 85%2 | Uchihara et al. [109] |
| 94/105 (CR) | MES = 01 MES ≤ 12 | na | 0.621 0.742 | 0.08 mg/dL1 0.04 mg/dL2 | na 68% and 70%2 | Yamaguchi et al. [65] |
| na/65 | mBS = 0 | r = 0.45, p < 0.001 | 0.68 | 5 mg/L | 80% and 50% | Falvey et al. [59] |
| 722 endoscopies in 552 patients | P-T = 01 MES ≤ 12 mBS = 03 HSI ≤ 44 RI ≤ 45 | r = 0.457, p < 0.0011 r = 0.503, p < 0.0012 r = 0.520, p < 0.0013 r = 0.507, p < 0.0014 r = 0.523, p < 0.0015 | 0.761 0.772 0.783 0.774 0.765 | 8 mg/L | 51% and 85%1 53% and 84%2 52% and 87%3 51% and 85%4 51% and 87%5 | Yoon et al. [110] |
| N MH/Total | MH Definition | Correlation with Endoscopic Score | Marker Performance | Authors | ||
|---|---|---|---|---|---|---|
| AUC | Cutoff | Sens. and Spec. | ||||
| 42/209 endoscopies | SES-CD ≤ 2 | r = 0.56, p < 0.001 | 0.64 | 3 mg/L | 50% and 24% | Björkesten et al. [26] |
| 31/50 | SES-CD ≤ 3 | r = 0.45, p = 0.07 | na | 5 mg/L | 65% and 56% | Bodelier et al. [81] |
| 20/34 (CR)1 21/56 (all)2 | SES-CD ≤ 3 | r = 0.658, p < 0.01 | 0.761 0.812 | 5 mg/L | 83% and 46%1 91% and 71%2 | Chen et al. [47] |
| 38/108 | Descriptive corresponding with SES-CD ≤ 2 | r= 0.307, p < 0.001 | 0.74 | 5 mg/L | 79% and 57% | de Bruyn et al. [111] |
| na/107 | SES-CD ≤ 2 | r = 0.44, p < 0.001 | 0.64 | 5 mg/L | 67% and 60% | Falvey et al. [59] |
| na/33 | SES-CD = 0 | r = 0.709, p < 0.001 | 0.92 | 0.03 mg/dL | 86% and 89% | Hosomi et al. [112] |
| 19/55 | SES-CD ≤ 3 | r = 0.61, p < 0.01 | 0.75 | 0.11 mg/dL | 68% and 78% | Ishihara et al. [113] |
| 6/34 | SES-CD ≤ 3 | r = 0.585, p < 0.001 | na | 0.4 mg/dL | 89% and 67% | Jung et al. [104] |
| 4/55 | CDEIS ≤ 2 | r = 0.672, p < 0.001 | na | 3 mg/L | 80% and 76% | Karczewski et al. [78] |
| 11/43 | Descriptive corresponding with SES-CD ≤ 3 | na | 0.78 | 1.1 mg/L | 100% and 38% | Miranda-García et al. [114] |
| 51/136 | SES-CD ≤ 3 | r = 0.458, p < 0.001 | 0.69 | 5 mg/L | 84% and 53% | Schaffer et al. [77] |
| 26/140 | SES-CD ≤ 3 | r = 0.53, p < 0.01 | na | 5 mg/L | 58% and 68% | Schoepfer et al. [30] |
| 39/94 | Descriptive corresponding with SES-CD ≤ 2 | na | 0.75 | 4 mg/L | 69% and 62% | Yarur et al. [115] |
| Marker | N MH/Total | MH Definition | ↑/↓ in Active Disease | Correlation with Endoscopic Score | Marker Performance | Authors | ||
|---|---|---|---|---|---|---|---|---|
| AUC | Cutoff | Sens. and Spec. | ||||||
| VEGF-A | 16/37 | MES = 0 | ↑ | r = 0.397, p = 0.015 | 0.72 | 341 ng/mL | 64% and 85% | Algaba et al. [126] |
| ADA | 20/43 | EAI ≤ 3 | ↑ | na | 0.87 | 9.45 U/L | 84% and 83% | Beyazit et al. [99] |
| ST2 | 25/83 | EAI ≤ 4 | ↑ | na | 0.81 | 47.1 pg/mL | 72% and 72% | Boga et al. [127] |
| ST2 | 44/84 | MES ≤ 1 | ↑ | r = 0.762, p < 0.001 | 0.92 | 74.87 ng/L | 83% and 83% | Díaz-Jiménez et al. [128] |
| ST2 | 18/24 | MES ≤ 1 | ↑ | r = 0.66, p < 0.001 | na | 74.87 ng/L | 44% and 95% | Díaz-Jiménez et al. [129] |
| NGAL | 14/41 | MES ≤ 1 | ↑ | r = 0.574, p < 0.05 | 0.76 | 43.6 ng/mL | 96% and 54% | Budzynska et al. [130] |
| NGAL-MMP9 complex | 28/66 (NT)1 88/132 (T)2 pooled3 | MES ≤ 1 | ↑ | r = 0.317, p < 0.011 r = 0.382, p < 0.012 r = 0.37, p < 0.013 | 0.751 0.782 0.783 | 97.7 ng/mL1 93.2 ng/mL2 97.7 ng/mL3 | 43% and 93%1 44% and 91%2 43% and 91%3 | de Bruyn et al. [100] |
| sIL2R | na/68 | MES = 0 | ↑ | r = 0.357, p = 0.003 | 0.65 | 274 U/mL | 60% and 76% | Hosomi et al. [112] |
| sTREM-1 | 10/85 | MES = 0 | ↑ | r = 0.498, p < 0.001 | na | 60 pg/mL | 59% and 80% | Jung et al. [103] |
| IL-6 | 5/45 | MES = 0 | ↑ | r = 0.596, p < 0.001 | 0.93 | 9.6 pg/mL | 80% and 95% | Mankowska-Wierzbicka et al. [116] |
| IL-17 | 5/45 | MES = 0 | ↑ | r = 0.578, p < 0.001 | 0.93 | 6.6 pg/mL | 60% and 92% | Mankowska-Wierzbicka et al. [116] |
| TNFα | 5/45 | MES = 0 | ↑ | r = 0.701, p < 0.001 | 0.98 | 7.6 pg/mL | 80% and 95% | Mankowska-Wierzbicka et al. [116] |
| IL-9 | na/53 | MES = 0 | ↑ | r = 0.74, p < 0.001 | 0.97 | 20.5 pg/mL | 94% and 92% | Matusiewicz et al. [119] |
| GM-CSF | 16/53 | MES = 0 | ↑ | r = 0.56, p < 0.001 | 0.91 | 16.7 pg/mL | 69% and 97% | Neubauer et al. [104] |
| IFNγ | 16/53 | MES = 0 | ↑ | r = 0.55, p < 0.001 | 0.78 | 83.2 pg/mL | 100% and 60% | Neubauer et al. [104] |
| IL-12(p70) | 16/53 | MES = 0 | ↑ | r = 0.49, p < 0.001 | 0.71 | 21.6 pg/mL | 50% and 100% | Neubauer et al. [104] |
| Nampt | 66/98 | MES ≤ 1 | ↑ | r = 0.47, p < 0.001 | 0.77 | 1.54 ng/mL | 76% and 75% | Neubauer et al. [131] |
| IL-8 | 22/67 | BS ≤ 1 (inactive + mild) | ↑ | na | na | 13.74 pg/mL | 69% and 55% | Rodriguez-Peralvarez et al. [132] |
| LRG | 16/1291 50/1292 | Matts’ = 11 Matts’ ≤ 22 | ↑ | na | 0.761 0.852 | na | na | Shinzaki et al. [107] |
| TTF3 | 43/76 (CR) | BS ≤ 1 | ↑ | na | 0.73 | 1.27 ng/mL | 70% and 68% | Srivastava et al. [133] |
| TTF3 | 50/116 | UCEIS = 0 and MES = 0 | ↑ | r = 0.82 for UCEIS, r = 0.811 for EMS, p < 0.001 | 0.927 | 6.74 ng/mL | 89.9% and 86.9% | Nakov et al. [66] |
| LL-37 | 9/34 (na)* | MES = 0 | ↓ | na | 0.76 | 32 mg/mL | 100% and 38% | Tran et al. [108] |
| Leptin | 8/23 UC1 5/11 CD2 | MES = 01 SES-CD = 02 | ↓ | na | 0.65 | 2.5 ng/mL | 88% and 45% | Trejo-Vasquez et al. [134] |
| ALB | 52/207 | mMES ≤ 11 cMES ≤ 82 | ↓ | r = −0.52, p < 0.051 r = −0.65, p < 0.052 | 0.771 0.902 | 4.2 g/dL | 73% and 72%1 79% and 88%2 | Uchihara et al. [109] |
| ALB | 94/105 (CR) | MES = 01 MES ≤ 12 | ↓ | na | 0.501 0.552 | 4.3 g/dL | na 34% and 90% | Yamaguchi et al. [65] |
| Marker | N MH/Total | MH Definition | ↑/↓ in Active Disease | Correlation with Endoscopic Score | Marker Performance | Authors | ||
|---|---|---|---|---|---|---|---|---|
| AUC | Cutoff | Sens. and Spec. | ||||||
| Ang1 | 19/35 | SES-CD ≤ 2 | ↑ | r = 0.362, p = 0.049 | 0.65 | 47.8 ng/mL | 52% and 67% | Algaba et al. [126] |
| ST2 | 52/8 | SES-CD ≤ 2 | ↑ | na | 0.73 | 57.6 pg/mL | 100% and 56% | Boga et al. [127] |
| NGAL | 45/79 | SES-CD ≤ 7 (inactive + mild) | ↑ | ns | 0.61 | 72.5 ng/mL | 48% and 83% | Budzynska et al. [130] |
| NGAL-MMP9 complex | 38/108 | Descriptive corresponding with SES-CD ≤ 2 | ↑ | r= 0.296, p < 0.001 | 0.77 | 45 ng/mL | 82% and 60% | de Bruyn et al. [111] |
| IL-9 | 23/50 | Descriptive corresponding with CDEIS ≤ 2 | ↑ | r = 0.395, p < 0.001 | 0.78 | 18.1 pg/mL | 52% and 100% | Feng et al. [138] |
| sIL2R | na/33 | SES-CD = 0 | ↑ | r = 0.516, p = 0.002 | 0.74 | 283 U/mL | 71% and 73% | Hosomi et al. [112] |
| sCD14-ST | na/33 | SES-CD = 0 | ↑ | r = 0.512, p = 0.002 | 0.85 | 89 pg/mL | 86% and 85% | Hosomi et al. [112] |
| SAA | 19/55 | SES-CD ≤ 3 | ↑ | r = 0.64, p < 0.01 | 0.77 | 5.9 μg/dL (mL)* | 68% and 83% | Ishihara et al. [113] |
| SAA | 39/94 | Descriptive corresponding with SES-CD ≤ 2 | ↑ | na | 0.77 | 28 mg/L | 95% and 64% | Yarur et al. [115] |
| sTREM-1 | 6/34 | SES-CD ≤ 3 | ↑ | ns | Na | 60 pg/mL | 29% and 83% | Jung et al. [103] |
| IL-17 | 4/55 | CDEIS ≤ 2 | ↑ | r = 0.296, p < 0.005 | Na | 7.05 pg/mL | 80% and 74% | Karczewski et al. [78] |
| ERβ/ERα | 11/31 | SES-CD ≤ 2 | na | 0.84 | 0.85 (ratio) | 91% and 70% | Linares et al. [139] | |
| IL-6 | 6/32 | CDEIS ≤ 7 | ↑ | r = 0.957, p < 0.001 | 1.00 | 15.9 pg/mL | 100% and 100% | Ljuca et al. [137] |
| Orosomucoid | 11/43 | Descriptive corresponding with SES-CD ≤ 3 | ↑ | na | 0.85 | 119.5 mg/dL | 100% and 57% | Miranda-García et al. [114] |
| Fibrinogen | 11/43 | Descriptive corresponding with SES-CD ≤ 3 | ↑ | na | 0.81 | 457 mg/dL | 100% and 65% | Miranda-García et al. [114] |
| Ficolin-2 | 51/136 | SES-CD ≤ 3 | ↑ | r = 0.171, p = 0.047 | 0.61 | 2.404 μg/mL | 51% and 68% | Schaffer et al. [87] |
| IL-1β | 25/47 (RP) | Descriptive corresponding with SES-CD ≤ 2 | ↑ | na | 0.88 | na | na | Yarur et al. [140] |
| ICAM | 25/47 (RP) | Descriptive corresponding with SES-CD ≤ 2 | ↑ | na | 0.89 | na | na | Yarur et al. [140] |
| Marker | N MH/Total | MH Definition | ↑/↓ in Active Disease | Correlation with Endoscopic Score | Marker Performance | Authors | ||
|---|---|---|---|---|---|---|---|---|
| AUC | Cutoff | Sens. and Spec. | ||||||
| PGE | 47/791 66/792 | MES = 01 MES ≤ 12 | ↑ | r = 0.85, p < 0.05 | 0.901 0.982 | 21.8 μg/g CR1 34.7 μg/g CR2 | 81% and 81%1 89% and 89%2 | Arai et al. [142] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krzystek-Korpacka, M.; Kempiński, R.; Bromke, M.; Neubauer, K. Biochemical Biomarkers of Mucosal Healing for Inflammatory Bowel Disease in Adults. Diagnostics 2020, 10, 367. https://doi.org/10.3390/diagnostics10060367
Krzystek-Korpacka M, Kempiński R, Bromke M, Neubauer K. Biochemical Biomarkers of Mucosal Healing for Inflammatory Bowel Disease in Adults. Diagnostics. 2020; 10(6):367. https://doi.org/10.3390/diagnostics10060367
Chicago/Turabian StyleKrzystek-Korpacka, Małgorzata, Radosław Kempiński, Mariusz Bromke, and Katarzyna Neubauer. 2020. "Biochemical Biomarkers of Mucosal Healing for Inflammatory Bowel Disease in Adults" Diagnostics 10, no. 6: 367. https://doi.org/10.3390/diagnostics10060367
APA StyleKrzystek-Korpacka, M., Kempiński, R., Bromke, M., & Neubauer, K. (2020). Biochemical Biomarkers of Mucosal Healing for Inflammatory Bowel Disease in Adults. Diagnostics, 10(6), 367. https://doi.org/10.3390/diagnostics10060367





