Biphasic Thyroid-Like Low-Grade Nasopharyngeal Papillary Adenocarcinoma with a Prominent Spindle Cell Component: A Case Report
Abstract
1. Introduction
2. Case Report
2.1. Clinical Presentation
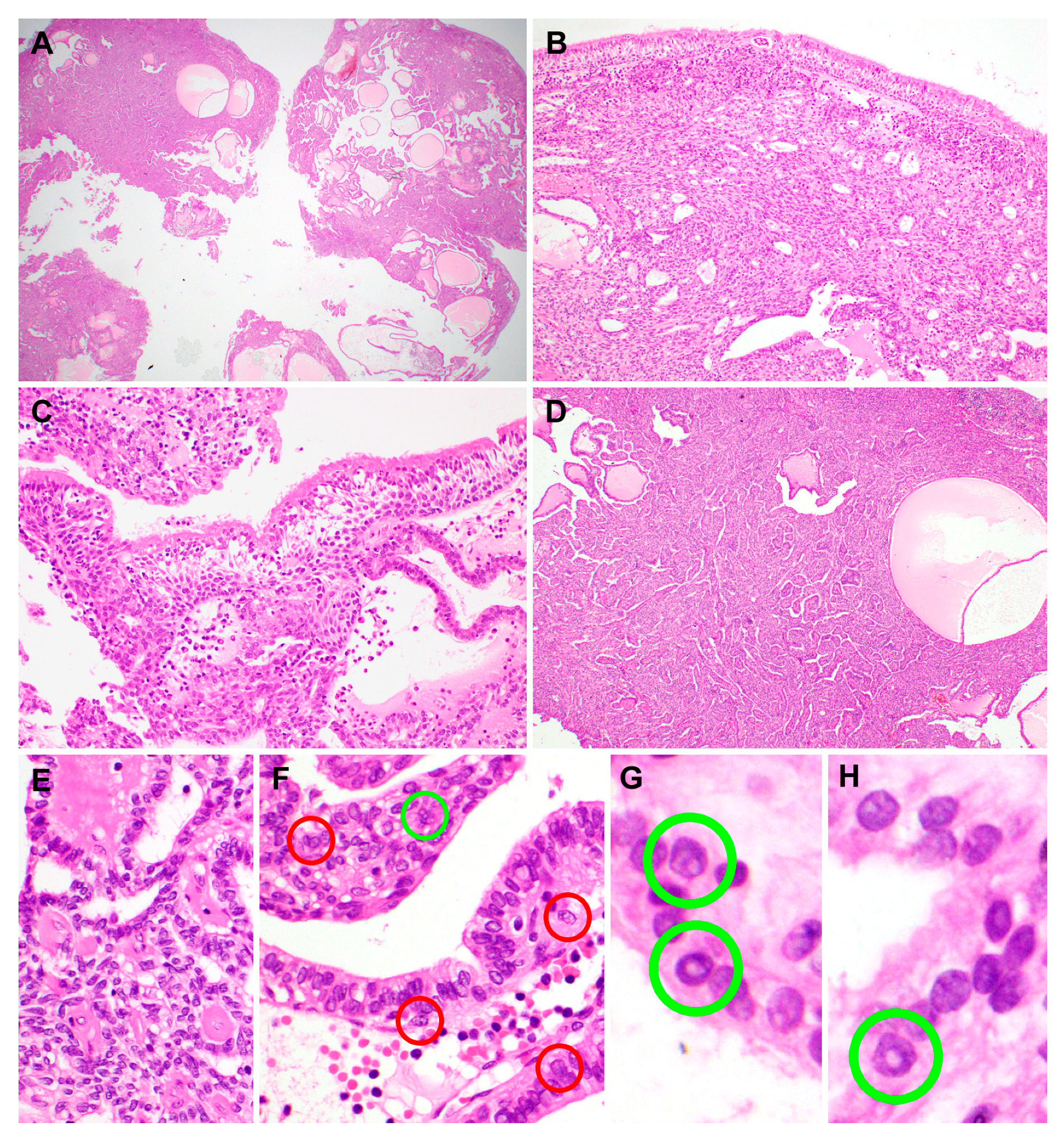
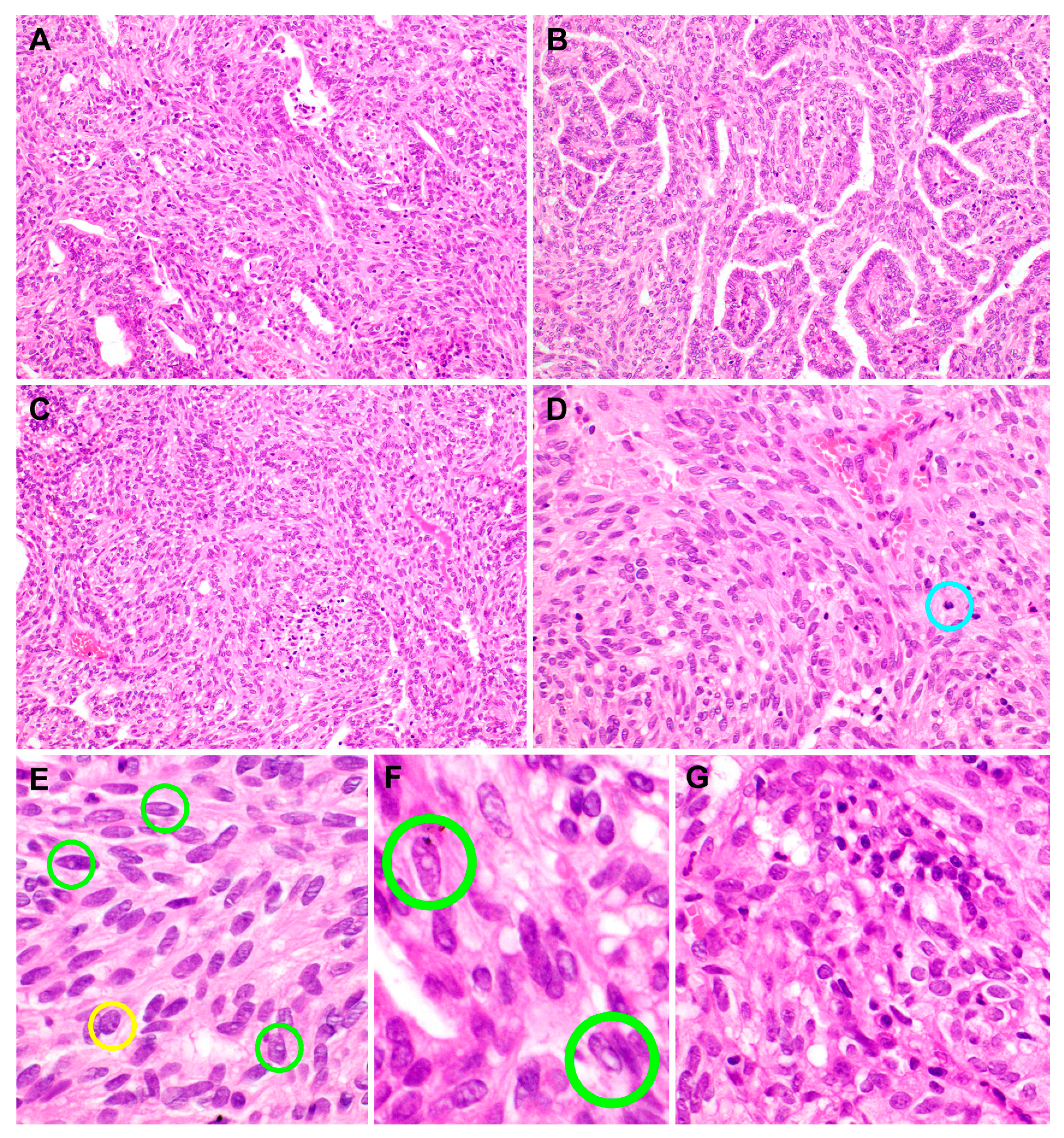
2.2. Pathological Features
2.3. Immunostaining Results
3. Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| TLLG-NPPA | Thyroid-like low-grade nasopharyngeal papillary adenocarcinoma |
| PTC | Papillary thyroid carcinoma |
References
- El-Naggar, A.K.; Chan, J.K.C.; Grandis, J.R.; Takata, T.; Slootweg, P.J. World Health Organization Classification of Head and Neck Tumours, 4th ed.; IARC: Lyon, France, 2017. [Google Scholar]
- Petersson, F.; Pang, B.; Loke, D.; Hao, L.; Yan, B. Biphasic low-grade nasopharyngeal papillary adenocarcinoma with a prominent spindle cell component: Report of a case localized to the posterior nasal septum. Head Neck Pathol. 2011, 5, 306–313. [Google Scholar] [CrossRef]
- Ohe, C.; Sakaida, N.; Tadokoro, C.; Fukui, H.; Asako, M.; Tomoda, K.; Uemura, Y. Thyroid-like low-grade nasopharyngeal papillary adenocarcinoma: Report of two cases. Pathol. Int. 2010, 60, 107–111. [Google Scholar] [CrossRef]
- Sillings, C.N.; Weathers, D.R.; Delgaudio, J.M. Thyroid-like papillary adenocarcinoma of the nasopharynx: A case report in a 19-year-old male. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2010, 110, e25–e28. [Google Scholar] [CrossRef]
- Oide, T.; Kadosono, O.; Matsushima, J.; Wu, D.; Nagashima, H.; Saigusa, H.; Masunaga, A.; Nakatani, Y.; Hiroshima, K. Thyroid-like low-grade nasopharyngeal papillary adenocarcinoma with squamous differentiation: A novel histological finding. Hum. Pathol. 2017, 70, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Wenig, B.M.; Hyams, V.J.; Heffner, D.K. Nasopharyngeal papillary adenocarcinoma. A clinicopathologic study of a low-grade carcinoma. Am. J. Surg. Pathol. 1988, 12, 946–953. [Google Scholar] [CrossRef] [PubMed]
- Carrizo, F.; Luna, M.A. Thyroid transcription factor-1 expression in thyroid-like nasopharyngeal papillary adenocarcinoma: Report of 2 cases. Ann. Diagn. Pathol. 2005, 9, 189–192. [Google Scholar] [CrossRef] [PubMed]
- Yokoi, H.; Terado, Y.; Fujiwara, M.; Matsumoto, Y.; Ikeda, T.; Saito, K. Biphasic low-grade nasopharyngeal papillary adenocarcinoma: A case report and literature review. BMC Clin. Pathol. 2018, 18, 10. [Google Scholar] [CrossRef] [PubMed]
- Mirza, R.; Dela Cruz, N.; Herrera, G.A. Thyroid-Like Low-Grade Nasopharyngeal Papillary Adenocarcinoma with Biphasic Histology. Case Rep. Pathol. 2020, 2020, 3275916. [Google Scholar] [CrossRef] [PubMed]
- Oishi, N.; Kondo, T.; Nakazawa, T.; Mochizuki, K.; Kasai, K.; Inoue, T.; Yamamoto, T.; Watanabe, H.; Hatsushika, K.; Masuyama, K.; et al. Thyroid-like low-grade nasopharyngeal papillary adenocarcinoma: Case report and literature review. Pathol. Res. Pract. 2014, 210, 1142–1145. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.L.; Ma, S.; Havrilla, L.; Cai, L.; Yu, C.Q.; Shen, S.; Xu, H.T.; Wang, L.; Yu, J.H.; Lin, X.Y.; et al. Primary thyroid-like low-grade nasopharyngeal papillary adenocarcinoma: A case report and literature review. Medicine 2017, 96, e8851. [Google Scholar] [CrossRef] [PubMed]
- Jones, T.D.; Kernek, K.M.; Yang, X.J.; Lopez-Beltran, A.; MacLennan, G.T.; Eble, J.N.; Lin, H.; Pan, C.X.; Tretiakova, M.; Baldridge, L.A.; et al. Thyroid transcription factor 1 expression in small cell carcinoma of the urinary bladder: An immunohistochemical profile of 44 cases. Hum. Pathol. 2005, 36, 718–723. [Google Scholar] [CrossRef] [PubMed]
- Comperat, E.; Zhang, F.; Perrotin, C.; Molina, T.; Magdeleinat, P.; Marmey, B.; Regnard, J.F.; Audouin, J.; Camilleri-Broet, S. Variable sensitivity and specificity of TTF-1 antibodies in lung metastatic adenocarcinoma of colorectal origin. Mod. Pathol. 2005, 18, 1371–1376. [Google Scholar] [CrossRef] [PubMed]
- Kakkar, A.; Sakthivel, P.; Mahajan, S.; Thakar, A. Nasopharyngeal Papillary Adenocarcinoma as a Second Head and Neck Malignancy. Head Neck Pathol. 2019, 13, 699–704. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Wei, J.; Yao, X.; Wang, C. Clinicopathological Features of Low-Grade Thyroid-like Nasopharyngeal Papillary Adenocarcinoma. Cancer Res. Treat. 2017, 49, 213–218. [Google Scholar] [CrossRef] [PubMed]
- Pineda-Daboin, K.; Neto, A.; Ochoa-Perez, V.; Luna, M.A. Nasopharyngeal adenocarcinomas: A clinicopathologic study of 44 cases including immunohistochemical features of 18 papillary phenotypes. Ann. Diagn. Pathol. 2006, 10, 215–221. [Google Scholar] [CrossRef] [PubMed]
- Evans, H.L.; Luna, M.A. Polymorphous low-grade adenocarcinoma: A study of 40 cases with long-term follow up and an evaluation of the importance of papillary areas. Am. J. Surg. Pathol. 2000, 24, 1319–1328. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.Y.; Huang, C.C.; Chen, H.K.; Chien, C.Y. Adult thyroid-like low-grade nasopharyngeal papillary adenocarcinoma with thyroid transcription factor-1 expression. Otolaryngol. Head Neck Surg. 2007, 137, 837–838. [Google Scholar] [CrossRef] [PubMed]
- Ozer, S.; Kayahan, B.; Cabbarzade, C.; Bugdayci, M.; Kosemehmetoglu, K.; Yucel, O.T. Thyroid-like papillary adenocarcinoma of the nasopharynx with focal thyroglobulin expression. Pathology 2013, 45, 622–624. [Google Scholar] [CrossRef] [PubMed]
- Fu, C.H.; Chang, K.P.; Ueng, S.H.; Wu, C.C.; Hao, S.P. Primary thyroid-like papillary adenocarcinoma of the nasopharynx. Auris Nasus Larynx 2008, 35, 579–582. [Google Scholar] [CrossRef] [PubMed]

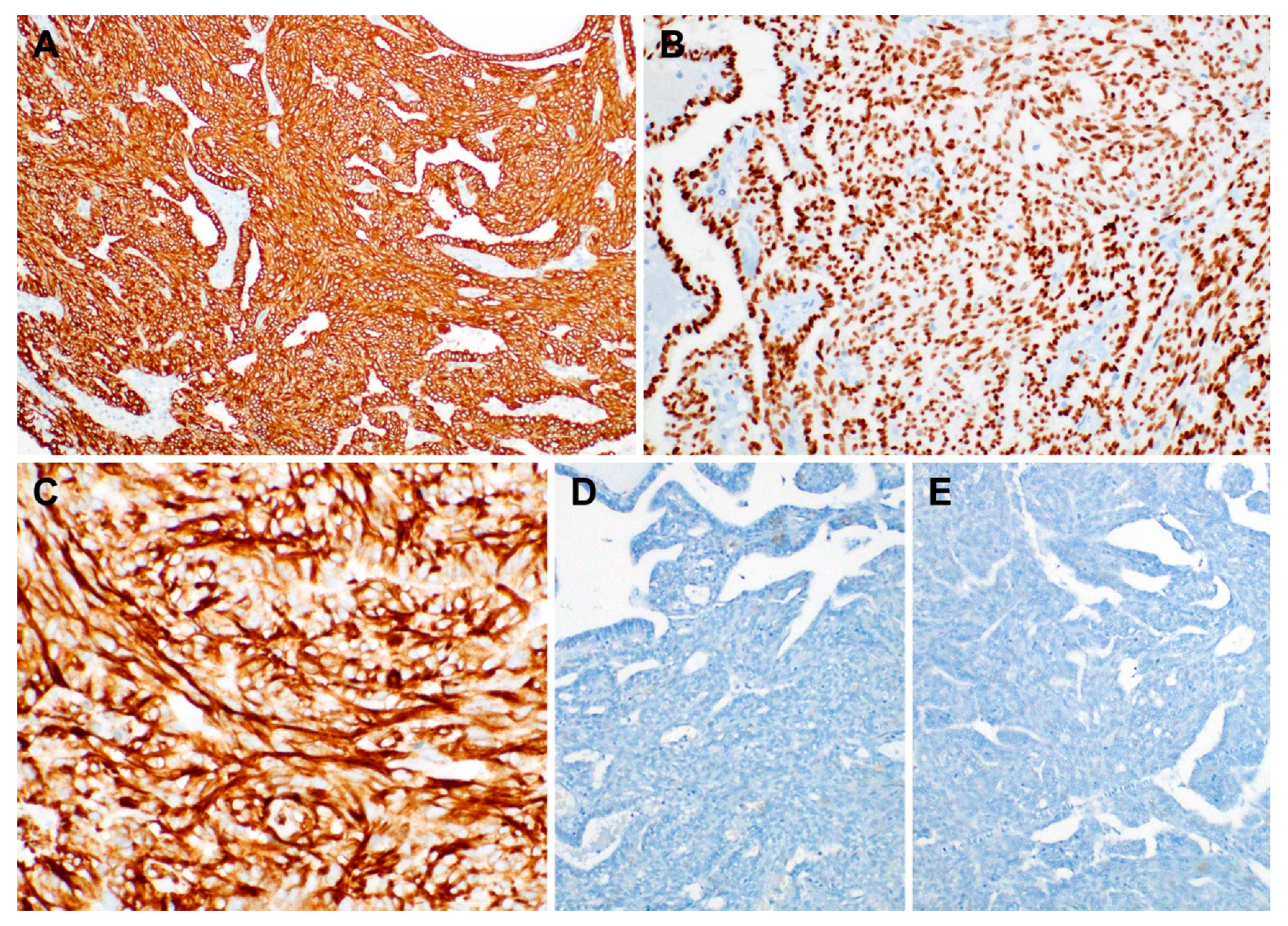
| Antibody | Immunostaining Result | |
|---|---|---|
| Papillary Component | Spindle Cell Component | |
| Thyroid transcription factor-1 | Positive | Positive |
| Vimentin | Positive | Positive |
| Hector Battifora mesothelial-1 | Positive | Positive |
| Cytokeratin 7 | Positive | Positive |
| Cytokeratin 19 | Positive | Positive |
| Paired box 8 | Negative | Negative |
| p63 | Negative | Negative |
| Smooth muscle actin | Negative | Negative |
| S-100 | Negative | Negative |
| Cytokeratin 5/6 | Negative | Negative |
| Thyroglobulin | Negative | Negative |
| BRAF V600E | Negative | Negative |
| Epstein–Barr virus-encoded small RNAs | Negative | Negative |
| No. | Reference | Age (Years) | Sex | Quantity of Spindle Cell Component | Current Status | Follow-Up (Months) |
|---|---|---|---|---|---|---|
| 1 | Petersson et al. [2] | 39 | F | Prominent | NA | Recent |
| 2 | Ohe et al. [3] | 25 | M | NA | NED | 13 |
| 3 | Ohe et al. [3] | 41 | M | NA | NED | 9 |
| 4 | Oishi et al. [10] | 47 | F | Focal | NED | 19 |
| 5 | Yokoi et al. [8] | 58 | M | Prominent | NED | 34 |
| 6 | Mirza et al. [9] | 54 | M | Several foci of solid aggregates | NED | 12 |
| 7 | The present report | 50 | F | Prominent | NA | Recent |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, S.H.; Kim, H.; Kim, M.J.; Kim, B.; Kim, H.-S. Biphasic Thyroid-Like Low-Grade Nasopharyngeal Papillary Adenocarcinoma with a Prominent Spindle Cell Component: A Case Report. Diagnostics 2020, 10, 323. https://doi.org/10.3390/diagnostics10050323
Lee SH, Kim H, Kim MJ, Kim B, Kim H-S. Biphasic Thyroid-Like Low-Grade Nasopharyngeal Papillary Adenocarcinoma with a Prominent Spindle Cell Component: A Case Report. Diagnostics. 2020; 10(5):323. https://doi.org/10.3390/diagnostics10050323
Chicago/Turabian StyleLee, Sang Hwa, Hyunjin Kim, Min Ju Kim, Byungwha Kim, and Hyun-Soo Kim. 2020. "Biphasic Thyroid-Like Low-Grade Nasopharyngeal Papillary Adenocarcinoma with a Prominent Spindle Cell Component: A Case Report" Diagnostics 10, no. 5: 323. https://doi.org/10.3390/diagnostics10050323
APA StyleLee, S. H., Kim, H., Kim, M. J., Kim, B., & Kim, H.-S. (2020). Biphasic Thyroid-Like Low-Grade Nasopharyngeal Papillary Adenocarcinoma with a Prominent Spindle Cell Component: A Case Report. Diagnostics, 10(5), 323. https://doi.org/10.3390/diagnostics10050323





