Optimization Protocol of Fixation Method for Trophoblast Retrieval from the Cervix (TRIC): A Preliminary Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Selection
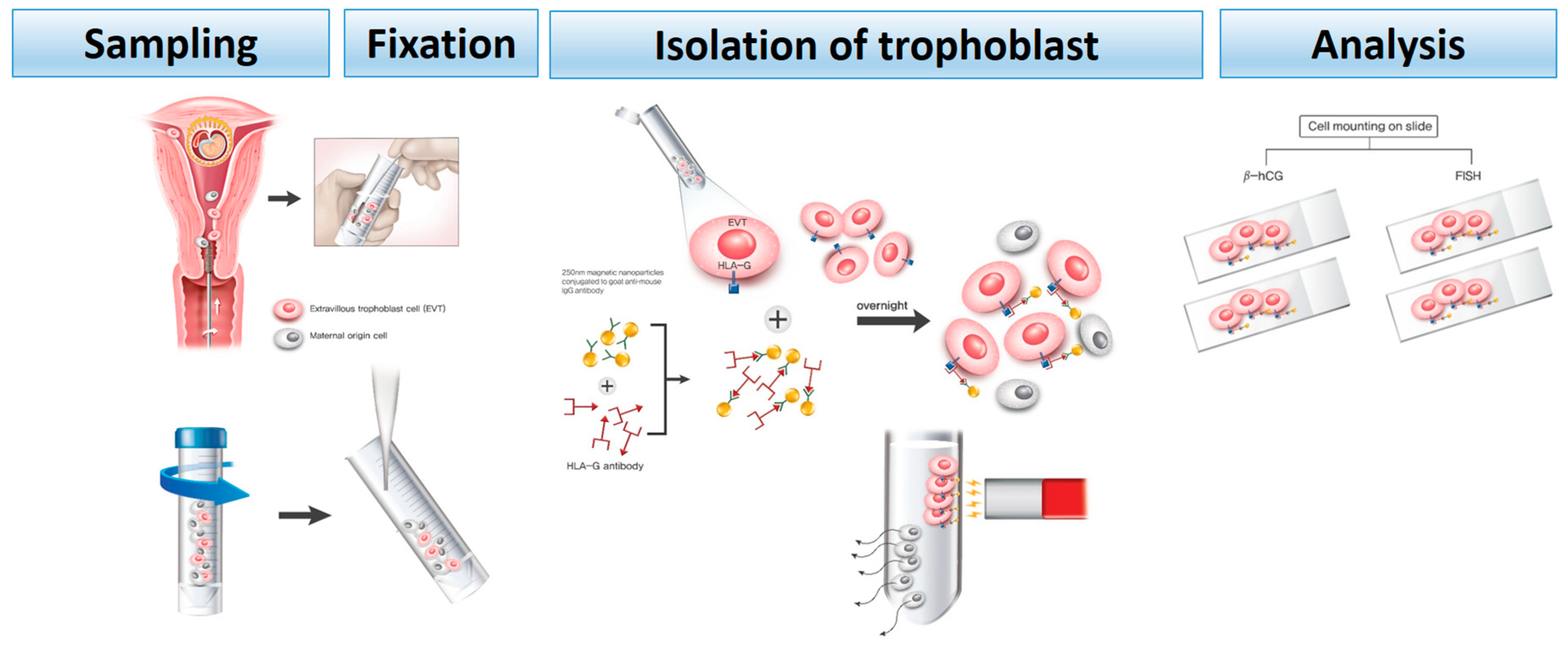
2.2. Endocervical Sampling
2.3. Immunomagnetic Isolation of Trophoblast Cells
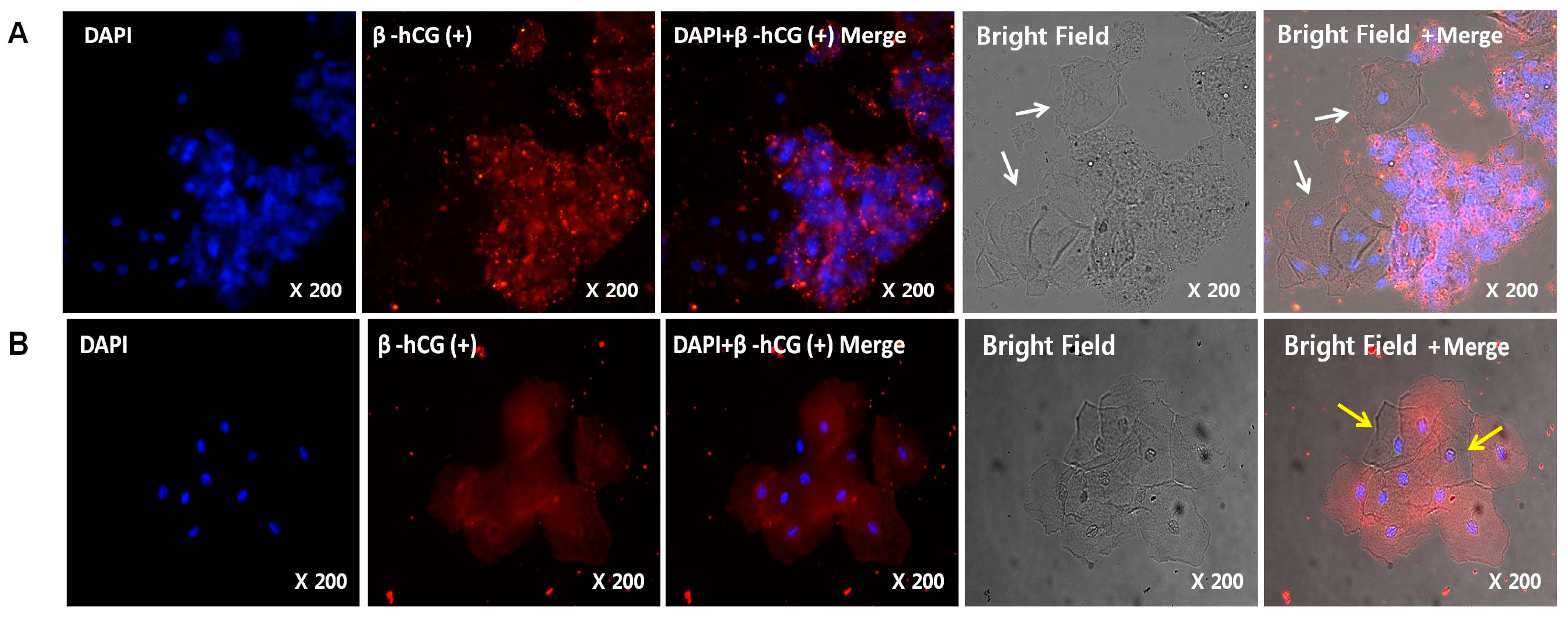
2.4. Immunohistochemistry
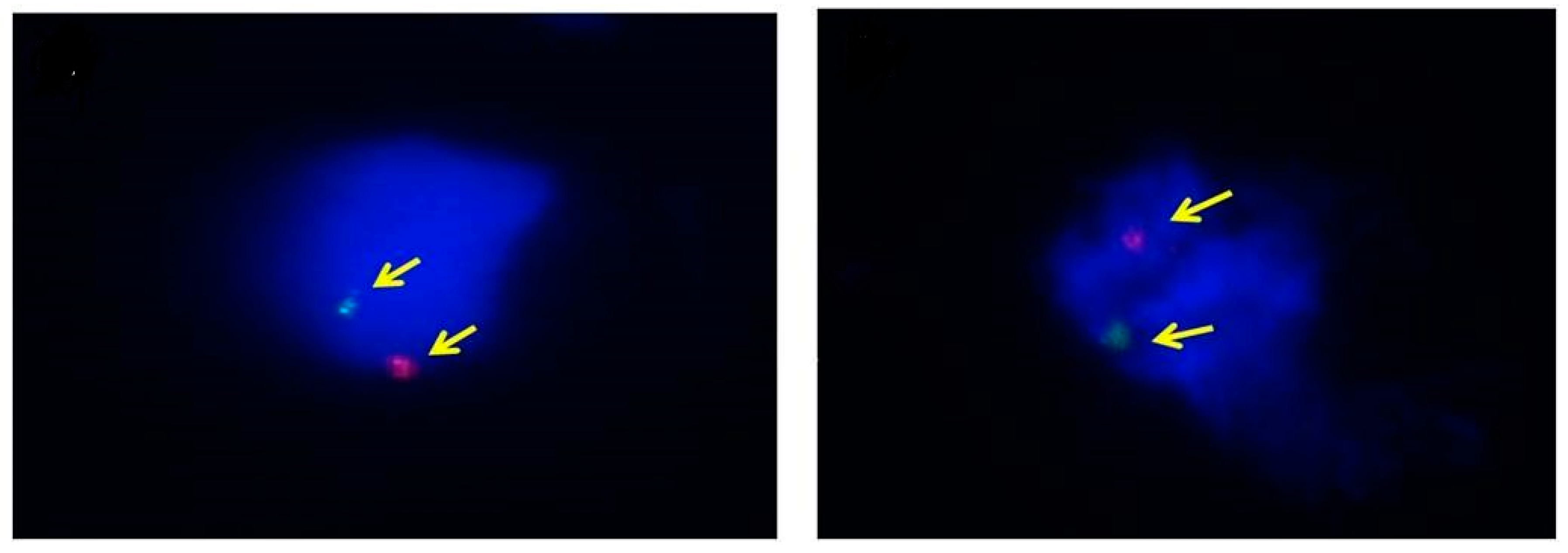
2.5. Fluorescence in Situ Hybridization (FISH)
2.6. Statistical Analysis
3. Results
4. Discussion
4.1. Main Findings
4.2. Strengths and Limitations
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- SF2.3: Age of Mothers at Childbirth and Age-Specific Fertility: The Organisation for Economic Co-Operation and Development (OECD). 2018. Available online: https://www.oecd.org/els/soc/SF_2_3_Age_mothers_childbirth.pdf (accessed on 29 May 2019).
- Centers for Disease Control and Prevention. Natality trends in the United States, 1909–2015 2015. Available online: https://www.cdc.gov/nchs/data-visualization/natality-trends/ (accessed on 12 July 2018).
- Cleary-Goldman, J.; Malone, F.D.; Vidaver, J.; Nyberg, D.A.; Comstock, C.H.; Saade, G.R.; Eddleman, K.A.; Klugman, S.; Dugoff, L.; Timor-Tritsch, I.E.; et al. Impact of maternal age on obstetric outcome. Obstet. Gynecol. 2005, 105, 983–990. [Google Scholar] [CrossRef] [PubMed]
- Akolekar, R.; Beta, J.; Picciarelli, G.; Ogilvie, C.; D’Antonio, F. Gynecology. Procedure-related risk of miscarriage following amniocentesis and chorionic villus sampling: A systematic review and meta-analysis. Ultrasound Obstet. Gynecol. 2015, 45, 16–26. [Google Scholar] [CrossRef] [PubMed]
- Caughey, A.B.; Hopkins, L.M.; Norton, M.E. Chorionic villus sampling compared with amniocentesis and the difference in the rate of pregnancy loss. Obstet. Gynecol. 2006, 108, 612–616. [Google Scholar] [CrossRef] [PubMed]
- Lo, Y.D.; Corbetta, N.; Chamberlain, P.F.; Rai, V.; Sargent, I.L.; Redman, C.W.; Wainscoat, J.S. Presence of fetal DNA in maternal plasma and serum. Lancet 1997, 350, 485–487. [Google Scholar] [CrossRef]
- Cheng, Y.K.; Leung, W.C.; Leung, T.Y.; Choy, K.W.; Chiu, R.W.; Lo, T.K.; Kwok, K.Y.; Sahota, D.S. Women’s preference for non-invasive prenatal DNA testing versus chromosomal microarray after screening for Down syndrome: A prospective study. BJOG 2018, 125, 451–459. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, F.; Leveno, K.; Bloom, S.; Spong, C.Y.; Dashe, J. Williams Obstetrics, 25th ed.; McGraw-Hill Education: New York, NY, USA, 2018; pp. 291–294. [Google Scholar]
- Jain, C.V.; Kadam, L.; van Dijk, M.; Kohan-Ghadr, H.R.; Kilburn, B.A.; Hartman, C.; Mazzorana, V.; Visser, A.; Hertz, M.; Bolnick, A.D.; et al. Fetal genome profiling at 5 weeks of gestation after noninvasive isolation of trophoblast cells from the endocervical canal. Sci. Transl. Med. 2016, 8, 363re4. [Google Scholar] [CrossRef]
- Beulen, L.; Faas, B.H.; Feenstra, I.; van Vugt, J.M.; Bekker, M.N. Clinical utility of non-invasive prenatal testing in pregnancies with ultrasound anomalies. Ultrasound Obstet. Gynecol. 2017, 49, 721–728. [Google Scholar] [CrossRef]
- Shettles, L.B. Use of the Y chromosome in prenatal sex determination. Nature 1971, 230, 52. [Google Scholar] [CrossRef]
- Rodeck, C.; Tutschek, B.; Sherlock, J.; Kingdom, J. Methods for the transcervical collection of fetal cells during the first trimester of pregnancy. Prenat Diagn. 1995, 15, 933–942. [Google Scholar] [CrossRef] [PubMed]
- Imudia, A.N.; Suzuki, Y.; Kilburn, B.A.; Yelian, F.D.; Diamond, M.P.; Romero, R.; Armant, D.R. Retrieval of trophoblast cells from the cervical canal for prediction of abnormal pregnancy: A pilot study. Hum. Reprod. 2009, 24, 2086–2092. [Google Scholar] [CrossRef] [PubMed]
- Moser, G.; Drewlo, S.; Huppertz, B.; Armant, D.R. Trophoblast retrieval and isolation from the cervix: Origins of cervical trophoblasts and their potential value for risk assessment of ongoing pregnancies. Hum. Reprod. Update 2018, 24, 484–496. [Google Scholar] [CrossRef] [PubMed]
- Pfeifer, I.; Benachi, A.; Saker, A.; Bonnefont, J.P.; Mouawia, H.; Broncy, L.; Frydman, R.; Brival, M.L.; Lacour, B.; Dachez, R.; et al. Cervical trophoblasts for non-invasive single-cell genotyping and prenatal diagnosis. Placenta 2016, 37, 56–60. [Google Scholar] [CrossRef] [PubMed]
- Bolnick, J.M.; Kilburn, B.A.; Bajpayee, S.; Reddy, N.; Jeelani, R.; Crone, B.; Simmerman, N.; Singh, M.; Diamond, M.P.; Armant, D.R. Trophoblast retrieval and isolation from the cervix (TRIC) for noninvasive prenatal screening at 5 to 20 weeks of gestation. Fertil. Steril. 2014, 102, 135–142.e6. [Google Scholar] [CrossRef] [PubMed]
- Bolnick, J.M.; Kohan-Ghadr, H.R.; Fritz, R.; Bolnick, A.D.; Kilburn, B.A.; Diamond, M.P.; Armant, D.R.; Drewlo, S. Altered biomarkers in trophoblast cells obtained noninvasively prior to clinical manifestation of perinatal disease. Sci. Rep. 2016, 6, 32382. [Google Scholar] [CrossRef] [PubMed]
- Fritz, R.; Kohan-Ghadr, H.R.; Sacher, A.; Bolnick, A.D.; Kilburn, B.A.; Bolnick, J.M.; Diamond, M.P.; Drewlo, S.; Armant, D.R. Trophoblast retrieval and isolation from the cervix (TRIC) is unaffected by early gestational age or maternal obesity. Prenat. Diagn. 2015, 35, 1218–1222. [Google Scholar] [CrossRef] [PubMed]
- Fritz, R.; Kohan-Ghadr, H.R.; Bolnick, J.M.; Bolnick, A.D.; Kilburn, B.A.; Diamond, M.P.; Drewlo, S.; Armant, D.R. Noninvasive detection of trophoblast protein signatures linked to early pregnancy loss using trophoblast retrieval and isolation from the cervix (TRIC). Fertil. Steril. 2015, 104, 339–346.e4. [Google Scholar] [CrossRef] [PubMed]
- Bulmer, J.N.; Cioni, R.; Bussani, C.; Cirigliano, V.; Sole, F.; Costa, C.; Garcia, P.; Adinolfi, M. HLA-G positive trophoblastic cells in transcervical samples and their isolation and analysis by laser microdissection and QF-PCR. Prenat. Diagn. 2003, 23, 34–39. [Google Scholar] [CrossRef] [PubMed]
- Imudia, A.N.; Kumar, S.; Diamond, M.P.; DeCherney, A.H.; Armant, D.R. Transcervical retrieval of fetal cells in the practice of modern medicine: A review of the current literature and future direction. Fertil. Steril. 2010, 93, 1725–1730. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.Y. Morphological Study of the Mouse Inner Ear. Korean J. Otorhinolaryngol. Head Neck Surg. 2011, 54, 445–453. [Google Scholar] [CrossRef]
| Sample ID | Maternal Age | Mean ± SD | p-Value | GA (Days) | Mean ± SD | p-Value | Gravidity/Parity | BMI (kg/m2) | |
|---|---|---|---|---|---|---|---|---|---|
| Pre-Fixation (ThinPrep) | 1 | 35 | 32.9 ± 4.1 | 0.356 | 119 | 71.6 ± 26.3 (63.0) | 0.260 | 2/1 | 22.6 |
| 2 | 27 | 87 | 1/0 | 15.9 | |||||
| 3 | 36 | 51 | 3/2 | 21.7 | |||||
| 4 | 32 | 50 | 2/1 | 22.2 | |||||
| 5 | 29 | 58 | 1/0 | 19.8 | |||||
| 6 | 36 | 112 | 2/1 | 20.4 | |||||
| 7 | 35 | 54 | 2/1 | 24.6 | |||||
| 8 | 36 | 45 | 1/0 | 23.0 | |||||
| 9 | 37 | 68 | 1/0 | 23.5 | |||||
| 10 | 26 | 72 | 1/0 | 20.7 | |||||
| Post-Fixation (Formalin) | 1 | 34 | 34.9 ± 5.4 | 77 | 85.2 ± 26.0 (89.3) | 1/0 | 20.0 | ||
| 2 | 35 | 54 | 2/1 | 25.9 | |||||
| 3 | 41 | 47 | 2/1 | 25.8 | |||||
| 4 | 31 | 55 | 1/0 | 17.4 | |||||
| 5 | 25 | 86 | 1/0 | 21.1 | |||||
| 6 | 40 | 112 | 1/0 | 17.5 | |||||
| 7 | 30 | 96 | 2/0 | 26.9 | |||||
| 8 | 38 | 112 | 3/2 | 20.7 | |||||
| 9 | 41 | 117 | 1/0 | 23.7 | |||||
| 10 | 34 | 96 | 1/0 | 19.6 |
| Sample ID | GA (Days) | Number. of Cells | β-hCG Detection | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total Endocervical Cells | Fixed Cells | Mean | p-Value | HLA-G-Positive Cells | Mean | p-Value | Positive (%) | Mean ± SD | p-Value | |||
| Pre- Fixation (ThinPrep) | 1 | 119 | 4.60 × 105 | 2.0 × 105 | 2.38 × 105 | 0.64 | 1000 | 2672.2 | 0.043 | 78.0 | 66.4 ± 13.3 | 0.003 |
| 2 | 87 | 5.35 × 105 | 2.0 × 105 | 4250 | 76.1 | |||||||
| 3 | 51 | 4.60 × 105 | 2.0 × 105 | 2550 | 73.9 | |||||||
| 4 | 50 | 2.58 × 106 | 2.0 × 105 | 867 | 78.4 | |||||||
| 5 | 58 | 3.25 × 105 | 5.1 × 105 | 1000 | 78.0 | |||||||
| 6 | 112 | 5.70 × 105 | 2.0 × 105 | 2333 | 71.7 | |||||||
| 7 | 54 | 2.40 × 105 | 2.0 × 105 | 6500 | 45.9 | |||||||
| 8 | 45 | 5.89 × 105 | 2.0 × 105 | 5750 | 46.7 | |||||||
| 9 | 68 | 1.37 × 106 | 2.3 × 105 | 1250 | 53.8 | |||||||
| 10 | 72 | 7.60 × 105 | 2.4 × 105 | 1222 | 61.3 | |||||||
| Post- Fixation (Formalin) | 1 | 77 | 1.60 × 106 | 3.2 × 105 | 2.67 × 105 | 575 | 1141.1 | 96.0 | 83.2 ± 8.1 | |||
| 2 | 54 | 3.48 × 106 | 3.4 × 105 | 2250 | 88.0 | |||||||
| 3 | 47 | 1.40 × 105 | 1.4 × 105 | 100 | 84.0 | |||||||
| 4 | 55 | 3.40 × 105 | 2.0 × 105 | 625 | 84.6 | |||||||
| 5 | 86 | 8.70 × 105 | 1.6 × 105 | 375 | 86.7 | |||||||
| 6 | 112 | 2.48 × 106 | 1.8 × 105 | 1277 | 88.9 | |||||||
| 7 | 96 | 7.10 × 105 | 2.0 × 105 | 1889 | 78.8 | |||||||
| 8 | 112 | 5.30 × 105 | 7.1 × 105 | 900 | 81.5 | |||||||
| 9 | 117 | 4.20 × 105 | 2.2 × 105 | 1670 | 65.7 | |||||||
| 10 | 96 | 4.50 × 105 | 2.0 × 105 | 1750 | 78.0 | |||||||
| Sample ID | GA (Days) | Karyotype (Genetic Test) | Karyotype (FISH) | Fetal Sex (after Delivery) | Number. of Cells (XY/Total) | Y Detection | |||
|---|---|---|---|---|---|---|---|---|---|
| Rate (%) | Mean ± SD | p-Value | |||||||
| Pre- Fixation (ThinPrep) | 1 | 119 | XX | XX | Female | 11.1 ± 2.1 | 0.001 | ||
| 2 | 87 | XY | XY | Male | 3/35 | 8.6 | |||
| 3 | 51 | XX | XX | Female | |||||
| 4 | 50 | XY | XY | Male | 7/52 | 13.5 | |||
| 5 | 58 | XY | XY | Male | 5/47 | 10.6 | |||
| 6 | 112 | XX | XX | Female | |||||
| 7 | 54 | XX | XX | Female | |||||
| 8 | 45 | XX | XX | Female | |||||
| 9 | 68 | XX | XX | Female | |||||
| 10 | 72 | XY | XY | Male | 6/51 | 11.8 | |||
| Post- Fixation (Formalin) | 1 | 77 | XY | XY | Male | 15/51 | 29.4 | 23.8 ± 4.8 | |
| 2 | 54 | XY | XY | Male | 12/52 | 17.8 | |||
| 3 | 47 | XX | XX | Female | |||||
| 4 | 55 | XX | XX | Female | |||||
| 5 | 86 | XX | XX | Female | |||||
| 6 | 112 | XY | XY | Male | 13/45 | 28.9 | |||
| 7 | 96 | XX | XX | Female | |||||
| 8 | 112 | XY | XY | Male | 12/50 | 24.0 | |||
| 9 | 117 | XY | XY | Male | 8/30 | 23.3 | |||
| 10 | 96 | XY | XY | Male | 12/62 | 19.4 | |||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, M.J.; Kim, S.H.; Shim, S.H.; Jang, H.Y.; Park, H.J.; Cha, D.H. Optimization Protocol of Fixation Method for Trophoblast Retrieval from the Cervix (TRIC): A Preliminary Study. Diagnostics 2020, 10, 300. https://doi.org/10.3390/diagnostics10050300
Lee MJ, Kim SH, Shim SH, Jang HY, Park HJ, Cha DH. Optimization Protocol of Fixation Method for Trophoblast Retrieval from the Cervix (TRIC): A Preliminary Study. Diagnostics. 2020; 10(5):300. https://doi.org/10.3390/diagnostics10050300
Chicago/Turabian StyleLee, Min Jin, Soo Hyun Kim, Sung Han Shim, Hee Yeon Jang, Hee Jin Park, and Dong Hyun Cha. 2020. "Optimization Protocol of Fixation Method for Trophoblast Retrieval from the Cervix (TRIC): A Preliminary Study" Diagnostics 10, no. 5: 300. https://doi.org/10.3390/diagnostics10050300
APA StyleLee, M. J., Kim, S. H., Shim, S. H., Jang, H. Y., Park, H. J., & Cha, D. H. (2020). Optimization Protocol of Fixation Method for Trophoblast Retrieval from the Cervix (TRIC): A Preliminary Study. Diagnostics, 10(5), 300. https://doi.org/10.3390/diagnostics10050300





