Serological Diagnosis of Flavivirus-Associated Human Infections
Abstract
1. Introduction
2. Laboratory Diagnosis of Flavivirus-Associated Human Diseases
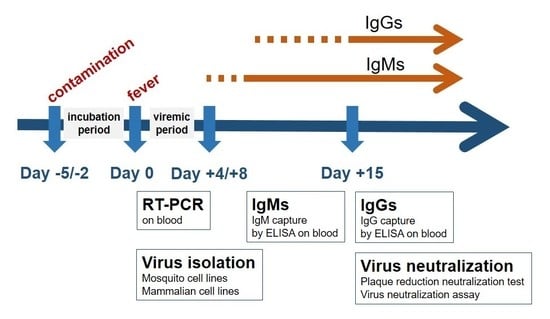
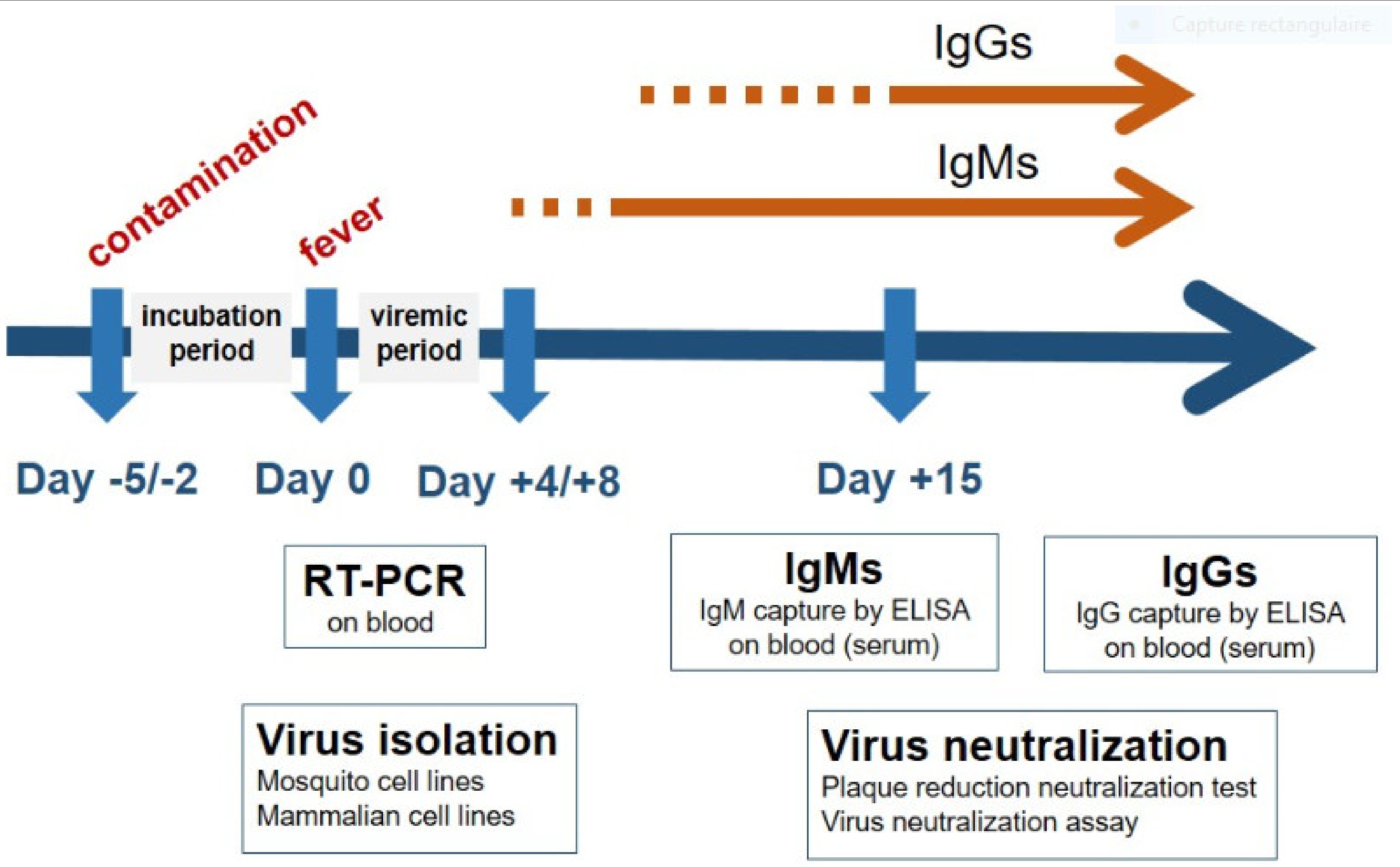
3. Early Diagnosis of Flavivirus-Associated Human Diseases
4. Detection of Flaviviruses Antibodies in Clinical Specimens
4.1. First Line Serological Assays
4.2. Confirmatory Serological Assays
4.3. Innovative Serological Assays
4.4. Serosurvey Studies
5. Challenges in the Serological Diagnosis of Human Flavivirus Infections
5.1. Access to Laboratory Tools
5.2. Co-Circulation of Flaviviruses
5.3. Prevalence of Flaviviruses
5.4. Determination of the Onset of Symptoms
5.5. Patient’s Past Exposure to Flaviviruses
5.6. Interpretation of Serological Results in Endemic Areas and in Travelers Returning from Endemic Areas
6. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Gubler, D.J. The global emergence/resurgence of arboviral diseases as public health problems. Arch. Med. Rest. 2002, 33, 330–342. [Google Scholar] [CrossRef]
- Calisher, C.H.; Gould, E.A. Taxonomy of the virus family Flaviviridae. Adv. Virus Res. 2003, 59, 1–19. [Google Scholar] [PubMed]
- Gubbler, D.J. Dengue and dengue hemorrhagic fever: Its history and resurgence as a global public health problem. In Dengue Dengue Hemorrhagic Fever; Gubler, D.J., Kuno, G., Eds.; CAB: Wallingford, UK, 1997; pp. 1–22. [Google Scholar]
- Gubler, D.J. The changing epidemiology of yellow fever and dengue, 1900 to 2003: Full circle? Comp. Immunol. Microbiol. Infect. Dis. 2004, 5, 319–330. [Google Scholar] [CrossRef] [PubMed]
- Roehrig, J.T. West nile virus in the United States-a historical perspective. Viruses 2013, 5, 3088–3108. [Google Scholar] [CrossRef]
- Petersen, L.R.; Brault, A.C.; Nasci, R.S. West Nile virus: Review of the literature. JAMA 2013, 310, 308–315. [Google Scholar] [CrossRef]
- Musso, D.; Ko, A.I.; Baud, D. Zika Virus Infection: After the Pandemic. N Engl. J. Med. 2019, 381, 1444–1457. [Google Scholar] [CrossRef]
- Musso, D.; Gubler, D.J. Zika virus. Clin. Microbiol. Rev. 2016, 29, 487–524. [Google Scholar] [CrossRef]
- Misra, U.K.; Kalita, J. Overview: Japanese encephalitis. Prog. Neurobiol. 2010, 91, 108–120. [Google Scholar] [CrossRef]
- Reusken, C.; Boonstra, M.; Rugebregt, S.; Scherbeijn, S.; Chandler, F.; Avšič-Županc, T.; Vapalahti, O.; Koopmans, M.; GeurtsvanKessel, C.H. An evaluation of serological methods to diagnose tick-borne encephalitis from serum and cerebrospinal fluid. J. Clin. Virol. 2019, 120, 78–83. [Google Scholar] [CrossRef]
- Musso, D.; Rodriguez-Morales, A.J.; Levi, J.E.; Cao-Lormeau, V.-M.; Gubler, D.J. Unexpected outbreaks of arboviruses infections: Lessons learned from the Pacific and tropical America. Lancet Infect. Dis. 2018, 18, e355–e361. [Google Scholar] [CrossRef]
- Charrel, R.N. Diagnosis of arboviral infections–A quagmire of cross reactions and complexities. Travel Med. Infect. Dis. 2016, 14, 11–12. [Google Scholar] [CrossRef] [PubMed]
- Nash, D.; Mostashari, F.; Fine, A.; Miller, J.; O’Leary, D.; Murray, K.; Huang, A.; Rosenberg, A.; Greenberg, A.; Sherman, M.; et al. The outbreak of West Nile virus infection in the New York City area in 1999. N. Engl. J. Med. 2001, 344, 1807–1814. [Google Scholar] [CrossRef] [PubMed]
- Tsai, T.F.; Popovici, F.; Cernescu, C.; Campbell, G.L.; Nedelcu, N.I. West Nile encephalitis epidemic in southeastern Romania. Lancet 1998, 352, 767–771. [Google Scholar] [CrossRef]
- European Centre for Disease Prevention and Control (ECDC). Local transmission of dengue fever in France and Spain. 2018. Available online: https://www.ecdc.europa.eu/en/publications-data/rapid-risk-assessment-local-transmission-dengue-fever-france-and-spain (accessed on 7 May 2020).
- Khawar, W.; Bromberg, R.; Moor, M.; Lyubynska, N.; Mahmoudi, H. Seven Cases of Zika Virus Infection in South Florida. Cureus 2017, 9, e1099. [Google Scholar] [CrossRef]
- Giron, S.; Franke, F.; Decoppet, A.; Cadiou, B.; Travaglini, T.; Thirion, L.; Durand, G.; Jeannin, C.; L’Ambert, G.; Grard, G.; et al. Vector-borne transmission of Zika virus in Europe, southern France, August 2019. Eurosurveillance 2019, 24, 1900655. [Google Scholar] [CrossRef]
- Moreira, J.; Peixoto, T.M.; Machado de Siqueira, A.; Lamas, C.C. Sexually acquired Zika virus: A systematic review. Clin. Microbiol. Infect. 2017, 23, 296–305. [Google Scholar] [CrossRef]
- Musso, D.; Stramer, S.L. International Society of Blood Transfusion Working Party on Transfusion-Transmitted Infectious Diseases. Zika virus: A new challenge for blood transfusion. Lancet 2016, 387, 1993–1994. [Google Scholar] [CrossRef]
- Busch, M.P.; Caglioti, S.; Robertson, E.F.; McAuley, J.D.; Tobler, L.H.; Kamel, H.; Linnen, J.M.; Shyamala, V.; Tomasulo, P.; Kleinman, S.H. Screening the blood supply for West Nile virus RNA by nucleic acid amplification testing. N. Engl. J. Med. 2005, 353, 460–467. [Google Scholar] [CrossRef]
- Levi, J.E. Dengue Virus and Blood Transfusion. J. Infect. Dis. 2016, 213, 689–690. [Google Scholar] [CrossRef]
- Center for disease control and prevention. Updated Guidance for U.S. Laboratories Testing for Zika Virus Infection. Center for disease control and prevention 2016. Available online: https://www.cdc.gov/zika/pdfs/laboratory-guidance-zika.pdf (accessed on 7 May 2020).
- Rathore, A.P.S.; St John, A.L. Cross-Reactive Immunity Among Flaviviruses. Front. Immunol. 2020, 11, 334. [Google Scholar] [CrossRef]
- Rabe, I.B.; Staples, J.E.; Villanueva, J.; Hummel, K.B.; Johnson, J.A.; Rose, L.; Hills, S.; Wasley, A.; Fischer, M.; Powers, A.M. Interim guidance for interpretation of Zika virus antibody test results. MMWR Morb. Mortal. Wkly. Rep. 2016, 21, 543–546. [Google Scholar] [CrossRef] [PubMed]
- Sambri, V.; Capobianchi, M.R.; Cavrini, F.; Charrel, R.; Donoso-Mantke, O.; Escadafal, C.; Franco, L.; Gaibani, P.; Gould, E.A.; Niedrig, M.; et al. Diagnosis of west nile virus human infections: Overview and proposal of diagnostic protocols considering the results of external quality assessment studies. Viruses 2013, 5, 2329–2348. [Google Scholar] [CrossRef] [PubMed]
- Johnson, B.W.; Goodman, C.H.; Jee, Y.; Featherstone, D.A. Differential Diagnosis of Japanese Encephalitis Virus Infections with the Inbios JE Detect TM and DEN Detect TM MAC-ELISA Kits. Am. J. Trop. Med. Hyg. 2016, 94, 820–828. [Google Scholar] [CrossRef] [PubMed]
- Allwinn, R.; Doerr, H.W.; Emmerich, P.; Schmitz, H.; Preiser, W. Cross-reactivity in flavivirus serology: New implications of an old finding? Med. Microbiol. Immunol. 2002, 190, 199–202. [Google Scholar] [CrossRef]
- Mansfield, K.L.; Horton, D.L.; Johnson, N.; Li, L.; Barrett, A.D.; Smith, D.J.; Solomom, T.; Fooks, A.R. Flavivirus-induced antibody cross-reactivity. J. Gen. Virol. 2011, 92, 2821–2829. [Google Scholar] [CrossRef]
- Papa, A.; Karabaxoglou, D.; Kansouzidou, A. Acute West Nile virus neuroinvasive infections: Cross-reactivity with dengue virus and tick-borne encephalitis virus. J. Med. Virol. 2011, 83, 1861–1865. [Google Scholar] [CrossRef]
- De Campos, M.R.; Cirne-Santos, C.; Meira, G.L.S.; Santos, L.L.; de Meneses, M.D.; Friedrich, J.; Jansen, S.; Ribeiro, M.S.; da Cruz, I.C.; Schmidt-Chanasit, J.; et al. Prolonged detection of Zika virus RNA in urine samples during the ongoing Zika virus epidemic in Brazil. J. Clin. Virol. 2016, 77, 69–70. [Google Scholar] [CrossRef]
- Gourinat, A.C.; O’Connor, O.; Calvez, E.; Goarant, C.; Dupont-Rouzeyrol, M. Detection of Zika virus in urine. Emerg. Infect. Dis. 2015, 21, 84–86. [Google Scholar] [CrossRef]
- Barzon, L.; Pacenti, M.; Franchin, E.; Pagni, S.; Martello, T.; Cattai, M.; Cusinato, R.; Palu, G. Excretion of West Nile virus in urine during acute infection. J. Infect. Dis. 2013, 208, 1086–1092. [Google Scholar] [CrossRef]
- Mead, P.S.; Duggal, N.K.; Hook, S.A.; Delorey, M.; Fischer, M.; Olzenak McGuire, D.; Becksted, H.; Max, R.J.; Anishchenko, M.; Schwartz, A.M.; et al. Zika Virus Shedding in Semen of Symptomatic Infected Men. N Engl. J. Med. 2018, 378, 1377–1385. [Google Scholar] [CrossRef]
- Musso, D.; Rouault, E.; Teissier, A.; Lanteri, M.; Zisou, K.; Broult, J.; Grange, E.; Nhan, T.-X.; Aubry, M. Molecular detection of Zika virus in blood and RNA load determination during the French Polynesian outbreak. J. Med. Virol. 2016, 89, 1505–1510. [Google Scholar] [CrossRef] [PubMed]
- Lanteri, M.C.; Lee, T.-H.; Wen, L.; Kaidarova, Z.; Bravo, M.D.; Kiely, N.E.; Kamel, H.T.; Tobler, L.H.; Norris, P.J.; Busch, M.P. West Nile virus nucleic acid persistence in whole blood months after clearance in plasma: Implication for transfusion and transplantation safety. Transfusion 2014, 54, 3232–3241. [Google Scholar] [CrossRef] [PubMed]
- Murray, K.O.; Gorchakov, R.; Carlson, A.R.; Berry, R.; Lai, L.; Natrajan, M.; Garcia, M.N.; Correa, A.; Patel, S.M.; Aagaard, K.; et al. Prolonged Detection of Zika Virus in Vaginal Secretions and Whole Blood. Emerg. Infect. Dis. 2017, 23, 99–101. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Dengue guidelines for diagnosis, treatment, prevention and control. World Heal Organization 2009. Available online: https://apps.who.int/iris/bitstream/handle/10665/44188/9789241547871_eng.pdf?sequence=1&isAllowed=y (accessed on 7 May 2020).
- White, L.A. Susceptibility of Aedes albopictus C6/36 cells to viral infection. J. Clin. Microbiol. 1987, 25, 1221–1224. [Google Scholar] [CrossRef]
- Lukman, N.; Salim, G.; Kosasih, H.; Susanto, N.H.; Parwati, I.; Fitri, S.; Alisjahbana, B.; Widjaja, S.; Williams, M. Comparison of the Hemagglutination Inhibition Test and IgG ELISA in Categorizing Primary and Secondary Dengue Infections Based on the Plaque Reduction Neutralization Test. Biomed Res. Int. 2016, 2016, 5253842. [Google Scholar] [CrossRef]
- Pavri, K.M.; Ghosh, S.N. Complement-fixation tests for simultaneous isolation and identification of Dengue viruses, using tissue cultures. Bull. World Health Organ. 1969, 40, 984–986. [Google Scholar] [PubMed]
- Buonora, S.N.; Passos, S.R.L.; do Carmo, C.N.; Quintela, F.M.; de Oliveira, D.N.R.; dos Santos, F.B.; Hokerberg, Y.H.M.; Nogueira, R.M.R.; Daumas, R.P. Accuracy of clinical criteria and an immunochromatographic strip test for dengue diagnosis in a DENV-4 epidemic. BMC Infect. Dis. 2016, 16, 37. [Google Scholar] [CrossRef]
- Niedrig, M.; Kursteiner, O.; Herzog, C.; Sonnenberg, K. Evaluation of an Indirect Immunofluorescence Assay for Detection of Immunoglobulin M (IgM) and IgG Antibodies against Yellow Fever Virus. Clin. Vaccine Immunol. 2008, 15, 177–181. [Google Scholar] [CrossRef]
- Welch, R.J.; Chang, G.-J.J.; Litwin, C.M. Comparison of a commercial dengue IgM capture ELISA with dengue antigen focus reduction microneutralization test and the centers for disease control dengue IgM capture-ELISA. J. Virol. Methods 2014, 195, 247–249. [Google Scholar] [CrossRef]
- Houghton-Triviño, N.; Montaña, D.; Castellanos, J. Dengue-yellow fever sera cross-reactivity; challenges for diagnosis. Rev. Salud. Publica. (Bogota) 2018, 10, 299–307. [Google Scholar] [CrossRef]
- Souza, N.C.S.; e Félix, A.C.; de Paula, A.V.; Levi, J.E.; Pannuti, C.S.; Romano, C.M. Evaluation of serological cross-reactivity between yellow fever and other flaviviruses. Int. J. Infect. Dis. 2019, 81, 4–5. [Google Scholar] [CrossRef] [PubMed]
- Henss, L.; Yue, C.; Kandler, J.; Faddy, H.M.; Simmons, G.; Panning, M.; Lewis-Ximenez, L.L.; Baylis, S.A.; Schnierle, B.S. Establishment of an Alphavirus-Specific Neutralization Assay to Distinguish Infections with Different Members of the Semliki Forest Complex. Viruses 2019, 11, 82. [Google Scholar] [CrossRef] [PubMed]
- Hassing, R.J.; Leparc-Goffart, I.; Tolou, H.; van Doornum, G.; van Genderen, P.J. Cross-reactivity of antibodies to viruses belonging to the Semliki forest serocomplex. Eurosurveillance 2010, 15, 19588. [Google Scholar] [PubMed]
- Yan, G.; Lee, C.K.; Lam, L.T.M.; Yan, B.; Chua, Y.X.; Lim, A.Y.N.; Phang, K.F.; Kew, G.S.; Teng, H.; Ngai, C.H.; et al. Covert COVID-19 and false-positive dengue serology in Singapore. Lancet Infect. Dis. 2020. [Google Scholar] [CrossRef]
- Sanchini, A.; Donoso-Mantke, O.; Papa, A.; Sambri, V.; Teichmann, A.; Niedrig, M. Second International Diagnostic Accuracy Study for the Serological Detection of West Nile Virus Infection. PLoS Negl. Trop. Dis. 2013, 7, e2184. [Google Scholar] [CrossRef] [PubMed]
- Domingo, C.; Alves, M.J.; de Ory, F.; Teichmann, A.; Schmita, H.; Muller, R.; Niedrig, M. International external quality control assessment for the serological diagnosis of dengue infections. BMC Infect. Dis. 2015, 15, 167. [Google Scholar] [CrossRef]
- Niedrig, M.; Avšič, T.; Aberle, S.W.; Ferenczi, E.; Labuda, M.; Rozentale, B.; Mantke, D. Quality control assessment for the serological diagnosis of tick borne encephalitis virus infections. J. Clin. Virol. 2007, 38, 260–264. [Google Scholar] [CrossRef]
- Gubler, D.J. Dengue and dengue hemorrhagic fever. Clin. Microbiol. Rev. 1998, 11, 480–496. [Google Scholar] [CrossRef]
- Halstead, S.B.; O’Rourke, E.J. Antibody-enhanced dengue virus infection in primate leukocytes. Nature 1977, 265, 739–741. [Google Scholar] [CrossRef]
- Roehrig, J.T.; Hombach, J.; Barrett, A.D.T. Guidelines for Plaque-Reduction Neutralization Testing of Human Antibodies to Dengue Viruses. Viral. Immunol. 2008, 21, 123–132. [Google Scholar] [CrossRef]
- Change for Centers for Disease Control and Prevention. National Institutes of Health. Biosafety in microbiological and biomedical laboratories, 5th ed. 2009. Available online: https://www.cdc.gov/labs/pdf/CDC-BiosafetyMicrobiologicalBiomedicalLaboratories-2009-P.PDF (accessed on 7 May 2020).
- Ward, M.; Alger, J.; Berrueta, M.; Bock, H.; Buekens, P.; Cafferata, M.L.; Ciganda, A.; Garcia, J.; Garcia, K.; Lopez, E.; et al. Zika Virus and the World Health Organization Criteria for Determining Recent Infection Using Plaque Reduction Neutralization Testing. Am. J. Trop. Med. Hyg. 2018, 99, 780–782. [Google Scholar] [CrossRef] [PubMed]
- Thomas, S.J.; Nisalak, A.; Anderson, K.B.; Libraty, D.H.; Kalayanarooj, S.; Vaughn, D.W.; Putnak, R.; Gibbons, R.V.; Jarman, R.; Endy, T.P. Dengue plaque reduction neutralization test (PRNT) in primary and secondary dengue virus infections: How alterations in assay conditions impact performance. Am. J. Trop. Med. Hyg. 2009, 81, 825–833. [Google Scholar] [CrossRef] [PubMed]
- Shan, C.; Xie, X.; Ren, P.; Loeffelholz, M.J.; Yang, Y.; Furuya, A.; Dupuis, A.P.; Kramer, L.D.; Wong, S.J.; Shi, P.-Y. A Rapid Zika Diagnostic Assay to Measure Neutralizing Antibodies in Patients. EBioMedicine 2017, 17, 157–162. [Google Scholar] [CrossRef] [PubMed]
- Frumence, E.; Viranaicken, W.; Gadea, G.; Desprès, P. A GFP Reporter MR766-Based Flow Cytometry Neutralization Test for Rapid Detection of Zika Virus-Neutralizing Antibodies in Serum Specimens. Vaccines 2019, 7, 66. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, M.; Yamanaka, A.; Yato, K.; Yoshii, K.; Watashi, K.; Aizaki, H.; Konishi, E.; Takasaki, T.; Kato, T.; Muramatsu, M.; et al. High-throughput neutralization assay for multiple flaviviruses based on single-round infectious particles using dengue virus type 1 reporter replicon. Sci. Rep. 2018, 8, 16624. [Google Scholar] [CrossRef] [PubMed]
- Koishi, A.C.; Suzukawa, A.A.; Zanluca, C.; Camacho, D.E.; Comach, G.; Duarte Dos Santos, C.N. Development and evaluation of a novel high-throughput image-based fluorescent neutralization test for detection of Zika virus infection. Singh SK, editor. PLoS Negl. Trop. Dis. 2018, 12, e0006342. [Google Scholar] [CrossRef]
- Kraus, A.A.; Messer, W.; Haymore, L.B.; de Silva, A.M. Comparison of plaque- and flow cytometry-based methods for measuring dengue virus neutralization. J. Clin. Microbiol. 2007, 45, 3777–3780. [Google Scholar] [CrossRef]
- De Alwis, R.; de Silva, A.M. Measuring antibody neutralization of dengue virus (DENV) using a flow cytometry-based technique. Methods Mol. Biol. 2014, 1138, 27–39. [Google Scholar]
- Lin, R.; Heeke, D.; Liu, H.; Rao, E.; Marshall, J.D.; Chio, V.; Cataniag, F.; Yu, L.; Zuo, F.; McCarthy, M.P. Development of a robust, higher throughput green fluorescent protein (GFP)-based Epstein-Barr Virus (EBV) micro-neutralization assay. J. Virol. Methods 2017, 247, 15–21. [Google Scholar] [CrossRef]
- Mishra, N.; Caciula, A.; Price, A.; Thakkar, R.; Ng, J.; Chauhan, L.V.; Jain, K.; Che, X.; Espinose, D.A.; Cruz, M.M.; et al. Diagnosis of Zika Virus Infection by Peptide Array and Enzyme-Linked Immunosorbent Assay. MBio 2018, 9, e00095. [Google Scholar] [CrossRef]
- Aubry, M.; Finke, J.; Teissier, A.; Roche, C.; Broult, J.; Paulous, S.; Despres, P.; Cao-Lormeau, V.-M.; Musso, D. Seroprevalence of arboviruses among blood donors in French Polynesia, 2011–2013. Int. J. Infect. Dis. 2015, 41, 11–12. [Google Scholar] [CrossRef] [PubMed]
- Aubry, M.; Teissier, A.; Huart, M.; Merceron, S.; Vanhomwegen, J.; Roche, C.; Vial, A.-L.; Teururai, S.; Sicard, S.; Paulous, S.; et al. Zika virus seroprevalence, French Polynesia, 2014–2015. Emerg. Infect. Dis. 2017, 23, 669–672. [Google Scholar] [CrossRef] [PubMed]
- Aubry, M.; Teissier, A.; Huart, M.; Merceron, S.; Vanhomwegen, J.; Roche, C.; Vial, A.-L.; Teururai, S.; Sicard, S.; Paulous, S.; et al. Ross River virus Seroprevalence, French Polynesia, 2014–2015. Emerg. Infect. 2017, 23, 1751–1753. [Google Scholar] [CrossRef] [PubMed]
- Lau, C.; Aubry, M.; Musso, D.; Teissier, A.; Paulous, S.; Desprès, P.; de-Lamballerie, X.; Pastorino, B.; Cao-Lormeau, V.-M.; Weinstein, P. New evidence for endemic circulation of Ross River virus in the Pacific Islands and the potential for emergence. Int. J. Infect. Dis. 2017, 57, 73–76. [Google Scholar] [CrossRef]
- Cao-Lormeau, V.-M. Tropical Islands as New Hubs for Emerging Arboviruses. Emerg. Infect. Dis. 2016, 22, 913–915. [Google Scholar] [CrossRef]
- Venkateswaran, K.N.; Sarwar, J.; Parameswaran, N.; Krishnan, K.; Nelson, W.M. Validation of a fluorescent microsphere multiplex serology assay for differential diagnosis of exposure to Zika virus and other closely related arboviruses. J. Immunol. 2018, 200, 25. [Google Scholar]
- Venkateswaran, N.; Sarwar, J.; Parameswaran, N.; Fecteau, T.; O’Connor, D.; Nelson, W.M.; Venkateswaran, K. Development and testing of a novel multiplex serodiagnostic assay for Zika and other arboviruses. J. Immunol. 2017, 198, 81.26. [Google Scholar]
- Basile, A.J.; Horiuchi, K.; Panella, A.J.; Laven, J.; Kosoy, O.; Lanciotti, R.S.; Venkateswaran, N.; Biggerstaff, G.J. Multiplex Microsphere Immunoassays for the Detection of IgM and IgG to Arboviral Diseases. PLoS ONE 2013, 8, e75670. [Google Scholar] [CrossRef]
- Tyson, J.; Tsai, W.-Y.; Tsai, J.-J.; Massgard, L.; Stramer, S.L.; Lehrer, A.T.; Nerurkar, V.R.; Wang, W.-K. A high-throughput and multiplex microsphere immunoassay based on non-structural protein 1 can discriminate three flavivirus infections. PLoS Negl. Trop. Dis. 2019, 13, e0007649. [Google Scholar] [CrossRef]
- Fritzell, C.; Rousset, D.; Adde, A.; Kazanji, M.; Van Kerkhove, M.D.; Flamand, C. Current challenges and implications for dengue, chikungunya and Zika seroprevalence studies worldwide: A scoping review. PLoS Negl. Trop. Dis. 2018, 12, e0006533. [Google Scholar] [CrossRef]
- The Academy of Medical Sciences. Improving the development and deployment of rapid diagnostic tests in LMICs. Workshop report, 21 November 2016, London, United Kingdom. Available online: https://www.interacademies.org/sites/default/files/publication/improving_the_development_and_deployment_of_rapid_diagnostic_tests_in_lmics.pdf (accessed on 7 May 2020).
- Pang, J.; Chia, P.Y.; Lye, D.C.; Leo, Y.S. Progress and Challenges towards Point-of-Care Diagnostic Development for Dengue. Kraft CS, editor. J. Clin. Microbiol. 2017, 55, 3339–3349. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.K.; Alexander, N.; Di Tanna, G.L. A systematic review of the economic impact of rapid diagnostic tests for dengue. BMC Health Serv. Res. 2017, 17, 850. [Google Scholar] [CrossRef] [PubMed]
- Blacksell, S.D. Commercial Dengue Rapid Diagnostic Tests for Point-of-Care Application: Recent Evaluations and Future Needs? J. Biomed. Biotechnol. 2012, 2012, 151967. [Google Scholar] [CrossRef] [PubMed]
- Fry, S.R.; Meyer, M.; Semple, M.G.; Simmons, C.P.; Sekaran, S.D.; Huang, J.X.; McElnea, C.; Huang, C.-Y.; Valks, A.; Yong, P.R.; et al. The diagnostic sensitivity of dengue rapid test assays is significantly enhanced by using a combined antigen and antibody testing approach. PLoS Negl. Trop. Dis. 2011, 5, e1199. [Google Scholar] [CrossRef] [PubMed]
- Gubler, D.J. Resurgent vector-borne diseases as a global health problem. Emerg. Infect. Dis. 1988, 4, 442–450. [Google Scholar] [CrossRef]
- De Oliveira, W.K.; de França, G.V.A.; Carmo, E.H.; Duncan, B.B.; de Souza Kuchenbecker, R.; Schmidt, M.I. Infection-related microcephaly after the 2015 and 2016 Zika virus outbreaks in Brazil: A surveillance-based analysis. Lancet 2017, 390, 861–870. [Google Scholar] [CrossRef]
- Rodriguez-Morales, A.J.; Villamil-Gómez, W.E.; Franco-Paredes, C. The arboviral burden of disease caused by co-circulation and co-infection of dengue, chikungunya and Zika in the Americas. Travel Med. Infect. Dis. 2016, 14, 177–179. [Google Scholar] [CrossRef]
- Wong, S.J.; Furuya, A.; Zou, J.; Xie, X.; Dupuis, A.P., II; Kramer, L.D.; Shi, P.-Y. A Multiplex Microsphere Immunoassay for Zika Virus Diagnosis. EBioMedicine 2017, 16, 136–140. [Google Scholar] [CrossRef]
- Cao-Lormeau, V.; Blake, A.; Mons, S.; Lastère, S.; Roche, C.; Vanhomwegen, J.; Dub, T.; Baudouin, L.; Teissier, A.; Larre, P.; et al. Guillain-Barré Syndrome outbreak associated with Zika virus infection in French Polynesia: A case control study. Lancet 2016, 387, 1531–1539. [Google Scholar] [CrossRef]
- Taylor, C.T.; Mackay, I.M.; McMahon, J.L.; Wheatley, S.L.; Moore, P.R.; Finger, M.L.J.; Hewitson, G.R.; Moore, F.A. Detection of specific ZIKV IgM in travelers using a multiplexed flavivirus microsphere immunoassay. Viruses 2018, 10, 253. [Google Scholar] [CrossRef]
- Trevethan, R. Commentary: Sensitivity, Specificity, and Predictive Values: Foundations, Pliabilities, and Pitfalls in Research and Practice. Front. Public Health 2017, 5. [Google Scholar] [CrossRef] [PubMed]
- Altman, D.G.; Bland, J.M. Statistics Notes: Diagnostic tests 1: Sensitivity and specificity. BMJ 1994, 308, 1552–1552. [Google Scholar] [CrossRef] [PubMed]
- Altman, D.G.; Bland, J.M. Statistics Notes: Diagnostic tests 2: Predictive values. BMJ 1994, 309, 102. [Google Scholar] [CrossRef] [PubMed]
- Musso, D.; Bossin, H.; Mallet, H.; Besnard, M.; Broult, J.; Baudouin, L.; Levi, J.E.; Sabino, E.C.; Ghawche, F.; Lanteri, M.C.; et al. Zika Virus in French Polynesia, 2013–2014: Anatomy of a completed outbreak. Lancet Infect. Dis. 2018, 18, e172–e182. [Google Scholar] [CrossRef]
| Methods | Advantages | Critical Evaluation |
|---|---|---|
| RT-PCR |
|
|
| Virus isolation |
|
|
| Viral antigen capture |
|
|
| Serology |
|
|
| Serological Diagnostic Methods | Virus-Specific Antibody Detection | Licensed Test Systems | Automation | General Remarks on the Method |
|---|---|---|---|---|
| Immuno-fluorescence tests (IFT) | Yes | Yes | Available IFT automation | IFT are referred as a conventional serological method |
| Enzyme-linked immunosorbent assays (ELISA) | Yes | Yes | Available ELISA automation | Conventional ELISA assays detect and measure a single analyte per plate |
| Virus neutralization tests (VNT) | Yes | No | No | VNT are performed to confirm the results of conventional serological methods |
| Lateral-flow immunoassays | Yes | Yes (only for DENV) | No | Rapid diagnostic tests but sensitivity and specificity are usually lower than for other methods. |
| Multiplex immunoassay (MIA) | Yes | No | Yes (MIA is based on the flow-cytometry technology) | MIA permits the multiplexing of many different assays within a single sample |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Musso, D.; Desprès, P. Serological Diagnosis of Flavivirus-Associated Human Infections. Diagnostics 2020, 10, 302. https://doi.org/10.3390/diagnostics10050302
Musso D, Desprès P. Serological Diagnosis of Flavivirus-Associated Human Infections. Diagnostics. 2020; 10(5):302. https://doi.org/10.3390/diagnostics10050302
Chicago/Turabian StyleMusso, Didier, and Philippe Desprès. 2020. "Serological Diagnosis of Flavivirus-Associated Human Infections" Diagnostics 10, no. 5: 302. https://doi.org/10.3390/diagnostics10050302
APA StyleMusso, D., & Desprès, P. (2020). Serological Diagnosis of Flavivirus-Associated Human Infections. Diagnostics, 10(5), 302. https://doi.org/10.3390/diagnostics10050302