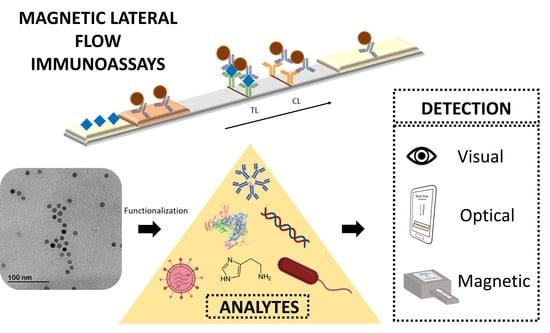
Magnetic Lateral Flow Immunoassays
Abstract
1. Introduction
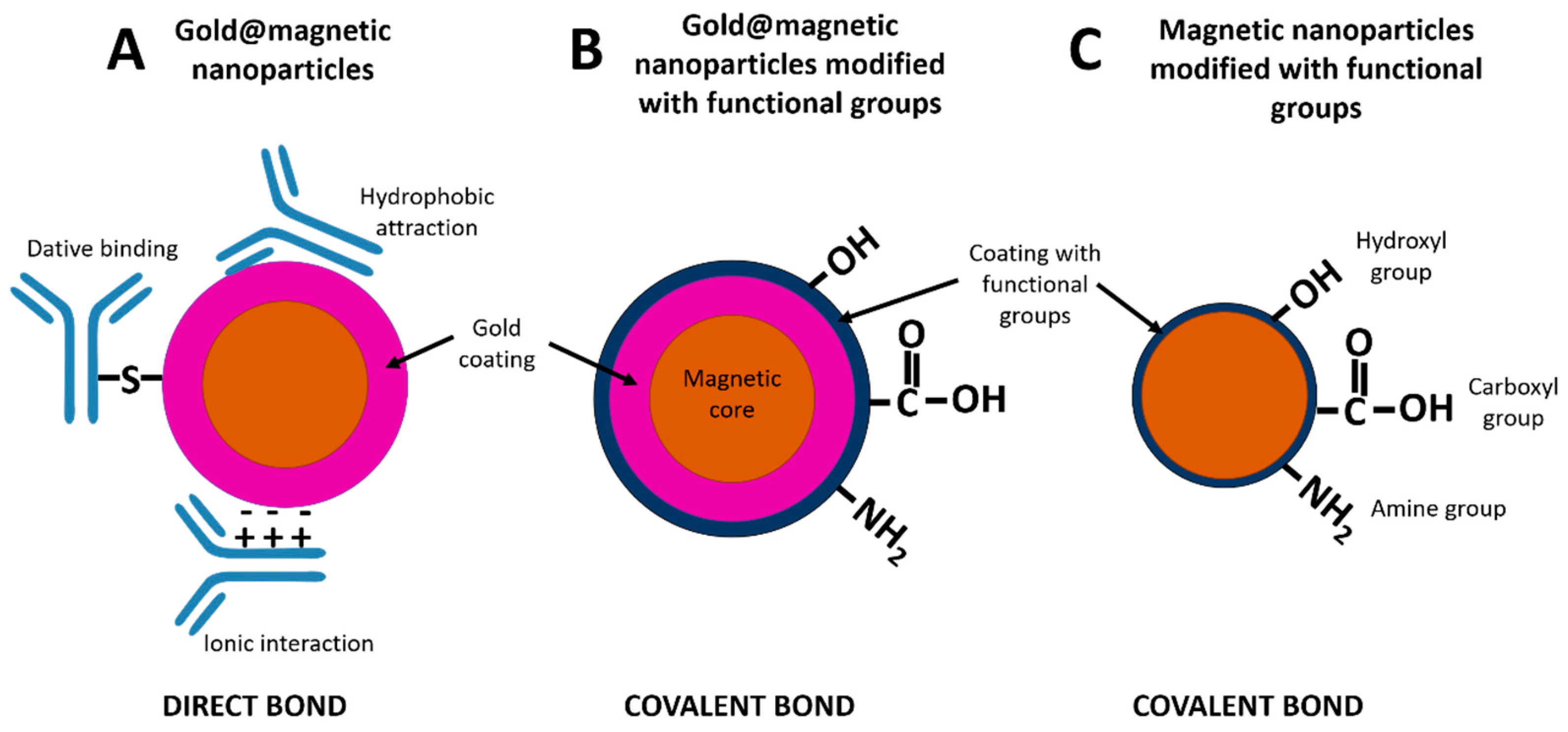
2. Magnetic Nanoparticles as Labels in LFIA
3. Detection of Magnetic Nanoparticles Used as Labels in LFIA
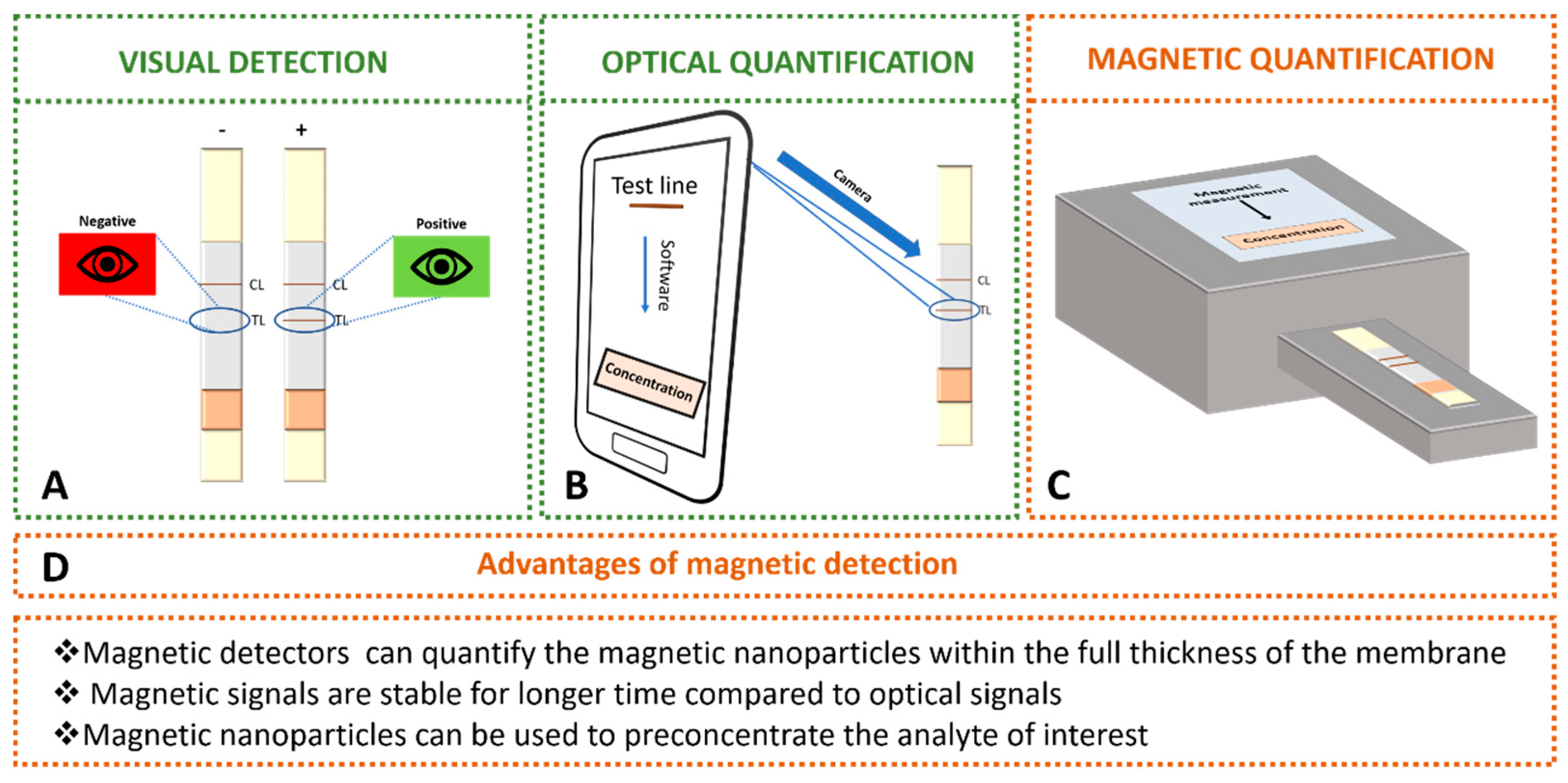
3.1. Optical Transduction
3.2. Magnetic Transduction
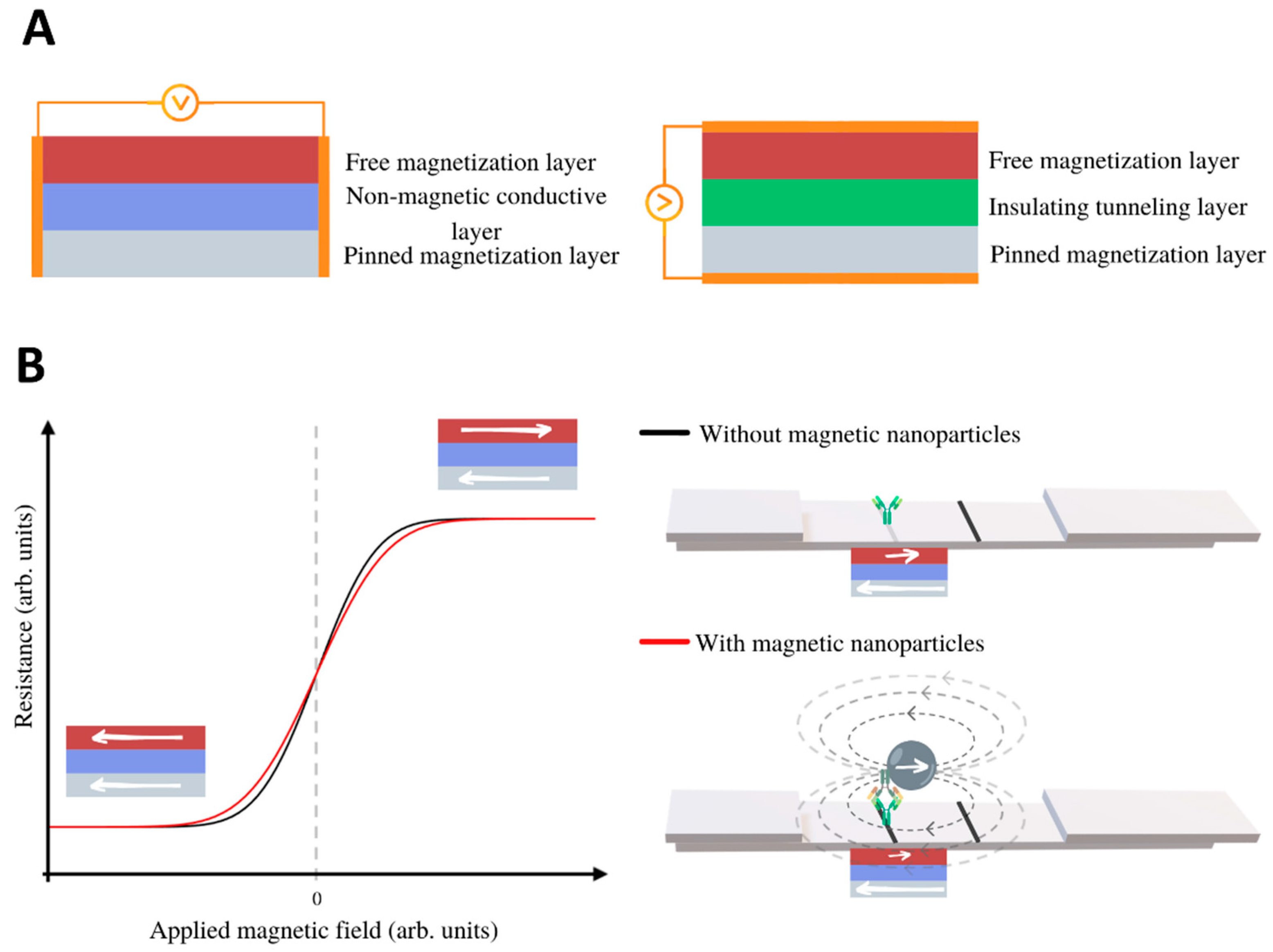
3.2.1. Magnetoresistive LFIA Readers
3.2.2. Inductive Readers
4. Fields of Application
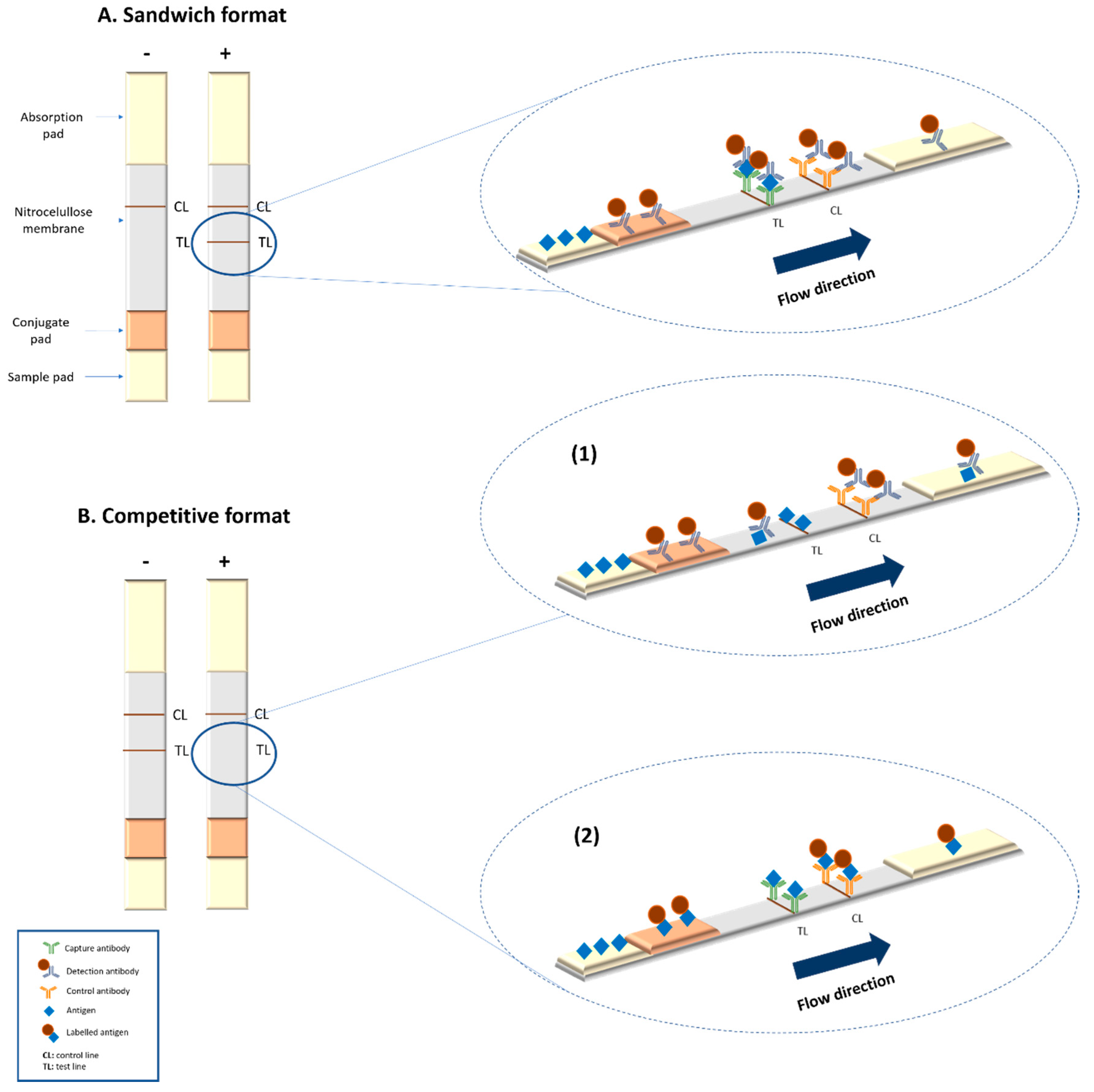
4.1. Conventional Magnetic LFIA for Analyte Detection
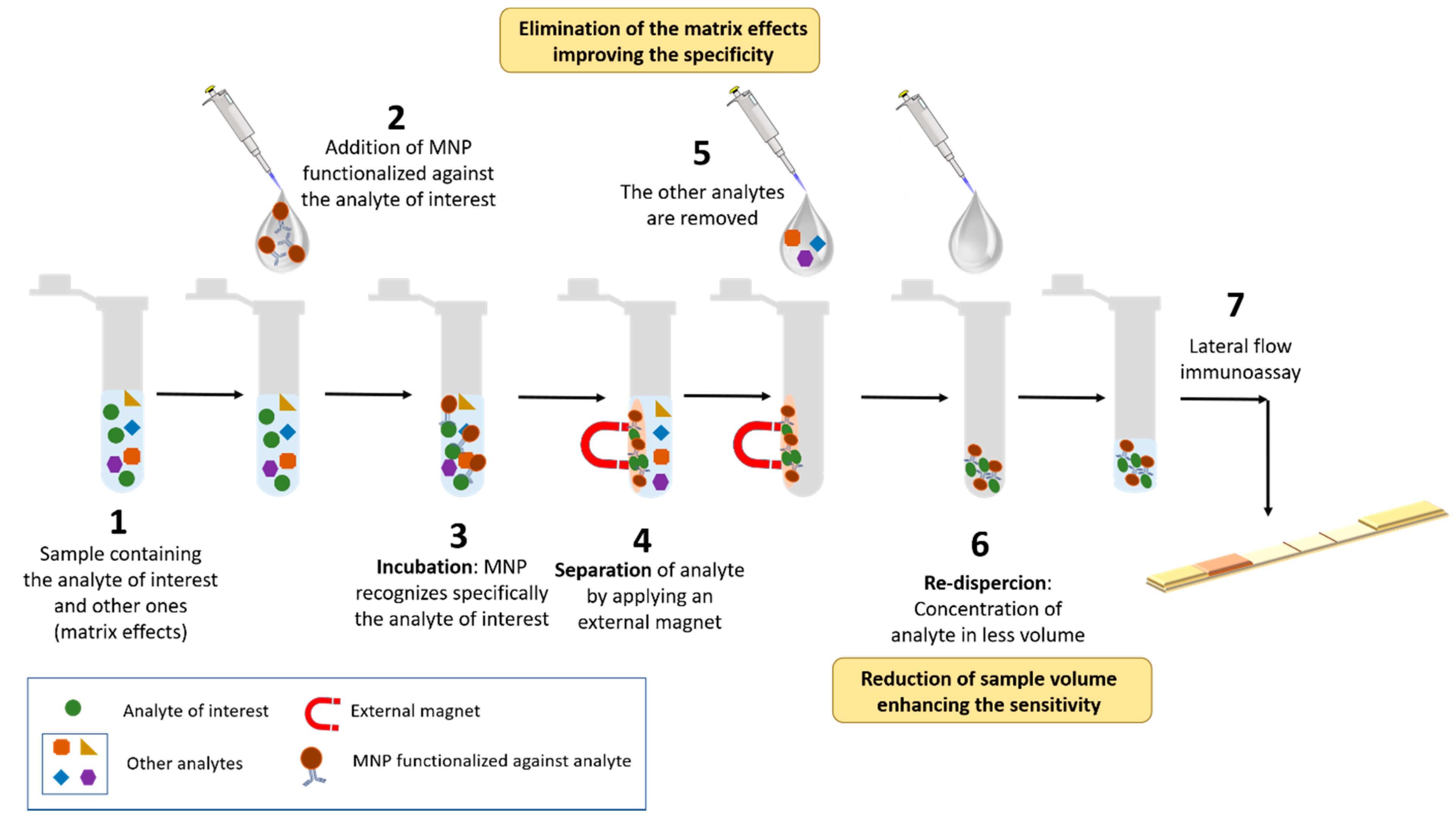
4.2. Immunomagnetic Separation in Combination with Other Transduction Systems
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Vashist, S.K.; Luppa, P.B.; Yeo, L.Y.; Ozcan, A.; Luong, J.H.T. Emerging technologies for next-generation point-of-care testing. Trends Biotechnol. 2015, 33, 692–705. [Google Scholar] [CrossRef] [PubMed]
- Klonoff, D.C. Point-of-care blood glucose meter accuracy in the hospital setting. Diabetes Spectr. 2014, 27, 174–179. [Google Scholar] [CrossRef] [PubMed]
- Dincer, C.; Bruch, R.; Kling, A.; Dittrich, P.S.; Urban, G.A. Multiplexed point-of-care testing—xPOCT. Trends Biotechnol. 2017, 35, 728–742. [Google Scholar] [CrossRef] [PubMed]
- Yager, P.; Domingo, G.J.; Gerdes, J. Point-of-care diagnostics for global health. Annu. Rev. Biomed. Eng. 2008, 10, 107–144. [Google Scholar] [CrossRef]
- Choi, J.R.; Yong, K.W.; Choi, J.Y.; Cowie, A.C. Emerging point-of-care technologies for food safety analysis. Sensors 2019, 19, 817. [Google Scholar] [CrossRef]
- Lau, H.Y.; Botella, J.R. Advanced DNA-based point-of-care diagnostic methods for plant diseases detection. Front. Plant Sci. 2017, 8, 1–14. [Google Scholar] [CrossRef]
- Cummins, B.M.; Ligler, F.S.; Walker, G.M. Point-of-care diagnostics for niche applications. Biotechnol. Adv. 2016, 34, 161–176. [Google Scholar] [CrossRef]
- Luzzi, V. Point of Care Devices for Drugs of Abuse Testing, 2nd ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2019; ISBN 9780128156070. [Google Scholar]
- Mandal, N.; Mitra, S.; Bandyopadhyay, D. Paper-sensors for point-of-care monitoring of drinking water quality. IEEE Sens. J. 2019, 19, 7936–7941. [Google Scholar] [CrossRef]
- Li, Z.; Yi, Y.; Luo, X.; Xiong, N.; Liu, Y.; Li, S.; Sun, R.; Wang, Y.; Hu, B.; Chen, W.; et al. Development and clinical application of a rapid IgM-IgG combined antibody test for SARS-CoV-2 infection diagnosis. J. Med. Virol. 2020. [Google Scholar] [CrossRef]
- Quesada-González, D.; Merkoçi, A. Nanoparticle-based lateral flow biosensors. Biosens. Bioelectron. 2015, 73, 47–63. [Google Scholar] [CrossRef]
- Petrakova, A.V.; Urusov, A.E.; Zherdev, A.V.; Dzantiev, B.B. Gold nanoparticles of different shape for bicolor lateral flow test. Anal. Biochem. 2019, 568, 7–13. [Google Scholar] [CrossRef]
- Available online: https://www.expedeon.com/resources/applications/lateral-flow-immunoassay/ (accessed on 5 February 2020).
- Sotnikov, D.V.; Barshevskaya, L.V.; Zherdev, A.V. Immunochromatographic system for serodiagnostics of cattle brucellosis using gold nanoparticles and signal amplification with quantum dots. Appl. Sci. 2020, 10, 738. [Google Scholar] [CrossRef]
- Yang, X.; Liu, L.; Hao, Q.; Zou, D.; Zhang, X.; Zhang, L.; Li, H.; Qiao, Y.; Zhao, H.; Zhou, L. Development and evaluation of Up-Converting phosphor technology-based lateral flow assay for quantitative detection of NT-proBNP in blood. PLoS ONE 2017, 12, e0171376. [Google Scholar] [CrossRef] [PubMed]
- Aktas, G.B.; Wichers, J.H.; Skouridou, V.; van Amerongen, A.; Masip, L. Nucleic acid lateral flow assays using a conjugate of a DNA binding protein and carbon nanoparticles. Microchim. Acta 2019, 186, 426. [Google Scholar] [CrossRef] [PubMed]
- Qiu, W.; Baryeh, K.; Takalkar, S.; Chen, W.; Liu, G. Carbon nanotube-based lateral flow immunoassay for ultrasensitive detection of proteins: Application to the determination of IgG. Microchim. Acta 2019, 186, 436. [Google Scholar] [CrossRef]
- Wang, Z.; Jing, J.; Ren, Y.; Guo, Y.; Tao, N.; Zhou, Q.; Zhang, H.; Ma, Y.; Wang, Y. Preparation and application of selenium nanoparticles in a lateral flow immunoassay for clenbuterol detection. Mater. Lett. 2019, 234, 212–215. [Google Scholar] [CrossRef]
- Liu, C.; Jia, Q.; Yang, C.; Qiao, R.; Jing, L.; Wang, L.; Xu, C.; Gao, M. Lateral flow immunochromatographic assay for sensitive pesticide detection by using Fe3O4 nanoparticle aggregates as color reagents. Anal. Chem. 2011, 83, 6778–6784. [Google Scholar] [CrossRef]
- Rodríguez, M.O.; Covián, L.B.; García, A.C.; Blanco-López, M.C. Silver and gold enhancement methods for lateral flow immunoassays. Talanta 2016, 148, 272–278. [Google Scholar] [CrossRef]
- Blanco-Covián, L.; Montes-García, V.; Girard, A.; Fernández-Abedul, M.T.; Pérez-Juste, J.; Pastoriza-Santos, I.; Faulds, K.; Graham, D.; Blanco-López, M.C. Au@Ag SERRS tags coupled to a lateral flow immunoassay for the sensitive detection of pneumolysin. Nanoscale 2017, 9, 2051–2058. [Google Scholar] [CrossRef]
- Park, J.M.; Jung, H.W.; Chang, Y.W.; Kim, H.S.; Kang, M.J.; Pyun, J.C. Chemiluminescence lateral flow immunoassay based on Pt nanoparticle with peroxidase activity. Anal. Chim. Acta 2015, 853, 360–367. [Google Scholar] [CrossRef]
- Leem, H.; Shukla, S.; Song, X.; Heu, S.; Kim, M. An Efficient Liposome-Based Immunochromatographic Strip Assay for the Sensitive Detection of SalmonellaTyphimurium in Pure Culture. J. Food Saf. 2014, 34, 239–248. [Google Scholar] [CrossRef]
- Poonlapdecha, W.; Seetang-Nun, Y.; Wonglumsom, W.; Tuitemwong, K.; Erickson, L.E.; Hansen, R.R.; Tuitemwong, P. Antibody-conjugated ferromagnetic nanoparticles with lateral flow test strip assay for rapid detection of Campylobacter jejuni in poultry samples. Int. J. Food Microbiol. 2018, 286, 6–14. [Google Scholar] [CrossRef]
- Razo, S.C.; Panferov, V.G.; Safenkova, I.V.; Varitsev, Y.A.; Zherdev, A.V.; Dzantiev, B.B. Double-enhanced lateral flow immunoassay for potato virus X based on a combination of magnetic and gold nanoparticles. Anal. Chim. Acta 2018, 1007, 50–60. [Google Scholar] [CrossRef] [PubMed]
- Anwar, S.; Khawja, M.; Ficiar, E.; Ruffinatti, F.A.; Stura, I.; Argenziano, M.; Abollino, O.; Cavalli, R.; Guiot, C.; Agata, F.D. Magnetic iron oxide nanoparticles: Synthesis, characterization and functionalization for biomedical applications in the central nervous system. Materials 2019, 12, 465. [Google Scholar]
- Mourdikoudis, S.; Pallares, R.M.; Thanh, N.T.K. Characterization techniques for nanoparticles: Comparison and complementarity upon studying nanoparticle properties. Nanoscale 2018, 10, 12871–12934. [Google Scholar] [CrossRef] [PubMed]
- Sandler, S.E.; Fellows, B.; Thompson Mefford, O. Best practices for characterization of magnetic nanoparticles for biomedical applications. Anal. Chem. 2019, 91, 14159–14169. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Zhao, Y.; Yang, Z.; Xu, C.; Lu, Y.; Pan, Y.; Shi, D.; Wang, Y. Influence of controlled surface functionalization of magnetic nanocomposites on the detection performance of immunochromatographic test. Sens. Actuators B Chem. 2016, 237, 817–825. [Google Scholar] [CrossRef]
- Hwang, J.; Kwon, D.; Lee, S.; Jeon, S. Detection of: Salmonella bacteria in milk using gold-coated magnetic nanoparticle clusters and lateral flow filters. RSC Adv. 2016, 6, 48445–48448. [Google Scholar] [CrossRef]
- Chen, Z.; Wu, C.; Zhang, Z.; Wu, W.; Wang, X.; Yu, Z. Synthesis, functionalization, and nanomedical applications of functional magnetic nanoparticles. Chin. Chem. Lett. 2018, 29, 1601–1608. [Google Scholar] [CrossRef]
- Wu, W.; Wu, Z.; Yu, T.; Jiang, C.; Kim, W.S. Recent progress on magnetic iron oxide nanoparticles: Synthesis, surface functional strategies and biomedical applications. Sci. Technol. Adv. Mater. 2015, 16, 023501. [Google Scholar] [CrossRef]
- Huang, J.; Xie, Z.; Xie, L.; Xie, Z.; Luo, S.; Deng, X.; Huang, L.; Zeng, T.; Zhang, Y.; Wang, S.; et al. Au/ Fe3O4 core-shell nanoparticles are an efficient immunochromatography test strip performance enhancer—A comparative study with Au and Fe3O4 nanoparticles. RSC Adv. 2018, 8, 14064–14071. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, Q.; Gao, M.; Luo, Z.; Zhang, Y.; Li, X.; Hua, K.; Zhang, C.; Lai, W.; Cui, Y. Clinical experimental study of GoldMag® immunochromatography in high sensitive C reactive protein detection from whole blood and plasma. J. Magn. Magn. Mater. 2019, 473, 68–73. [Google Scholar] [CrossRef]
- Wu, Z.; He, D.; Xu, E.; Jiao, A.; Chughtai, M.F.J.; Jin, Z. Rapid detection of β-conglutin with a novel lateral flow aptasensor assisted by immunomagnetic enrichment and enzyme signal amplification. Food Chem. 2018, 269, 375–379. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhang, C.; Liu, K.; Wang, H.; Lu, C.; Li, H.; Hua, K.; Zhu, J.; Hui, W.; Cui, Y.; et al. Multiple SNPs detection based on lateral flow assay for phenylketonuria diagnostic. Anal. Chem. 2018, 90, 3430–3436. [Google Scholar] [CrossRef] [PubMed]
- Xia, S.; Yu, Z.; Liu, D.; Xu, C.; Lai, W. Developing a novel immunochromatographic test strip with gold magnetic bifunctional nanobeads (GMBN) for efficient detection of Salmonella choleraesuis in milk. Food Control 2016, 59, 507–512. [Google Scholar] [CrossRef]
- Thobhani, S.; Attree, S.; Boyd, R.; Kumarswami, N.; Noble, J.; Szymanski, M.; Porter, R.A. Bioconjugation and characterisation of gold colloid-labelled proteins. J. Immunol. Methods 2010, 356, 60–69. [Google Scholar] [CrossRef]
- Wu, J.; Dong, M.; Zhang, C.; Wang, Y.; Xie, M.; Chen, Y. Magnetic lateral flow strip for the detection of cocaine in urine by naked eyes and smart phone camera. Sensors 2017, 17, 1286. [Google Scholar] [CrossRef]
- Yan, L.; Dou, L.; Bu, T.; Huang, Q.; Wang, R.; Yang, Q.; Huang, L.; Wang, J.; Zhang, D. Highly sensitive furazolidone monitoring in milk by a signal amplified lateral flow assay based on magnetite nanoparticles labeled dual-probe. Food Chem. 2018, 261, 131–138. [Google Scholar] [CrossRef]
- Pilavaki, E.; Demosthenous, A. Optimized lateral flow immunoassay reader for the detection of infectious diseases in developing countries. Sensors 2017, 17, 2673. [Google Scholar] [CrossRef]
- Eltzov, E.; Guttel, S.; Low Yuen Kei, A.; Sinawang, P.D.; Ionescu, R.E.; Marks, R.S. Lateral flow immunoassays - from paper strip to smartphone technology. Electroanalysis 2015, 27, 2116–2130. [Google Scholar] [CrossRef]
- Ruppert, C.; Phogat, N.; Laufer, S.; Kohl, M.; Deigner, H.P. A smartphone readout system for gold nanoparticle-based lateral flow assays: Application to monitoring of digoxigenin. Microchim. Acta 2019, 186, 119. [Google Scholar] [CrossRef]
- Saisin, L.; Amarit, R.; Somboonkaew, A.; Gajanandana, O.; Himananto, O.; Sutapun, B. Significant sensitivity improvement for camera-based lateral flow immunoassay readers. Sensors 2018, 18, 4026. [Google Scholar] [CrossRef] [PubMed]
- Park, J. A giant magnetoresistive reader platform for quantitative lateral flow immunoassays. Sens. Actuators A Phys. 2016, 250, 55–59. [Google Scholar] [CrossRef]
- Marquina, C.; De Teresa, J.M.; Serrate, D.; Marzo, J.; Cardoso, F.A.; Saurel, D.; Cardoso, S.; Freitas, P.P.; Ibarra, M.R. GMR sensors and magnetic nanoparticles for immuno-chromatographic assays. J. Magn. Magn. Mater. 2012, 324, 3495–3498. [Google Scholar] [CrossRef]
- Taton, K.; Johnson, D.; Guire, P.; Lange, E.; Tondra, M. Lateral flow immunoassay using magnetoresistive sensors. J. Magn. Magn. Mater. 2009, 321, 1679–1682. [Google Scholar] [CrossRef]
- Serrate, D.; De Teresa, J.M.; Marquina, C.; Marzo, J.; Saurel, D.; Cardoso, F.A.; Cardoso, S.; Freitas, P.P.; Ibarra, M.R. Quantitative biomolecular sensing station based on magnetoresistive patterned arrays. Biosens. Bioelectron. 2012, 35, 206–212. [Google Scholar] [CrossRef]
- Ryu, Y.; Jin, Z.; Kang, M.S.; Kim, H.S. Increase in the detection sensitivity of a lateral flow assay for a cardiac marker by oriented immobilization of antibody. Biochip J. 2011, 5, 193–198. [Google Scholar] [CrossRef]
- Chicharo, A.; Cardoso, F.; Cardoso, S.; Freitas, P.P. Dynamical detection of magnetic nanoparticles in paper microfluidics with spin valve sensors for point-of-care applications. IEEE Trans. Magn. 2014, 50, 1–4. [Google Scholar] [CrossRef]
- Chicharo, A.; Cardoso, F.; Cardoso, S.; Freitas, P.J.P. Real-time monitoring of magnetic nanoparticles diffusion in lateral flow microporous membrane using spin valve sensors. IEEE Trans. Magn. 2015, 51, 1–4. [Google Scholar] [CrossRef]
- Lei, H.; Wang, K.; Ji, X.; Cui, D. Contactless measurement of magnetic nanoparticles on lateral flow strips using tunneling magnetoresistance (TMR) sensors in differential configuration. Sensors 2016, 16, 2130. [Google Scholar] [CrossRef]
- Guteneva, N.V.; Znoyko, S.L.; Orlov, A.V.; Nikitin, M.P.; Nikitin, P.I. Volumetric registration of magnetic nanoparticles for optimization of quantitative immunochromatographic assays for detection of small molecules. EPJ Web Conf. 2018, 185, 10006. [Google Scholar] [CrossRef][Green Version]
- Barnett, J.M.; Wraith, P.; Kiely, J.; Persad, R.; Hurley, K.; Hawkins, P.; Luxton, R. An inexpensive, fast and sensitive quantitative lateral flow magneto-immunoassay for total prostate specific antigen. Biosensors 2014, 4, 204–220. [Google Scholar] [CrossRef] [PubMed]
- Nikitin, M.P.; Orlov, A.V.; Znoyko, S.L.; Bragina, V.A.; Gorshkov, B.G.; Ksenevich, T.I.; Cherkasov, V.R.; Nikitin, P.I. Multiplex biosensing with highly sensitive magnetic nanoparticle quantification method. J. Magn. Magn. Mater. 2018, 459, 260–264. [Google Scholar] [CrossRef]
- Orlov, A.V.; Znoyko, S.L.; Cherkasov, V.R.; Nikitin, M.P.; Nikitin, P.I. Multiplex Biosensing Based on Highly Sensitive Magnetic Nanolabel Quantification: Rapid Detection of Botulinum Neurotoxins A, B, and e in Liquids. Anal. Chem. 2016, 88, 10419–10426. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.; Wang, K.; Xiao, K.; Qin, W.; Hou, Y.; Xu, H.; Yan, X.; Chen, Y.; Cui, D.; He, J. Dual Immunomagnetic Nanobeads-Based Lateral Flow Test Strip for Simultaneous Quantitative Detection of Carcinoembryonic Antigen and Neuron Specific Enolase. Sci. Rep. 2017, 7, 1–10. [Google Scholar] [CrossRef]
- Shi, L.; Wu, F.; Wen, Y.; Zhao, F.; Xiang, J.; Ma, L. A novel method to detect Listeria monocytogenes via superparamagnetic lateral flow immunoassay. Anal. Bioanal. Chem. 2015, 407, 529–535. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, K.; Liu, Z.; Sun, R.; Cui, D.; He, J. Rapid detection and quantification of tumor marker carbohydrate antigen 72-4 (CA72-4) using a superparamagnetic immunochromatographic strip. Anal. Bioanal. Chem. 2016, 408, 2319–2327. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, Z.; Wang, Y.; Zhao, Y.; Lu, Y.; Xu, X.; Yan, J.; Pan, Y. A highly sensitive and flexible magnetic nanoprobe labeled immunochromatographic assay platform for pathogen Vibrio parahaemolyticus. Int. J. Food Microbiol. 2015, 211, 109–116. [Google Scholar] [CrossRef]
- Hu, J.; Jiang, Y.Z.; Tang, M.; Wu, L.L.; Xie, H.Y.; Zhang, Z.L.; Pang, D.W. Colorimetric-fluorescent-magnetic nanosphere-based multimodal assay platform for salmonella detection. Anal. Chem. 2019, 91, 1178–1184. [Google Scholar] [CrossRef]
- Motte, L.; Benyettou, F.; De Beaucorps, C.; Lecouvey, M.; Milesovic, I.; Lalatonne, Y. Multimodal superparamagnetic nanoplatform for clinical applications: Immunoassays, imaging & therapy. Faraday Discuss. 2011, 149, 211–225. [Google Scholar]
- Gas, F.; Baus, B.; Queré, J.; Chapelle, A.; Dreanno, C. Rapid detection and quantification of the marine toxic algae, Alexandrium minutum, using a super-paramagnetic immunochromatographic strip test. Talanta 2016, 147, 581–589. [Google Scholar] [CrossRef]
- Lago-Cachón, D.; Oliveira-Rodríguez, M.; Rivas, M.; Blanco-López, M.C.; Martínez-García, J.C.; Moyano, A.; Salvador, M.; García, J.A. Scanning Magneto-Inductive Sensor for Quantitative Assay of Prostate-Specific Antigen. IEEE Magn. Lett. 2017, 8, 1–5. [Google Scholar] [CrossRef]
- Moyano, A.; Salvador, M.; Martínez-García, J.C.; Socoliuc, V.; Vékás, L.; Peddis, D.; Alvarez, M.A.; Fernández, M.; Rivas, M.; Blanco-López, M.C. Magnetic immunochromatographic test for histamine detection in wine. Anal. Bioanal. Chem. 2019, 411, 6615–6624. [Google Scholar] [CrossRef] [PubMed]
- Hall, D.A.; Gaster, R.S.; Lin, T.; Osterfeld, S.J.; Han, S.; Murmann, B.; Wang, S.X. GMR biosensor arrays: A system perspective. Biosens. Bioelectron. 2010, 25, 2051–2057. [Google Scholar] [CrossRef]
- Makiranta, J.J.; Lekkala, J.O. Modeling and Simulation of Magnetic Nanoparticle Sensor. In Proceedings of the 2005 IEEE Engineering in Medicine and Biology 27th Annual Conference, Shanghai, China, 17–18 January 2006; pp. 1256–1259. [Google Scholar]
- MagnaBioSciences, LLC. Available online: http://www.magnabiosciences.com/ (accessed on 5 February 2020).
- Lago-Cachón, D.; Rivas, M.; Martínez-García, J.C.; García, J.A. Cu impedance-based detection of superparamagnetic nanoparticles. Nanotechnology 2013, 24, 245501. [Google Scholar] [CrossRef]
- Rivas, M.; Lago-Cachón, D.; Martínez-García, J.C.; García, J.A.; Calleja, A.J. Eddy-current sensing of superparamagnetic nanoparticles with spiral-like copper circuits. Sens. Actuators A Phys. 2014, 216, 123–127. [Google Scholar] [CrossRef]
- Panferov, V.G.; Safenkova, I.V.; Zherdev, A.V.; Dzantiev, B.B. Setting up the cut-off level of a sensitive barcode lateral flow assay with magnetic nanoparticles. Talanta 2017, 164, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.-C.; Wu, K.-H.; Hung, H.-C.; Wang, J.-C.; Chang, S.-C. Magnetic nanoparticle-based lateral flow immunochromatographic strip as a reporter for rapid detection of melamine. J. Nanosci. Nanotechnol. 2018, 18, 7190–7196. [Google Scholar] [CrossRef]
- Liu, F.; Zhang, H.; Wu, Z.; Dong, H.; Zhou, L.; Yang, D.; Ge, Y.; Jia, C.; Liu, H.; Jin, Q.; et al. Highly sensitive and selective lateral flow immunoassay based on magnetic nanoparticles for quantitative detection of carcinoembryonic antigen. Talanta 2016, 161, 205–210. [Google Scholar] [CrossRef]
- Oliveira-Rodríguez, M.; Serrano-Pertierra, E.; García, A.C.; Martín, S.L.; Mo, M.Y.; Cernuda-Morollón, E.; Blanco-López, M.C. Point-of-care detection of extracellular vesicles: Sensitivity optimization and multiple-target detection. Biosens. Bioelectron. 2017, 87, 38–45. [Google Scholar] [CrossRef]
- Hui, W.; Zhang, S.; Zhang, C.; Wan, Y.; Zhu, J.; Zhao, G.; Wu, S.; Xi, D.; Zhang, Q.; Li, N.; et al. A novel lateral flow assay based on GoldMag nanoparticles and its clinical applications for genotyping of MTHFR C677T polymorphisms. Nanoscale 2016, 8, 3579–3587. [Google Scholar] [CrossRef]
- Lian, T.; Hui, W.; Li, X.; Zhang, C.; Zhu, J.; Li, R.; Wan, Y.; Cui, Y. Apolipoprotein e genotyping using PCR-GoldMag lateral flow assay and its clinical applications. Mol. Med. Rep. 2016, 14, 4153–4161. [Google Scholar] [CrossRef]
- Li, X.; Zhang, Q.; Hou, P.; Chen, M.; Hui, W.; Vermorken, A.; Luo, Z.; Li, H.; Li, Q.; Cui, Y. Gold magnetic nanoparticle conjugate-based lateral flow assay for the detection of IgM class antibodies related to TORCH infections. Int. J. Mol. Med. 2015, 36, 1319–1326. [Google Scholar] [CrossRef]
- Jacinto, M.J.; Trabuco, J.R.C.; Vu, B.V.; Garvey, G.; Khodadady, M.; Azevedo, A.M.; Aires-Barros, M.R.; Chang, L.; Kourentzi, K.; Litvinov, D.; et al. Enhancement of lateral flow assay performance by electromagnetic relocation of reporter particles. PLoS ONE 2018, 13, e0186782. [Google Scholar] [CrossRef]
- Znoyko, S.L.; Orlov, A.V.; Pushkarev, A.V.; Mochalova, E.N.; Guteneva, N.V.; Lunin, A.V.; Nikitin, M.P.; Nikitin, P.I. Ultrasensitive quantitative detection of small molecules with rapid lateral-flow assay based on high-affinity bifunctional ligand and magnetic nanolabels. Anal. Chim. Acta 2018, 1034, 161–167. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Ma, J.; Xue, C.; Wang, L.; Wang, X. One-pot synthesis of poly (acrylic acid)-stabilized Fe3O4 nanocrystal clusters for the simultaneously qualitative and quantitative detection of biomarkers in lateral flow immunoassay. J. Pharm. Biomed. Anal. 2018, 159, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Hong, L.; Wang, K.; Yan, W.; Xu, H.; Chen, Q.; Zhang, Y.; Cui, D.; Jin, Q.; He, J. High performance immunochromatographic assay for simultaneous quantitative detection of multiplex cardiac markers based on magnetic nanobeads. Theranostics 2018, 8, 6121–6131. [Google Scholar] [CrossRef]
- Wang, C.; Guan, D.; Chen, C.; He, S.; Liu, X.; Wang, C.; Wu, H. Rapid detection of unconjugated estriol in the serum via superparamagnetic lateral flow immunochromatographic assay. Anal. Bioanal. Chem. 2018, 410, 123–130. [Google Scholar] [CrossRef]
- Li, X.; Wang, Y.; Tang, Q.; Li, Q. MnFe 2 O 4 nanoclusters as labels for the quantitative detection of D-dimer in a lateral-flow immunochromatographic assay. J. Chinese Chem. Soc. 2019, 66, 297–302. [Google Scholar] [CrossRef]
- Guo, L.; Shao, Y.; Duan, H.; Ma, W.; Leng, Y.; Huang, X.; Xiong, Y. Magnetic Quantum Dot Nanobead-Based Fluorescent Immunochromatographic Assay for the Highly Sensitive Detection of Aflatoxin B 1 in Dark Soy Sauce. Anal. Chem. 2019, 91, 4727–4734. [Google Scholar] [CrossRef]
- Li, F.; Li, F.; Luo, D.; Lai, W.; Xiong, Y.; Xu, H. Biotin-exposure-based immunomagnetic separation coupled with nucleic acid lateral flow biosensor for visibly detecting viable Listeria monocytogenes. Anal. Chim. Acta 2018, 1017, 48–56. [Google Scholar] [CrossRef]
- Huang, H.; Zhao, G.; Dou, W. Portable and quantitative point-of-care monitoring of Escherichia coli O157:H7 using a personal glucose meter based on immunochromatographic assay. Biosens. Bioelectron. 2018, 107, 266–271. [Google Scholar] [CrossRef]
- Ren, W.; Cho, I.H.; Zhou, Z.; Irudayaraj, J. Ultrasensitive detection of microbial cells using magnetic focus enhanced lateral flow sensors. Chem. Commun. 2016, 52, 4930–4933. [Google Scholar] [CrossRef]
- Zhang, X.; Zhou, J.; Zhang, C.; Zhang, D.; Su, X. Rapid detection of Enterobacter cloacae by immunomagnetic separation and a colloidal gold-based immunochromatographic assay. RSC Adv. 2016, 6, 1279–1287. [Google Scholar] [CrossRef]
- Li, Q.; Zhang, S.; Cai, Y.; Yang, Y.; Hu, F.; Liu, X.; He, X. Rapid detection of Listeria monocytogenes using fluorescence immunochromatographic assay combined with immunomagnetic separation technique. Int. J. Food Sci. Technol. 2017, 52, 1559–1566. [Google Scholar] [CrossRef]
- Zhao, Y.; Chen, X.; Lin, S.; Du, D.; Lin, Y. Integrated immunochromatographic strip with glucometer readout for rapid quantification of phosphorylated proteins. Anal. Chim. Acta 2017, 964, 1–6. [Google Scholar] [CrossRef]
- Xu, F.; Xu, D.; Ming, X.; Xu, H.; Li, B.; Li, P.; Aguilar, Z.P.; Cheng, T.; Wu, X.; Wei, H. Quantum dot-based immunochromatography test strip for rapid detection of campylobacter jejuni. J. Nanosci. Nanotechnol. 2013, 13, 4552–4559. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Wu, X.; Li, B.; Li, P.; Ming, X.; Chen, T.; Wei, H.; Xu, F. Rapid detection of Campylobacter jejuni using fluorescent microspheres as label for immunochromatographic strip test. Food Sci. Biotechnol. 2013, 22, 585–591. [Google Scholar] [CrossRef]
- Wang, W.; Liu, L.; Song, S.; Xu, L.; Kuang, H.; Zhu, J.; Xu, C. Identification and quantification of eight Listeria monocytogene serotypes from Listeria spp. using a gold nanoparticle-based lateral flow assay. Microchim. Acta 2017, 184, 715–724. [Google Scholar] [CrossRef]
- Moongkarndi, P.; Rodpai, E.; Kanarat, S. Evaluation of an immunochromatographic assay for rapid detection of salmonella enterica serovars typhimurium and enteritidis. J. Vet. Diagn. Investig. 2011, 23, 797–801. [Google Scholar] [CrossRef]
- Xie, Q.Y.; Wu, Y.H.; Xiong, Q.R.; Xu, H.Y.; Xiong, Y.H.; Liu, K.; Jin, Y.; Lai, W.H. Advantages of fluorescent microspheres compared with colloidal gold as a label in immunochromatographic lateral flow assays. Biosens. Bioelectron. 2014, 54, 262–265. [Google Scholar] [CrossRef] [PubMed]
- Liao, J.Y.; Li, H. Lateral flow immunodipstick for visual detection of aflatoxin B1 in food using immuno-nanoparticles composed of a silver core and a gold shell. Microchim. Acta 2010, 171, 289–295. [Google Scholar] [CrossRef]
- Moon, J.; Kim, G.; Lee, S. A Gold nanoparticle and aflatoxin B1-BSA conjugates based lateral flow assay method for the analysis of aflatoxin B1. Materials 2012, 5, 634–643. [Google Scholar] [CrossRef] [PubMed]
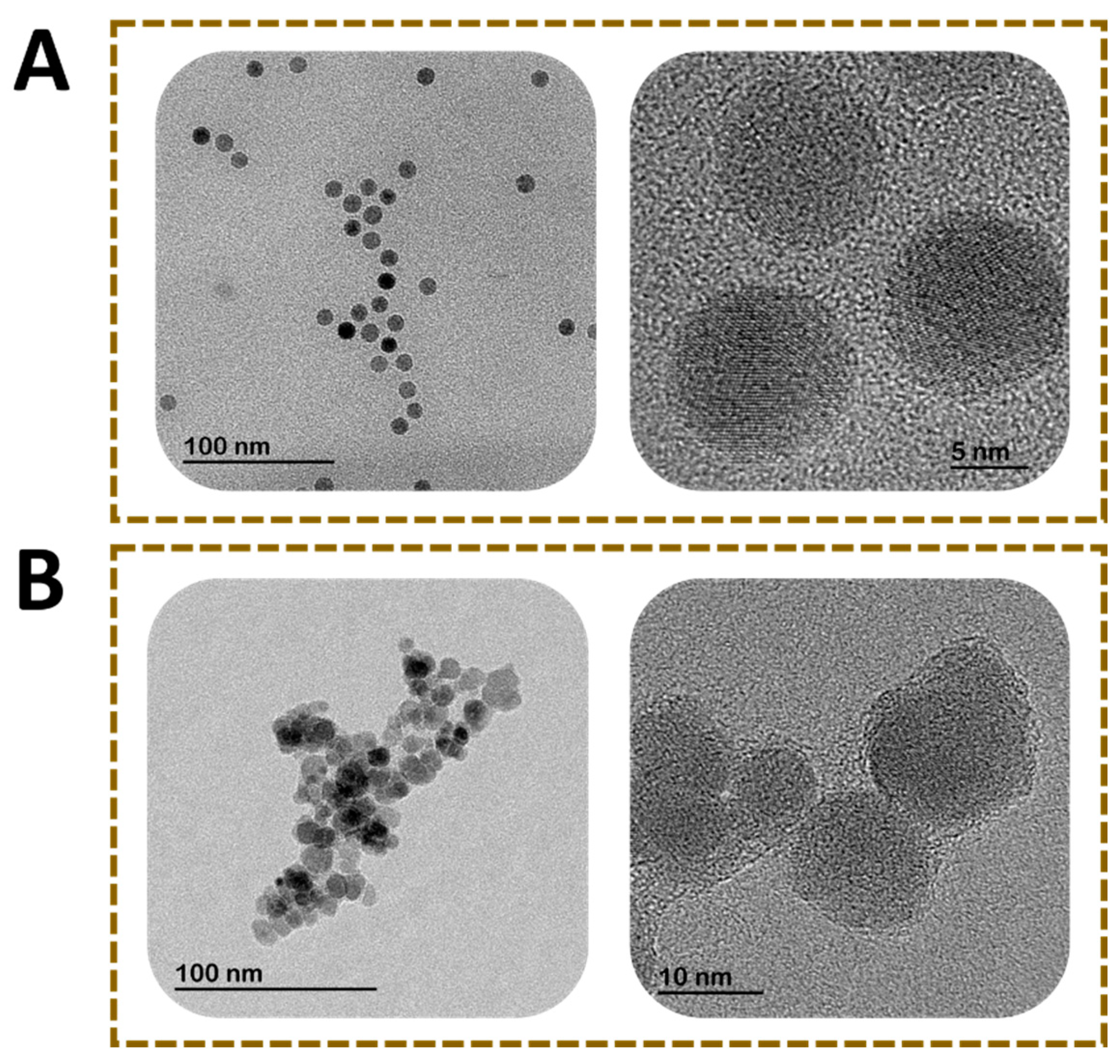

| Techniques | Characterization Information |
|---|---|
| Microscopy: Transmission electron microscopy and Scanning electron microscopy | Morphology, size distribution, crystallinity and composition |
| X-ray diffraction (XRD) | Crystal structure and size |
| Dynamic light scattering (DLS) | Hydrodynamic size |
| Infrared spectroscopy (IR) | Nature of surface and functional groups on surface |
| Zeta potential | Surface charge and stability |
| Thermal analysis | Concentration and thermal stability |
| Mass spectroscopy | Concentration |
| Superconducting quantum interference device (SQUID)/Vibrating sample magnetometry (VSM) | Magnetic properties |
| Transducing Principle | Nanoparticles | References |
|---|---|---|
| Magnetoresistive LFIA readers | Superparamagnetic nanoparticles | [45] |
| Superparamagnetic maghemite nanoparticles | [46] | |
| Beads of 440 nm in diameter | [47] | |
| Superparamagnetic Maghemite nanoparticles (200 nm) | [48] | |
| Magnetic beads of 200 nm | [49] | |
| Superparamagnetic nanoparticles (10.5 nm) | [50] | |
| [51] | ||
| Superparamagnetic nanoparticles (80 nm) | [52] | |
| Inductive LFIA readers | Superparamagnetic nanoparticles encapsulated in microspheres (198 nm) | [53] |
| Paramagnetic particles (760 nm) | [54] | |
| Superparamagnetic nanoparticles (50 nm) | [55] | |
| Superparamagnetic nanoparticles encapsulated in microspheres (198 nm) | [56] | |
| Magnetic nanobeads (15, 80 and 200 nm) | [57] | |
| Superparamagnetic nanoparticles (140 nm) | [58] | |
| Superparamagnetic nanoparticles (15 nm) | [59] | |
| Superparamagnetic nanoparticles (140 nm) | [60] | |
| Colorimetric-Fluorescent-Magnetic nanospheres (300 nm) | [61] | |
| Superparamagnetic nanoparticles (10 nm) | [62] | |
| Superparamagnetic nanoparticles (200 nm) | [63] | |
| Superparamagnetic nanoparticles (10.5 nm) | [64] | |
| Superparamagnetic magnetite nanoparticles | [65] |
| Nanoparticles | Conjugation | Detection | Analyte | Limit of Detection | Reference |
|---|---|---|---|---|---|
| Pathogens | |||||
| Gold magnetic nanoparticles | Via Au–S bonds | Visual detection | Salmonella choleraesuis | 5 × 105 CFU/mL | [37] |
| Gold magnetic nanoparticles | Via Au–S bonds | Colour intensity. ImageJ density analysis | Salmonella | 103 CFU/mL | [30] |
| Magnetic nanoparticles | EDC/NHS Chemistry | Colour intensity. TotalLab TL120 | Potato virus X | 0.5 ng/mL | [71] |
| Magnetic nanoparticles | EDC/NHS Chemistry | Colour intensity. TotalLab TL120 | Potato virus X | 0.25 ng/mL | [25] |
| Gold magnetic nanoparticles | Via Au–S bonds | Visual detection | Avian influenza virus subtype H7 (AIV H7) | 103 5 EID50 | [33] |
| Toxins and allergens | |||||
| Gold magnetic nanoparticles | Via Au–S bonds | Visual detection | β-Conglutin | 8 fM | [35] |
| Magnetic nanoparticles | EDC/NHS Chemistry | Visual detection | Melamine | 0.4 ppm for Fe2O3PEG 2.2 ppm for Fe3O4-PEG | [72] |
| Magnetic nanoparticles | EDC/NHS Chemistry | Visual detection | Furazolidone metabolite of 3-amino-2-oxazolidinone (AOZ) | 0.044 ng/mL | [40] |
| Biomarkers | |||||
| Magnetic nanoparticles | EDC chemistry | Visual detection | Carcinoembryonic antigen (CEA) | 0.25 ng/mL | [73] |
| Magnetic nanoparticles | EDC/NHS Chemistry | Reflectance measurements. ESE Quant LR3 (Qiagen Inc., Germany) | Extracellular vesicles (EVs) | 107 EVs/mL | [74] |
| Gold magnetic nanoparticles | EDC chemistry | Visual detection | Genotyping of MTHFR C677T | 5 ng | [75] |
| Gold magnetic nanoparticles | CTAB-PSS modification (direct bonds) | Visual detection | Genotype Apolipoprotein E | 10 ng | [76] |
| Gold magnetic nanoparticles | EDC chemistry | Visual detection | IgM class antibodies related infections | - | [77] |
| Magnetic nanoparticles | Periodate-based oxidation of the glycosylated Fc residues | Colour intensity. ImageJ density analysis | Human chorionic gonadotropin (hCG) | 0.31 ng/mL | [78] |
| Drugs | |||||
| Magnetic nanoparticles | EDC/NHS Chemistry | Smart phone camera was used for quantitative analysis | Cocaine | 5 ng/mL | [39] |
| Nanoparticles | Conjugation | Detection | Analyte | Limit of Detection | Reference |
|---|---|---|---|---|---|
| Pathogens | |||||
| Magnetic nanoparticles | EDC/NHS Chemistry | MAR system (MagnaBioSciences, CA, USA) | Vibrio parahaemolyticus | 4.73 × 103 CFU/mL | [60] |
| Colorimetric-Fluorescent-Magnetic nanoparticles | EDC/NHS Chemistry | MAR system (MagnaBioSciences, CA, USA) Fibre optic spectrometer | Salmonella typhimurium | 1.88 × 104 CFU/mL: naked detection 3.75 × 103 CFU/mL: magnetic and fluorescent detection | [61] |
| Magnetic nanoparticles | EDC/NHS Chemistry | Reader Miateks (Magnisense) | Alexandrium minutum | 105 cells/L | [63] |
| Toxins and allergens | |||||
| Magnetic nanoparticles | EDC/NHS Chemistry | Magnetic particle quantification (MPQ) method | Botulinum neurotoxin (BoNT) types A, B and E | 0.22 ng/mL for BoNT-A 0.11 ng/mL for BoNT-B 0.32 ng/mL for BoNT-E | [56] |
| Magnetic nanoparticles | EDC/NHS Chemistry | Novel sensor developed by authors Reflectance measurements. ESE Quant LR3 (Qiagen Inc., Germany) | Histamine | 1.2 mg/L for magnetic sensor 1.5 mg/L for optical reader | [65] |
| Biomarkers | |||||
| Magnetic nanoparticles | EDC/NHS Chemistry | MAR system (MagnaBioSciences, CA, USA) | Carbohydrate antigen 72-4 (CA72-4) | 0.38 IU/mL | [59] |
| Magnetic nanoparticles | EDC/NHS Chemistry | Novel sensor developed by authors Reflectance measurements. ESE Quant LR3 (Qiagen Inc., Germany) | Prostate-Specific Antigen | 0.25 ng/mL | [64] |
| Magnetic nanoparticles | EDC/NHS Chemistry | MAR system (MagnaBioSciences, CA, USA) | Neuron specific enolase (NSE) carcinoembryonic antigen (CEA). | 0.094 ng/mL for NSE 0.045 ng/mL for CEA | [57] |
| Magnetic nanoparticles | EDC/NHS Chemistry | Magnetic particle quantification (MPQ) method | Thyroxine | 20 fM | [79] |
| Magnetic nanoparticles | EDC Chemistry | MAR system (MagnaBioSciences, CA, USA) | Amino-terminal pro-B-type natriuretic peptide (NT-proBNP) | 100 pg/mL | [80] |
| Gold magnetic nanoparticles | EDC Chemistry | MAR system (MagnaBioSciences, CA, USA) | Single nucleotide polymorphisms (SNPs) | 0.04 pg/μL with plasmid | [36] |
| Magnetic nanoparticles | EDC/NHS Chemistry | MIR system developed by authors | Troponin I (cTnI) Creatine kinase isoenzyme MB (CKMB) Myoglobin (Myo) | 0.0089 ng/mL for cTnI 0.063 ng/mL for CKMB 0.05 ng/mL for Myo | [81] |
| Magnetic nanoparticles | EDC/NHS Chemistry | MAR system (MagnaBioSciences, CA, USA) | Unconjugated estriol (uE3) | 0.86 nmol/L | [82] |
| Magnetic nanoparticles | EDC/NHS Chemistry | MAR system (MagnaBioSciences, CA, USA) | D-dimer | 0.05 μg/mL | [83] |
| Gold magnetic nanoparticles | - | Magnetic quantitative immunoanalyzer | C-reactive protein (CRP) | 0.15 mg/mL | [34] |
| Nanoparticles | Conjugation | Detection | Analyte | Limit of Detection | Reference |
|---|---|---|---|---|---|
| Magnetic nanoparticles | Glutaraldehyde chemistry | Visual detection | Campylobacter jejuni | 100 with pure culture 101 with poultry samples | [24] |
| Magnetic nanoparticles | Biotin-streptavidin affinity | Visual detection | Listeria monocytogenes | 3.5 × 103 CFU/mL for standards 3.5 × 104 CFU/g in real samples | [85] |
| Magnetic nanoparticles | EDC/NHS Chemistry | Electrochemical detection. Glucose meter | Escherichia coli O157:H7 | 6.2 × 104 CFU/mL | [86] |
| Gold magnetic nanoparticles | Via Au–S bonds | Visual detection | Escherichia coli O157:H7 and Salmonella typhimurium | 23 CFU/mL for E. coli 17 CFU/mL for Salmonella | [87] |
| Magnetic nanoparticles | Glutaraldehyde chemistry | Visual detection. | Enterobacter cloacae | 102 CFU/mL | [88] |
| Magnetic nanoparticles | EDC/NHS Chemistry | Fluorescent detection | Listeria monocytogenes | 104 CFU/mL | [89] |
| Fluorescent magnetic nanoparticles | EDC chemistry | Fluorescent detection. Fluorescent strip reader (Suzhou Hemai Precision Instrument Co., Ltd. Jiangsu, China). | Aflatoxin B1 (AFB1) | 3 pg/mL in sauce extract 51 pg/mL in real dark soy sauce | [84] |
| Magnetic nanoparticles | EDC/NHS Chemistry | Electrochemical detection. Glucose meter | Phospho-p5315 | 50 pg/mL | [90] |
| Analyte | Limit of Detection Using LFIA | Limit of Detection Using LFIA in Combination with IMS | References |
|---|---|---|---|
| Gold nanoparticles as label | |||
| Campylobacter jejuni | 105 cfu/mL for gold nanoparticles (pure culture) 104 cfu/mL for quantum dots (pure culture) | 100 cfu/mL (pure culture) 101 cfu/mL (poultry sample) | [24,91,92] |
| Listeria monocytogenes | 104 cfu/mL for superparamagnetic nanoparticles 3.7 × 106 cfu/mL for gold nanoparticles | 3.5 × 103 cfu/mL (buffer) 3.5 × 104 cfu/g (lettuce samples) | [58,85,93] |
| E. coli O157:H7 and Salmonella typhimurium | E. coli O157:H7 105 cfu/mL for gold nanoparticles 104 cfu/mL for fluorescent microspheres Salmonella typhimurium 104 cfu/mL for gold nanoparticles | 23 CFU/mL for E. coli 17 CFU/mL for Salmonella | [87,94,95] |
| Potato virus X | 0.25 ng/mL for combination of magnetic nanoparticles with gold nanoparticles | 8 ng/mL | [25] |
| Enterobacter cloacae | 103 cfu/mL for gold nanoparticles | 102 cfu/mL | [88] |
| β-conglutin | 5 nM for gold nanoparticles | 8 fM | [35] |
| Fluorescent nanoparticles | |||
| Aflatoxin B1 | 10 µg/mL for gold nanoparticles 0.1 ng/mL for silver@gold nanoparticles | 3 pg/mL in sauce extract 51 pg/mL in real dark soy sauce | [84,96,97] |
| Listeria monocytogenes | 3.7 × 106 cfu/mL for gold nanoparticles | 104 CFU/mL | [89,93] |
| Electrochemical detection | |||
| Escherichia coli O157:H7 | 105 cfu/mL for gold nanoparticles 104 cfu/mL for fluorescent microspheres | 6.2 × 104 CFU/mL | [86,95] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moyano, A.; Serrano-Pertierra, E.; Salvador, M.; Martínez-García, J.C.; Rivas, M.; Blanco-López, M.C. Magnetic Lateral Flow Immunoassays. Diagnostics 2020, 10, 288. https://doi.org/10.3390/diagnostics10050288
Moyano A, Serrano-Pertierra E, Salvador M, Martínez-García JC, Rivas M, Blanco-López MC. Magnetic Lateral Flow Immunoassays. Diagnostics. 2020; 10(5):288. https://doi.org/10.3390/diagnostics10050288
Chicago/Turabian StyleMoyano, Amanda, Esther Serrano-Pertierra, María Salvador, José Carlos Martínez-García, Montserrat Rivas, and M. Carmen Blanco-López. 2020. "Magnetic Lateral Flow Immunoassays" Diagnostics 10, no. 5: 288. https://doi.org/10.3390/diagnostics10050288
APA StyleMoyano, A., Serrano-Pertierra, E., Salvador, M., Martínez-García, J. C., Rivas, M., & Blanco-López, M. C. (2020). Magnetic Lateral Flow Immunoassays. Diagnostics, 10(5), 288. https://doi.org/10.3390/diagnostics10050288