Circulating Monocyte Count as a Surrogate Marker for Ventricular-Arterial Remodeling and Incident Heart Failure with Preserved Ejection Fraction
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Subjects
2.2. Determination of Ventricular-Arterial Remodeling
2.2.1. Assessment of Ventricular Remodeling
2.2.2. Assessment of Carotid Arterial Remodeling
2.3. Determination of the Serum Biomarker, N-Terminal Pro-Brain Natriuretic Peptide (Nt-ProBNP), and the Inflammatory Marker, High Sensitivity C-Reactive Protein (hs-CRP)
2.4. Statistical Analysis
3. Results
3.1. Baseline Demographics and Cardiac Remodeling with CCAD
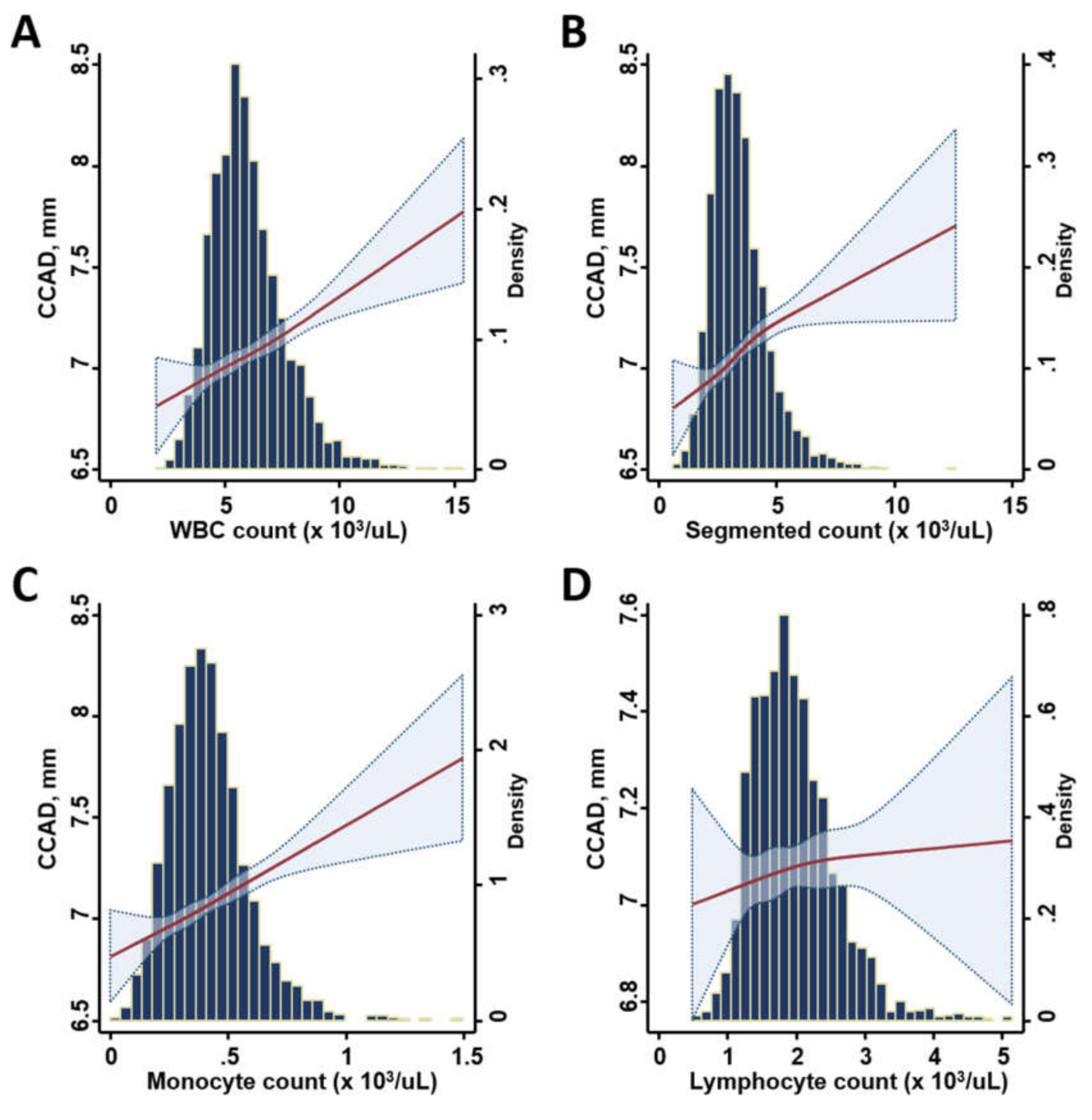
3.2. Association of Various Leukocyte Counts with the Inflammatory Marker, CCAD, and Cardiac Remodeling
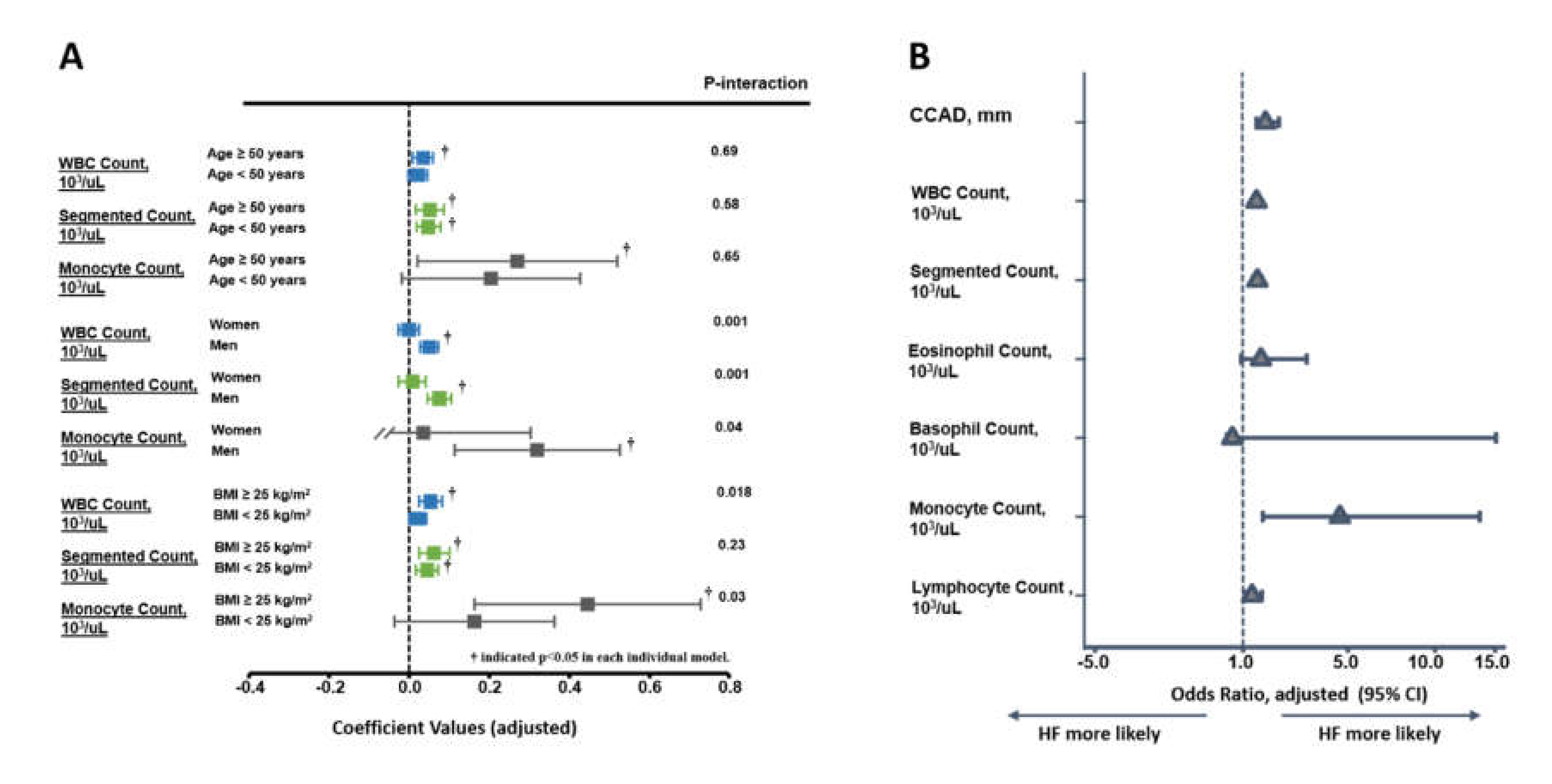
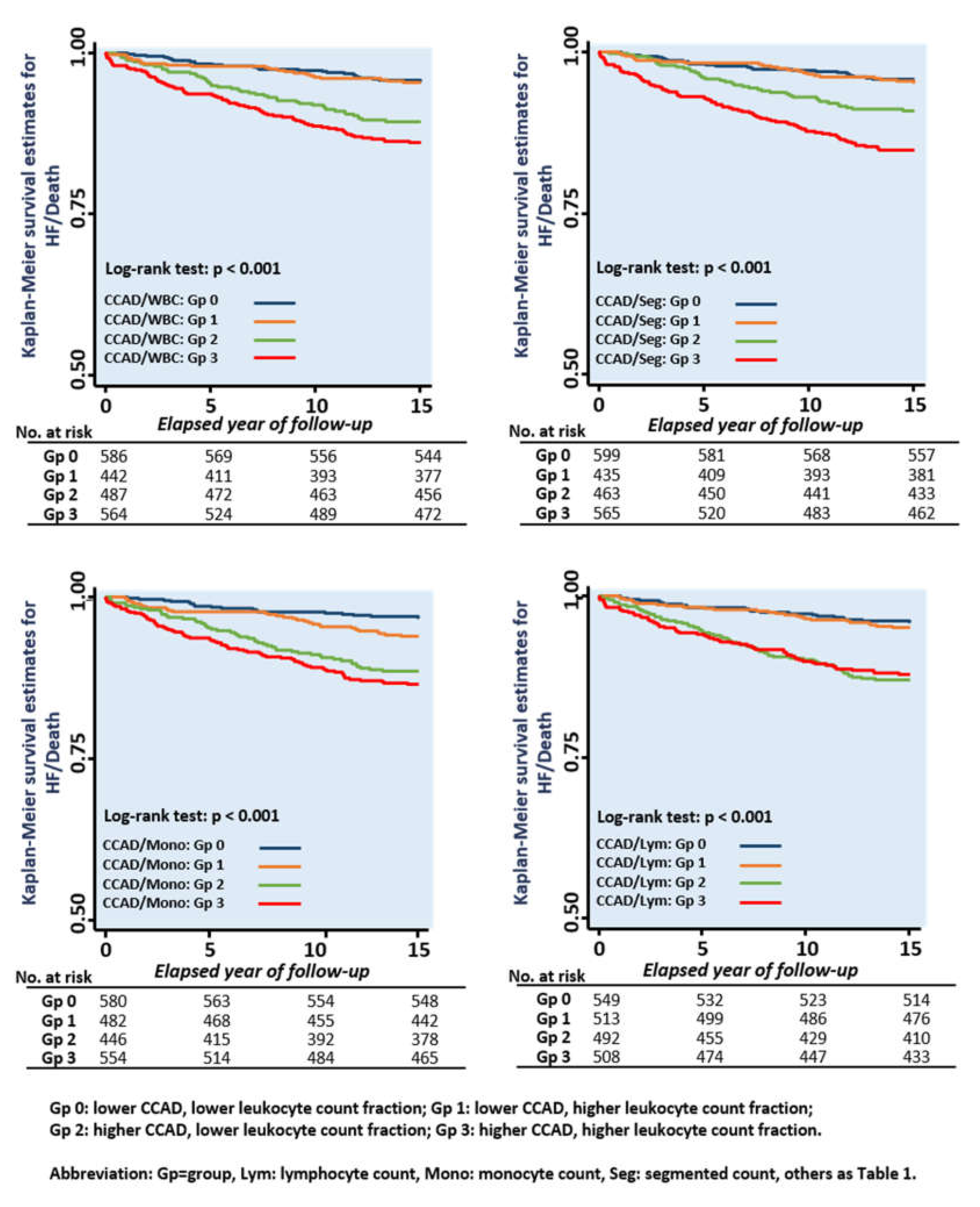
3.3. Associations of Various Leukocyte Counts with CCAD and Clinical Outcomes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Redfield, M.M. Heart failure with preserved ejection fraction. N. Engl. J. Med. 2016, 375, 1868–1877. [Google Scholar] [CrossRef] [PubMed]
- Owan, T.E.; Redfield, M.M. Epidemiology of diastolic heart failure. Prog. Cardiovasc. Dis. 2005, 47, 320–332. [Google Scholar] [CrossRef] [PubMed]
- Upadhya, B.; Kitzman, D.W. Heart Failure with Preserved Ejection Fraction in Older Adults. Heart Fail. Clin. 2017, 13, 485–502. [Google Scholar] [CrossRef] [PubMed]
- Borlaug, B.A.; Lam, C.S.; Roger, V.L.; Rodeheffer, R.J.; Redfield, M.M. Contractility and ventricular systolic stiffening in hypertensive heart disease insights into the pathogenesis of heart failure with preserved ejection fraction. J. Am. Coll. Cardiol. 2009, 54, 410–418. [Google Scholar] [CrossRef] [PubMed]
- Velagaleti, R.S.; Gona, P.; Pencina, M.J.; Aragam, J.; Wang, T.J.; Levy, D.; D’Agostino, R.B.; Lee, D.S.; Kannel, W.B.; Benjamin, E.J.; et al. Left ventricular hypertrophy patterns and incidence of heart failure with preserved versus reduced ejection fraction. Am. J. Cardiol. 2014, 113, 117–122. [Google Scholar] [CrossRef]
- Liao, Z.Y.; Peng, M.C.; Yun, C.H.; Lai, Y.H.; Po, H.L.; Hou, C.J.; Kuo, J.Y.; Hung, C.L.; Wu, Y.J.; Bulwer, B.E.; et al. Relation of carotid artery diameter with cardiac geometry and mechanics in heart failure with preserved ejection fraction. J. Am. Heart Assoc. 2012, 1, e003053. [Google Scholar] [CrossRef]
- Chen, C.H.; Nakayama, M.; Nevo, E.; Fetics, B.J.; Maughan, W.L.; Kass, D.A. Coupled systolic ventricular and vascular stiffening with age implications for pressure regulation and cardiac reserve in the elderly. J. Am. Coll. Cardiol. 1998, 32, 1221–1227. [Google Scholar] [CrossRef]
- Glezeva, N.; Baugh, J.A. Role of inflammation in the pathogenesis of heart failure with preserved ejection fraction and its potential as a therapeutic target. Heart Fail. Rev. 2014, 19, 681–694. [Google Scholar] [CrossRef]
- Mozos, I.; Malainer, C.; Horbańczuk, J.; Gug, C.; Stoian, D.; Luca, C.T.; Atanasov, A.G. Inflammatory Markers for Arterial Stiffness in Cardiovascular Diseases. Front. Immunol. 2017, 8, 1058. [Google Scholar] [CrossRef]
- Shah, S.J.; Kitzman, D.W.; Borlaug, B.A.; van Heerebeek, L.; Zile, M.R.; Kass, D.A. Phenotype-Specific Treatment of Heart Failure With Preserved Ejection Fraction: A Multiorgan Roadmap. Circulation 2016, 134, 73–90. [Google Scholar] [CrossRef]
- Nishida, K.; Otsu, K. Inflammation and metabolic cardiomyopathy. Cardiovasc. Res. 2017, 113, 389–398. [Google Scholar] [CrossRef]
- Lim, G.B. Heart failure: Macrophages promote cardiac fibrosis and diastolic dysfunction. Nat. Rev. Cardiol. 2018, 15, 196–197. [Google Scholar]
- Shahid, F.; Lip, G.Y.H.; Shantsila, E. Role of Monocytes in Heart Failure and Atrial Fibrillation. J. Am. Heart Assoc. 2018, 7, e007849. [Google Scholar] [CrossRef]
- Hung, C.L.; Po, H.L.; Liu, C.C.; Yen, C.H.; Wu, Y.J.; Hou, C.J.; Kuo, J.Y.; Yeh, H.I.; Su, S. The utilization of carotid artery imaging beyond metabolic scores and high-sensitivity CRP in screening intermediate-to-high Framingham risk of asymptomatic Taiwanese population. Int. J. Cardiovasc. Imaging 2013, 29, 765–775. [Google Scholar] [CrossRef] [PubMed]
- Lai, Y.H.; Yun, C.H.; Yang, F.S.; Liu, C.C.; Wu, Y.J.; Kuo, J.Y.; Yeh, H.I.; Lin, T.Y.; Bezerra, H.G.; Shih, S.C.; et al. Epicardial adipose tissue relating to anthropometrics, metabolic derangements and fatty liver disease independently contributes to serum high-sensitivity C-reactive protein beyond body fat composition: A study validated with computed tomography. J. Am. Soc. Echocardiogr. 2012, 25, 234–241. [Google Scholar] [CrossRef]
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. 2015, 28, 1–39. [Google Scholar] [CrossRef]
- Xu, X.; Wang, B.; Ren, C.; Hu, J.; Greenberg, D.A.; Chen, T.; Xie, L.; Jin, K. Age-related Impairment of Vascular Structure and Functions. Aging Dis. 2017, 8, 590–610. [Google Scholar] [CrossRef] [PubMed]
- Virmani, R.; Avolio, A.P.; Mergner, W.J.; Robinowitz, M.; Herderick, E.E.; Cornhill, J.F.; Guo, S.Y.; Liu, T.H.; Ou, D.Y.; O’Rourke, M. Effect of aging on aortic morphology in populations with high and low prevalence of hypertension and atherosclerosis. Comparison between occidental and Chinese communities. Am. J. Pathol. 1991, 139, 1119–1129. [Google Scholar] [PubMed]
- Bonithon-Kopp, C.; Touboul, P.J.; Berr, C.; Magne, C.; Ducimetière, P. Factors of carotid arterial enlargement in a population aged 59 to 71 years: The EVA study. Stroke 1996, 27, 654–660. [Google Scholar] [CrossRef] [PubMed]
- Laurent, S. Arterial wall hypertrophy and stiffness in essential hypertensive patients. Hypertension 1995, 26, 355–362. [Google Scholar] [CrossRef] [PubMed]
- Benetos, A.; Laurent, S.; Hoeks, A.P.; Boutouyrie, P.H.; Safar, M.E. Arterial alterations with aging and high blood pressure. A noninvasive study of carotid and femoral arteries. Arterioscler. Thromb. 1993, 13, 90–97. [Google Scholar] [CrossRef] [PubMed]
- Crouse, J.R.; Goldbourt, U.; Evans, G.; Pinsky, J.; Sharrett, A.R.; Sorlie, P.; Riley, W.; Heiss, G. Risk factors and segment-specific carotid arterial enlargement in the Atherosclerosis Risk in Communities (ARIC) cohort. Stroke 1996, 27, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Chironi, G.; Gariepy, J.; Denarie, N.; Balice, M.; Megnien, J.L.; Levenson, J.; Simon, A. Influence of hypertension on early carotid artery remodeling. Arterioscler. Thromb. Vasc. Biol. 2003, 23, 1460–1464. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.J.; Lee, J.W.; Kim, J.K.; Lee, J.H.; Kim, J.H.; Kwon, K.Y.; Lee, H.R.; Lee, D.C.; Shim, J.Y. Elevated white blood cell count is associated with arterial stiffness. Nutr. Metab. Cardiovasc. Dis. 2009, 19, 3–7. [Google Scholar] [CrossRef] [PubMed]
- Glezeva, N.; Voon, V.; Watson, C.; Horgan, S.; McDonald, K.; Ledwidge, M.; Baugh, J. Exaggerated inflammation and monocytosis associate with diastolic dysfunction in heart failure with preserved ejection fraction: Evidence of M2 macrophage activation in disease pathogenesis. J. Card. Fail. 2015, 21, 167–177. [Google Scholar] [CrossRef] [PubMed]
- Duprez, D.A.; Somasundaram, P.E.; Sigurdsson, G.; Hoke, L.; Florea, N.; Cohn, J.N. Relationship between C-reactive protein and arterial stiffness in an asymptomatic population. J. Hum. Hypertens. 2005, 19, 515–519. [Google Scholar] [CrossRef]
- Ikeda, U.; Takahashi, M.; Shimada, K. C-reactive protein directly inhibits nitric oxide production by cytokine stimulated vascular smooth muscle cells. J. Cardiovasc. Pharmacol. 2003, 42, 607–611. [Google Scholar] [CrossRef]
- King, D.E.; Egan, B.M.; Mainous, I.I.I.A.G.; Geesey, M.E. Elevation of C-reactive protein in people with prehypertension. J. Clin. Hypertens. (Greenwich) 2004, 6, 562–568. [Google Scholar] [CrossRef]
- Pyo, R.; Lee, J.K.; Shipley, J.M.; Curci, J.A.; Mao, D.; Ziporin, S.J.; Ennis, T.L.; Shapiro, S.D.; Senior, R.M.; Thompson, R.W. Targeted gene disruption of matrix metalloproteinase-9 (gelatinase B) suppresses development of experimental abdominal aortic aneurysms. J. Clin. Investig. 2000, 105, 1641–1649. [Google Scholar] [CrossRef]
- Ismahil, M.A.; Hamid, T.; Bansal, S.S.; Patel, B.; Kingery, J.R.; Prabhu, S.D. Remodeling of the mononuclear phagocyte network underlies chronic inflammation and disease progression in heart failure: Critical importance of the cardiosplenic axis. Circ. Res. 2014, 114, 266–282. [Google Scholar] [CrossRef]
- Földes, G.; von Haehling, S.; Okonko, D.O.; Jankowska, E.A.; Poole-Wilson, P.A.; Anker, S.D. Fluvastatin reduces increased blood monocyte Toll-like receptor 4 expression in whole blood from patients with chronic heart failure. Int. J. Cardiol. 2008, 124, 80–85. [Google Scholar] [CrossRef] [PubMed]
- Kapellos, T.S.; Bonaguro, L.; Gemünd, I.; Reusch, N.; Saglam, A.; Hinkley, E.R.; Schultze, J.L. Human Monocyte Subsets and Phenotypes in Major Chronic Inflammatory Diseases. Front. Immunol. 2019, 10, 2035. [Google Scholar] [CrossRef] [PubMed]
- Gu, L.; Okada, Y.; Clinton, S.K.; Gerard, C.; Sukhova, G.K.; Libby, P.; Rollins, B.J. Absence of monocyte chemoattractant protein-1 reduces atherosclerosis in low density lipoprotein receptor-deficient mice. Mol. Cell 1998, 2, 275–281. [Google Scholar] [CrossRef]
- Mewhort, H.E.; Lipon, B.D.; Svystonyuk, D.A.; Teng, G.; Guzzardi, D.G.; Silva, C.; Yong, V.W.; Fedak, P.W. Monocytes increase human cardiac myofibroblast-mediated extracellular matrix remodeling through TGF β1. Am. J. Physiol. Heart Circ. Physiol. 2016, 310, H716–H724. [Google Scholar] [CrossRef]
- Newby, A.C. Metalloproteinase expression in monocytes and macrophages and its relationship to atherosclerotic plaque instability. Arterioscler. Thromb. Vasc. Biol. 2008, 28, 2108–2114. [Google Scholar] [CrossRef]
- Thompson, R.W.; Holmes, D.R.; Mertens, R.A.; Liao, S.; Botney, M.D.; Mecham, R.P.; Welgus, H.G.; Parks, W.C. Production and localization of 92 kilodalton gelatinase in abdominal aortic aneurysms. An elastolytic metalloproteinase expressed by aneurysm-infiltrating macrophages. J. Clin. Investig. 1995, 96, 318–326. [Google Scholar] [CrossRef]
- Pasterkamp, G.; Galis, Z.S.; de Kleijn, D.P. Expansive arterial remodeling: Location, location, location. Arterioscler. Thromb. Vasc. Biol. 2004, 24, 650–657. [Google Scholar] [CrossRef]
- Franssen, C.; Chen, S.; Unger, A.; Korkmaz, H.I.; De Keulenaer, G.W.; Tschöpe, C.; Leite-Moreira, A.F.; Musters, R.; Niessen, H.W.; Linke, W.A.; et al. Myocardial Microvascular Inflammatory Endothelial Activation in Heart Failure With Preserved Ejection Fraction. JACC Heart Fail. 2016, 4, 312–324. [Google Scholar] [CrossRef]
- Jensen-Urstad, K.; Jensen-Urstad, M.; Johansson, J. Carotid artery diameter correlates with risk factors for cardiovascular disease in a population of 55-year-old subjects. Stroke 1999, 30, 1572–1576. [Google Scholar] [CrossRef]
- Crouse, J.R.; Goldbourt, U.; Evans, G.; Pinsky, J.; Sharrett, A.R.; Sorlie, P.; Riley, W.; Heiss, G. Arterial enlargement in the atherosclerosis risk in communities (ARIC) cohort. In vivo quantification of carotid arterial enlargement. The ARIC Investigators. Stroke 1994, 25, 1354–1359. [Google Scholar] [CrossRef]
- Eigenbrodt, M.L.; Sukhija, R.; Rose, K.M.; Tracy, R.E.; Couper, D.J.; Evans, G.W.; Bursac, Z.; Mehta, J.L. Common carotid artery wall thickness and external diameter as predictors of prevalent and incident cardiac events in a large population study. Cardiovasc. Ultrasound 2007, 5, 11. [Google Scholar] [CrossRef] [PubMed]
- Yetkin, E.; Ozturk, S.; Yetkin, G.I. Inflammation in Coronary Artery Ectasia Compared to Atherosclerosis. Int. J. Mol. Sci. 2018, 19, 2971. [Google Scholar] [CrossRef] [PubMed]
- Walker, A.E.; Seibert, S.M.; Donato, A.J.; Pierce, G.L.; Seals, D.R. Vascular endothelial function is related to white blood cell count and myeloperoxidase among healthy middle-aged and older adults. Hypertension 2010, 55, 363–369. [Google Scholar] [CrossRef] [PubMed]
| Metabolic Score Categories | All Subjects | CCAD Quartiles | p (Trend) | |||||
|---|---|---|---|---|---|---|---|---|
| (n = 2085) | Pearson (R) | p Value | Q1 (n = 546) | Q2 (n = 530) | Q3 (n = 506) | Q4 (n = 503) | ||
| Baseline Demographics | ||||||||
| Age, yrs. | 51.03 (10.73) | 0.40 | < 0.001 | 46.28 (9.15) | 49.32 (9.34) | 51.76 (9.94) | 57.25 (11.34) | < 0.001 |
| Female sex, n (%) | 873 (41.20) | — | — | 347 (63.55) | 234 (44.15) | 147 (29.05) | 132 (26.24) | < 0.001 |
| Systolic blood pressure, mm Hg | 121.55 (17.55) | 0.42 | < 0.001 | 112.94 (14.62) | 118.94 (15.46) | 123.81 (15.90) | 131.38 (18.75) | < 0.001 |
| Diastolic blood pressure, mm Hg | 75.51 (10.49) | 0.31 | < 0.001 | 71.05 (10.09) | 74.70 (9.73) | 77.29 (9.56) | 79.43 (10.67) | < 0.001 |
| Pulse pressure, mm Hg | 46.05 (12.03) | 0.34 | < 0.001 | 41.89 (9.221) | 44.24 (10.29) | 46.52 (11.42) | 51.92 (14.36) | < 0.001 |
| Heart rate, min−1 | 74.71 (10.11) | 0.02 | 0.47 | 74.39 (9.63) | 74.41 (9.91) | 75.19 (19.41) | 74.90 (10.53) | 0.246 |
| Waist circumference, cm | 82.37 (10.60) | 0.39 | < 0.001 | 76.86 (9.79) | 80.69 (9.05) | 84.85 (9.55) | 87.62 (10.69) | < 0.001 |
| Weight, kg | 65.25 (12.27) | 0.32 | < 0.001 | 59.59 (10.44) | 63.62 (10.61) | 68.46 (12.43) | 69.86 (12.78) | < 0.001 |
| BMI, kg/m2 | 24.30 (3.65) | 0.31 | < 0.001 | 22.78 (3.15) | 23.85 (3.19) | 24.97 (3.58) | 25.74 (3.94) | < 0.001 |
| Body fat, % | 26.85 (7.40) | 0.04 | < 0.001 | 26.67 (6.93) | 26.88 (7.74) | 26.58 (7.29) | 27.30 (7.61) | 0.277 |
| Laboratory Data | ||||||||
| Fasting glucose, mg/dL | 100.36 (23.77) | 0.21 | < 0.001 | 94.42 (15.69) | 97.92 (20.46) | 101.67 (22.83) | 108.18 (31.81) | < 0.001 |
| Total cholesterol, mg/dL | 199.05 (37.68) | 0.07 | 0.002 | 195.16 (35.67) | 199.56 (40.96) | 199.18 (32.81) | 202.58 (40.42) | 0.003 |
| Triglyceride, mg/dL | 136.15 (115.02) | 0.15 | < 0.001 | 113.50 (84.06) | 132.20 (149.04) | 141.96 (85.31) | 159.14 (124.29) | < 0.001 |
| HDL, mg/dL | 55.30 (15.86) | −0.21 | < 0.001 | 60.47 (17.05) | 56.26 (15.48) | 52.83 (14.21) | 51.19 (14.84) | < 0.001 |
| LDL, mg/dL | 129.95 (33.15) | 0.10 | < 0.001 | 124.28 (32.15) | 129.78 (32.40) | 131.95 (29.84) | 134.25 (37.13) | < 0.001 |
| Uric acid, mg/dL | 5.88 (1.48) | 0.25 | < 0.001 | 5.37 (1.38) | 5.81 (1.38) | 6.08 (1.43) | 6.32 (1.55) | < 0.001 |
| e-GFR, ml/min/1.73 m2 | 87.57 (17.69) | −0.17 | < 0.001 | 91.13 (16.72) | 88.08 (16.50) | 87.84 (17.17) | 82.86 (19.41) | < 0.001 |
| Leukocyte Counts | ||||||||
| WBC count, 103/µL | 6.01 (1.62) | 0.15 | < 0.001 | 5.78 (1.48) | 5.83 (1.58) | 6.08 (1.61) | 6.36 (1.77) | < 0.001 |
| Segmented count, 103/µL | 3.43 (1.21) | 0.15 | < 0.001 | 3.27 (1.14) | 3.26 (1.12) | 3.52 (1.27) | 3.69 (1.29) | < 0.001 |
| Monocyte count, 103/µL | 0.42 (0.17) | 0.15 | < 0.001 | 0.39 (0.15) | 0.41 (0.17) | 0.43 (0.17) | 0.45 (0.18) | < 0.001 |
| Lymphocyte count, 103/µL | 1.96 (0.60) | 0.03 | 0.22 | 1.94 (0.58) | 1.95 (0.62) | 1.94 (0.58) | 1.99 (0.61) | 0.15 |
| Biomarkers | ||||||||
| hs-CRP (median, 25th–75th), mg/L | 0.090 (0.043–0.210) | 0.11 | < 0.001 | 0.069 (0.030–0.155) | 0.079 (0.040–0.165) | 0.103 (0.050–0.230) | 0.130 (0.070–0.270) | < 0.001 |
| Nt-ProBNP (median, 25th–75th), pg/mL | 28.05 (14.98–55.93) | 0.15 | < 0.001 | 31.15 (18.68–54.83) | 26.95 (14.55–57.73) | 22.60 (10.85–41.60) | 33.55 (15.08–73.80) | < 0.001 |
| Medical Histories | ||||||||
| Hypertension, n (%) | 311 (14.68) | — | — | 30 (5.49) | 66 (12.45) | 80 (15.81) | 135 (26.84) | < 0.001 |
| Diabetes, n (%) | 113 (5.33) | — | — | 14 (2.56) | 23 (4.34) | 27 (5.34) | 49 (9.74) | < 0.001 |
| CVD, n (%) | 93 (5.63) | — | — | 15 (3.52) | 19 (4.49) | 17 (4.12) | 42 (10.77) | < 0.001 |
| Regular exercise, n (%) | 219 (21.88) | — | — | 49 (18.49) | 49 (19.68) | 57 (24.57) | 58 (26.01) | 0.021 |
| Active smoking, n (%) | 187 (8.82) | — | — | 37 (15.68) | 44 (20.00) | 40 (20.83) | 62 (33.88) | < 0.001 |
| Cardiac Structure and Function (n = 1805) | ||||||||
| IVS, mm | 9.96 (1.53) | 0.34 | < 0.001 | 9.29 (1.39) | 9.81 (1.48) | 10.13 (1.41) | 10.65 (1.52) | < 0.001 |
| LVPW, mm | 9.80 (1.39) | 0.34 | < 0.001 | 9.16 (1.26) | 9.72 (1.34) | 9.92 (1.26) | 10.44 (1.38) | < 0.001 |
| LVIDd, mm | 46.67 (3.85) | 0.30 | < 0.001 | 45.18 (3.59) | 46.22 (3.56) | 47.18 (3.83) | 48.19 (3.77) | < 0.001 |
| LVEF, % | 67.18 (4.84) | −0.05 | < 0.001 | 67.41 (4.58) | 67.31 (4.94) | 67.29 (4.78) | 66.70 (5.02) | 0.038 |
| RWT | 42.52 (6.10) | 0.18 | < 0.001 | 40.99 (5.35) | 42.49 (6.51) | 42.73 (5.90) | 43.96 (6.23) | < 0.001 |
| LV mass, gm | 152.62 (39.79) | 0.43 | < 0.001 | 131.27 (33.97) | 146.99 (33.91) | 157.94 (36.08) | 175.83 (41.42) | < 0.001 |
| LVMi, gm/m2 | 59.00 (15.80) | 0.38 | < 0.001 | 51.89 (13.45) | 57.33 (14.28) | 59.66 (14.11) | 67.64 (17.12) | < 0.001 |
| LVH, n (%) | 278 (15.5) | — | — | 38 (8.3) | 61 (13.2) | 68 (15.3) | 111 (25.8) | < 0.001 |
| Independent Variables | hs-CRP, mg/L | CCAD, mm | LVMi, gm/m2 † | RWT | ||||
|---|---|---|---|---|---|---|---|---|
| Univariate (unit: 103/µL) | ß-Coef. | p value | ß-Coef. | p value | ß-Coef. | p value | ß-Coef. | p value |
| WBC Count | 0.25 | < 0.001 | 0.07 | < 0.001 | 0.03 | 0.15 | 0.1 | < 0.001 |
| Segmented Count | 0.27 | < 0.001 | 0.09 | < 0.001 | 0.03 | 0.29 | 0.09 | < 0.001 |
| Monocyte Count | 0.16 | < 0.001 | 0.15 | < 0.001 | 0.11 | < 0.001 | 0.12 | < 0.001 |
| Lymphocyte Count | 0.08 | 0.001 | 0.03 | 0.222 | −0.005 | 0.84 | 0.06 | 0.008 |
| Multivariate Model 1 | ß-Coef. | p value | ß-Coef. | p value | ß-Coef. | p value | ß-Coef. | p value |
| WBC Count | 0.24 | < 0.001 | 0.04 | < 0.001 | 0.03 | 0.26 | 0.09 | < 0.001 |
| Segmented Count | 0.26 | < 0.001 | 0.06 | < 0.001 | 0.01 | 0.6 | 0.06 | 0.006 |
| Monocyte Count | 0.15 | < 0.001 | 0.06 | 0.002 | 0.08 | < 0.001 | 0.09 | < 0.001 |
| Lymphocyte Count | 0.08 | 0.002 | 0.004 | 0.87 | 0.01 | 0.64 | 0.06 | 0.008 |
| Multivariate Model 2 | ß-Coef. | p value | ß-Coef. | p value | ß-Coef. | p value | ß-Coef. | p value |
| WBC Count | 0.24 | < 0.001 | 0.03 | 0.001 | −0.01 | 0.73 | 0.06 | 0.009 |
| Segmented Count | 0.25 | < 0.001 | 0.05 | < 0.001 | −0.01 | 0.52 | 0.05 | 0.037 |
| Monocyte Count | 0.15 | < 0.001 | 0.05 | 0.007 | 0.06 | 0.01 | 0.08 | 0.001 |
| Lymphocyte Count | 0.07 | 0.007 | 0.004 | 0.88 | −0.01 | 0.66 | 0.04 | 0.064 |
| Multivariate Cox Regression Models | ||||||||
|---|---|---|---|---|---|---|---|---|
| (WBC Count) | (Segmented) | (Monocyte) | (Lymphocyte) | |||||
| HR (95% CI) | p Value | HR (95% CI) | p Value | HR (95% CI) | p Value | HR (95% CI) | p Value | |
| CCAD | 1.33 (1.08, 1.65) | 0.008 | 1.34 (1.08, 1.66) | 0.007 | 1.33 (1.08, 1.65) | 0.009 | 1.35 (1.09, 1.68) | 0.006 |
| WBC Count, 103/µL | 1.11 (1.02, 1.22) | 0.018 | — | — | — | — | — | — |
| P interaction (CCAD) | — | 0.56 | — | — | — | — | — | — |
| Segmented Count, 103/µL | — | — | 1.15 (1.03, 1.29) | 0.016 | — | — | — | — |
| P interaction (CCAD) | — | — | — | 0.90 | — | — | — | — |
| Monocyte Count, 103/µL | — | — | — | — | 2.56 (1.08, 6.04) | 0.032 | — | — |
| P interaction (CCAD) | — | — | — | — | — | 0.035 | — | — |
| Lymphocyte Count, 103/µL | — | — | — | — | — | — | 1.10 (0.80, 1.51) | 0.55 |
| P interaction (CCAD) | — | — | — | — | — | — | — | 0.87 |
| Age, +10 year | 1.66 (1.40, 1.97) | < 0.001 | 1.65 (1.38, 1.96) | < 0.001 | 1.64 (1.38, 1.95) | < 0.001 | 1.63 (1.37, 1.95) | < 0.001 |
| Sex (men), n % | 0.65 (0.46, 0.91) | 0.012 | 0.65 (0.46, 0.91) | 0.011 | 0.62 (0.44, 0.88) | 0.006 | 0.67 (0.48, 0.93) | 0.017 |
| BMI, +1 kg/m2 | 1.05 (1.01, 1.09) | 0.023 | 1.05 (1.01, 1.09) | 0.021 | 1.05 (1.01, 1.09) | 0.02 | 1.05 (1.01, 1.10) | 0.02 |
| Hypertension | 1.50 (1.03, 2.18) | 0.036 | 1.48 (1.02, 2.17) | 0.041 | 1.53 (1.05, 2.22) | 0.027 | 1.55 (1.06, 2.26) | 0.023 |
| CVD | 1.71 (1.05, 2.81) | 0.032 | 1.70 (1.04, 2.79) | 0.034 | 1.70 (1.04, 2.78) | 0.033 | 1.69 (1.03, 2.75) | 0.037 |
| Glucose, +10 mg/dL | 1.05 (0.99, 1.11) | 0.089 | 1.05 (1.00, 1.11) | 0.078 | 1.05 (1.00, 1.12) | 0.062 | 1.06 (1.00, 1.12) | 0.042 |
| Cox Regression Models | CCAD and Leukocyte Counts (103/µL) Categories | |||
|---|---|---|---|---|
| CCAD/WBC Categories | CCAD ≤ 7, WBC ≤ 5.8 | CCAD ≤ 7, WBC > 5.8 | CCAD >7, WBC ≤ 5.8 | CCAD > 7, WBC > 5.8 |
| Crude HR | (Reference) | 1.02 (0.58, 1.79), p = 0.96 | 2.45 (1.51, 3.96), p < 0.001 | 3.30 (2.11, 5.14), p < 0.001 |
| Adjusted HR | (Reference) | 0.94 (0.52, 1.69), p = 84 | 1.31 (0.77, 2.21), p = 0.32 | 1.98 (1.20, 3.28), p = 0.008 |
| CCAD/Segmented Count Categories | CCAD ≤ 7, Seg ≤ 3.25 | CCAD ≤ 7, Seg > 3.25 | CCAD > 7, Seg ≤ 3.25 | CCAD > 7, Seg > 3.25 |
| Crude HR | (Reference) | 1.13 (0.64, 2.01), p = 0.67 | 2.21 (1.34, 3.66), p = 0.002 | 3.85 (2.47, 6.02), p < 0.001 |
| Adjusted HR | (Reference) | 0.96 (0.53, 1.74), p = 0.90 | 1.22 (0.71, 2.10), p = 0.48 | 2.18 (1.31, 3.62), p = 0.003 |
| CCAD/Monocyte Count Categories | CCAD ≤ 7, Mono ≤ 0.4 | CCAD ≤ 7, Mono > 0.4 | CCAD > 7, Mono ≤ 0.4 | CCAD > 7, Mono > 0.4 |
| Crude HR | (Reference) | 1.95 (1.08, 3.51), p = 0.026 | 3.80 (2.22, 6.51), p < 0.001 | 4.56 (2.73, 7.64), p < 0.001 |
| Adjusted HR | (Reference) | 2.01 (1.09, 3.69), p = 0.025 | 2.38 (1.33, 4.24), p = 0.003 | 2.81 (1.57, 5.03), p < 0.001 |
| CCAD/Lymphocyte Count Categories | CCAD ≤ 7, Lym ≤ 1.88 | CCAD ≤ 7, Lym > 1.88 | CCAD > 7, Lym ≤ 1.88 | CCAD > 7, Lym > 1.88 |
| Crude HR | (Reference) | 1.22 (0.69, 2.16), p = 0.50 | 3.38 (2.08, 5.49), p < 0.001 | 3.15 (1.93, 5.13), p < 0.001 |
| Adjusted HR | (Reference) | 1.23 (0.68, 2.21), p = 0.49 | 1.87 (1.10, 3.17), p = 0.021 | 2.00 (1.18, 3.40), p = 0.01 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, K.-T.; Liu, Y.-Y.; Sung, K.-T.; Liu, C.-C.; Su, C.-H.; Hung, T.-C.; Hung, C.-L.; Chien, C.-Y.; Yeh, H.-I. Circulating Monocyte Count as a Surrogate Marker for Ventricular-Arterial Remodeling and Incident Heart Failure with Preserved Ejection Fraction. Diagnostics 2020, 10, 287. https://doi.org/10.3390/diagnostics10050287
Wang K-T, Liu Y-Y, Sung K-T, Liu C-C, Su C-H, Hung T-C, Hung C-L, Chien C-Y, Yeh H-I. Circulating Monocyte Count as a Surrogate Marker for Ventricular-Arterial Remodeling and Incident Heart Failure with Preserved Ejection Fraction. Diagnostics. 2020; 10(5):287. https://doi.org/10.3390/diagnostics10050287
Chicago/Turabian StyleWang, Kuang-Te, Yen-Yu Liu, Kuo-Tzu Sung, Chuan-Chuan Liu, Cheng-Huang Su, Ta-Chuan Hung, Chung-Lieh Hung, Chen-Yen Chien, and Hung-I Yeh. 2020. "Circulating Monocyte Count as a Surrogate Marker for Ventricular-Arterial Remodeling and Incident Heart Failure with Preserved Ejection Fraction" Diagnostics 10, no. 5: 287. https://doi.org/10.3390/diagnostics10050287
APA StyleWang, K.-T., Liu, Y.-Y., Sung, K.-T., Liu, C.-C., Su, C.-H., Hung, T.-C., Hung, C.-L., Chien, C.-Y., & Yeh, H.-I. (2020). Circulating Monocyte Count as a Surrogate Marker for Ventricular-Arterial Remodeling and Incident Heart Failure with Preserved Ejection Fraction. Diagnostics, 10(5), 287. https://doi.org/10.3390/diagnostics10050287






