Association of Plasma Oligomerized Beta Amyloid with Neurocognitive Battery Using Korean Version of Consortium to Establish a Registry for Alzheimer’s Disease in Health Screening Population
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Plasma Oligomerized Beta Amyloid Assay
2.3. Clinical Variables
2.4. Statistics
3. Results
3.1. Aging Increases the Level of Oligomerized Beta Amyloid in Blood
3.2. Comparison of High vs. Low OAβ
3.3. OAβ Is Well Correlated with the Consortium to Establish a Registry for Alzheimer’s Disease (CERAD-K) and its Subdomains
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Cummings, J.L.; Lee, G.; Ritter, A.; Sabbagh, M.; Zhong, K. Alzheimer’s disease drug development pipeline: 2019. Alzheimers Dement. 2019, 5, 272–293. [Google Scholar] [CrossRef]
- Alzheimer’s Association. 2015 Alzheimer’s disease facts and figures. Alzheimers Dement. 2015, 11, 332–384. [Google Scholar] [CrossRef] [PubMed]
- Rajan, K.B.; Wilson, R.S.; Weuve, J.; Barnes, L.L.; Evans, D.A. Cognitive impairment 18 years before clinical diagnosis of Alzheimer disease dementia. Neurology 2015, 85, 898–904. [Google Scholar] [CrossRef] [PubMed]
- Jack, C.R., Jr.; Albert, M.S.; Knopman, D.S.; McKhann, G.M.; Sperling, R.A.; Carrillo, M.C.; Thies, B.; Phelps, C.H. Introduction to the recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011, 7, 257–262. [Google Scholar] [CrossRef] [PubMed]
- Jack, C.R., Jr.; Bennett, D.A.; Blennow, K.; Carrillo, M.C.; Dunn, B.; Haeberlein, S.B.; Holtzman, D.M.; Jagust, W.; Jessen, F.; Karlawish, J.; et al. NIA-AA Research Framework: Toward a biological definition of Alzheimer’s disease. Alzheimers Dement. 2018, 14, 535–562. [Google Scholar] [CrossRef]
- Um, J.W.; Nygaard, H.B.; Heiss, J.K.; Kostylev, M.; Stagi, M.; Vortmeyer, A.; Wisniewski, T.; Gunther, E.C.; Strittmatter, S.M. Alzheimer amyloid-β oligomer bound to postsynaptic prion protein activates Fyn to impair neurons. Nat. Neurosci. 2012, 15, 1227–1235. [Google Scholar] [CrossRef]
- Cline, E.N.; Bicca, M.A.; Viola, K.L.; Klein, W.L. The Amyloid- β Oligomer Hypothesis: Beginning of the Third Decade. J. Alzheimers Dis. 2018, 64, S567–S610. [Google Scholar] [CrossRef]
- Koffie, R.M.; Meyer-Luehmann, M.; Hashimoto, T.; Adams, K.W.; Mielke, M.L.; Garcia-Alloza, M.; Micheva, K.D.; Smith, S.J.; Kim, M.L.; Lee, V.M.; et al. Oligomeric amyloid beta associates with postsynaptic densities and correlates with excitatory synapse loss near senile plaques. Proc. Natl. Acad. Sci. USA 2009, 106, 4012–4017. [Google Scholar] [CrossRef]
- Haass, C.; Selkoe, D.J. Soluble protein oligomers in neurodegeneration: Lessons from the Alzheimer’s amyloid β-peptide. Nat. Rev. Mol. Cell Boil. 2007, 8, 101–112. [Google Scholar] [CrossRef]
- Fukumoto, H.; Tokuda, T.; Kasai, T.; Ishigami, N.; Hidaka, H.; Kondo, M.; Allsop, D.; Nakagawa, M. High-molecular-weight beta-amyloid oligomers are elevated in cerebrospinal fluid of Alzheimer patients. FASEB J. 2010, 24, 2716–2726. [Google Scholar] [CrossRef]
- Sun, L.; Zhong, Y.; Gui, J.; Wang, X.; Zhuang, X.; Weng, J. A hydrogel biosensor for high selective and sensitive detection of amyloid-beta oligomers. Int. J. Nanomed. 2018, 13, 843–856. [Google Scholar] [CrossRef] [PubMed]
- An, S.S.A.; Lee, B.S.; Yu, J.S.; Lim, K.; Kim, G.J.; Lee, R.; Kim, S.; Kang, S.; Park, Y.H.; Wang, M.J.; et al. Dynamic changes of oligomeric amyloid beta levels in plasma induced by spiked synthetic Aβ42. Alzheimers Res. Ther. 2017, 9, 86. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.J.; Yi, S.H.; Han, J.; Park, S.Y.; Jang, J.-W.; Chun, I.K.; Kim, S.E.; Lee, B.S.; Kim, G.J.; Yu, J.S.; et al. Oligomeric forms of amyloid-beta protein in plasma as a potential blood-based biomarker for Alzheimer’s disease. Alzheimers Res. Ther. 2017, 9, 98. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Li, T.; Wang, X.; Lv, X.; Sun, Z.; Zhang, J.; Yu, X. Association between increased levels of amyloid-beta oligomers in plasma and episodic memory loss in Alzheimer’s disease. Alzheimers Res. Ther. 2019, 11, 89. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Youn, Y.C.; Lee, B.S.; Kim, G.J.; Ryu, J.S.; Lim, K.; Ryu, N. Blood amyloid-β oligomerization as a biomarker of Alzheimer’s disease: A blinded validation study. J. Alzheimers Dis. 2020, in press. [Google Scholar]
- Youn, Y.C.; Kang, S.; Suh, J.; Park, Y.H.; Kang, M.J.; Pyun, J.M.; An, S.S.A. Blood amyloid-beta oligomerization associated with neurodegeneration of Alzheimer’s disease. Alzheimers Res. Ther. 2019, 11, 40. [Google Scholar] [CrossRef]
- National Institute on Alcohol Abuse and Alcoholism (NIAAA). Drinking Levels Defined. 2016. Available online: https://www.niaaa.nih.gov/alcohol-health/overview-alcohol-consumption/moderate-binge-drinking (accessed on 25 March 2020).
- Rush, A.J.; Trivedi, M.H.; Ibrahim, H.M.; Carmody, T.J.; Arnow, B.; Klein, D.N.; Markowitz, J.C.; Ninan, P.T.; Kornstein, S.; Manber, R.; et al. The 16-Item Quick Inventory of Depressive Symptomatology (QIDS), clinician rating (QIDSC), and self-report (QIDS-SR): A psychometric evaluation in patients with chronic major depression. Biol. Psychiatry 2003, 54, 573e83. [Google Scholar] [CrossRef]
- Janelidze, S.; Stomrud, E.; Palmqvist, S.; Zetterberg, H.; Van Westen, D.; Jeromin, A.; Blennow, K. Plasma β-amyloid in Alzheimer’s disease and vascular disease. Sci. Rep. 2016, 6, 1–11. [Google Scholar] [CrossRef]
- Nakamura, A.; Kaneko, N.; Villemagne, V.L.; Kato, T.; Doecke, J.; Doré, V.; Tomita, T. High performance plasma amyloid-β biomarkers for Alzheimer’s disease. Nature 2018, 554, 249. [Google Scholar] [CrossRef]
- Lue, L.F.; Guerra, A.; Walker, D.G. Amyloid Beta and Tau as Alzheimer’s Disease Blood Biomarkers: Promise From New Technologies. Neurol. Ther. 2017, 6 (Suppl. 1), 25–36. [Google Scholar] [CrossRef]
- Zhou, L.; Chan, K.H.; Chu, L.W.; Kwan, J.S.; Song, Y.Q.; Chen, L.H.; Ho, P.W.; Cheng, O.Y.; Ho, J.W.; Lam, K.S. Plasma amyloid-beta oligomers level is a biomarker for Alzheimer’s disease diagnosis. Biochem. Biophys. Res. Commun. 2012, 423, 697–702. [Google Scholar] [CrossRef]
- Moms, J.C.; Heyman, A.; Mohs, R.C.; Hughes, J.P.; van Belle, G.; Fillenbaum, G.; Mellits, E.D.; Clark, C. The Consortium to Establish a Registry for Alzheimer’s Disease (CERAD). Part I. Clinical and neuropsychological assessment of Alzheimer’s disease. Neurology 1989, 39, 1159–1165. [Google Scholar] [CrossRef] [PubMed]
- Rossetti, H.C.; Cullum, C.M.; Hynan, L.S.; Lacritz, L. The CERAD Neuropsychologic Battery Total Score and the progression of Alzheimer disease. Alzheimer Dis. Assoc. Disord. 2010, 24, 138–142. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.; Jeong, J.H.; Yoon, S.J.; Park, K.W.; Kim, E.J.; Yoon, B.; Jang, J.W.; Kim, H.J.; Hong, J.Y.; Lee, J.M.; et al. Clinical and Biomarker Characteristics According to Clinical Spectrum of Alzheimer’s Disease (AD) in the Validation Cohort of Korean Brain Aging Study for the Early Diagnosis and Prediction of AD. JCM 2019, 8, 341. [Google Scholar] [CrossRef] [PubMed]
- Karrasch, M.; Sinervä, E.; Grönholm, P.; Rinne, J.; Laine, M. CERAD test performances in amnestic mild cognitive impairment and Alzheimer’s disease. Acta Neurol. Scand. 2005, 111, 172–179. [Google Scholar] [CrossRef]
- Bilello, M.; Doshi, J.; Nabavizadeh, S.A.; Toledo, J.B.; Erus, G.; Xie, S.X.; Trojanowski, J.Q.; Han, X.; Davatzikos, C. Correlating Cognitive Decline with White Matter Lesion and Brain Atrophy Magnetic Resonance Imaging Measurements in Alzheimer’s Disease. J. Alzheimers Dis. 2015, 48, 987–994. [Google Scholar] [CrossRef]
- Kwon, J.W.; Kim, H.; Lee, K.J. Association between Cognitive Function, Behavioral and Psychological Symptoms of Dementia and White Matter Hyperintensities in Patients with Alzheimer’s Disease and Mild Cognitive. KJPM 2018, 26, 2119. [Google Scholar]
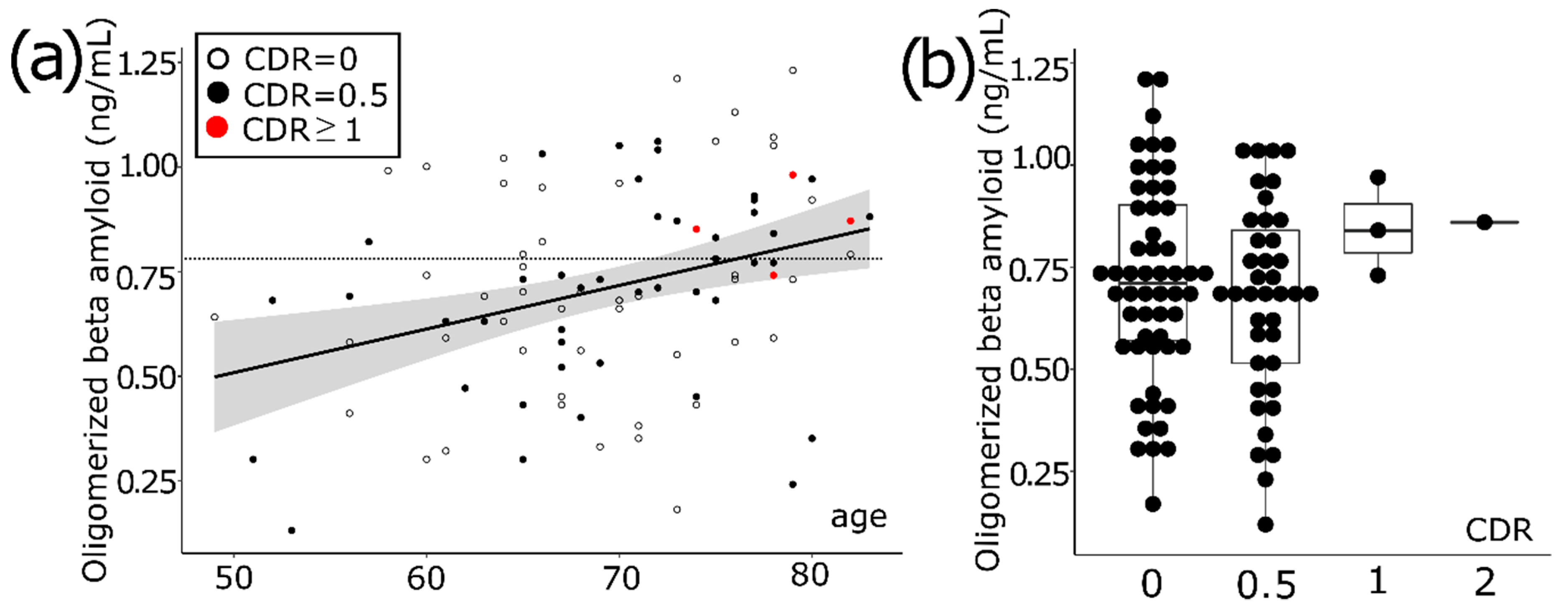
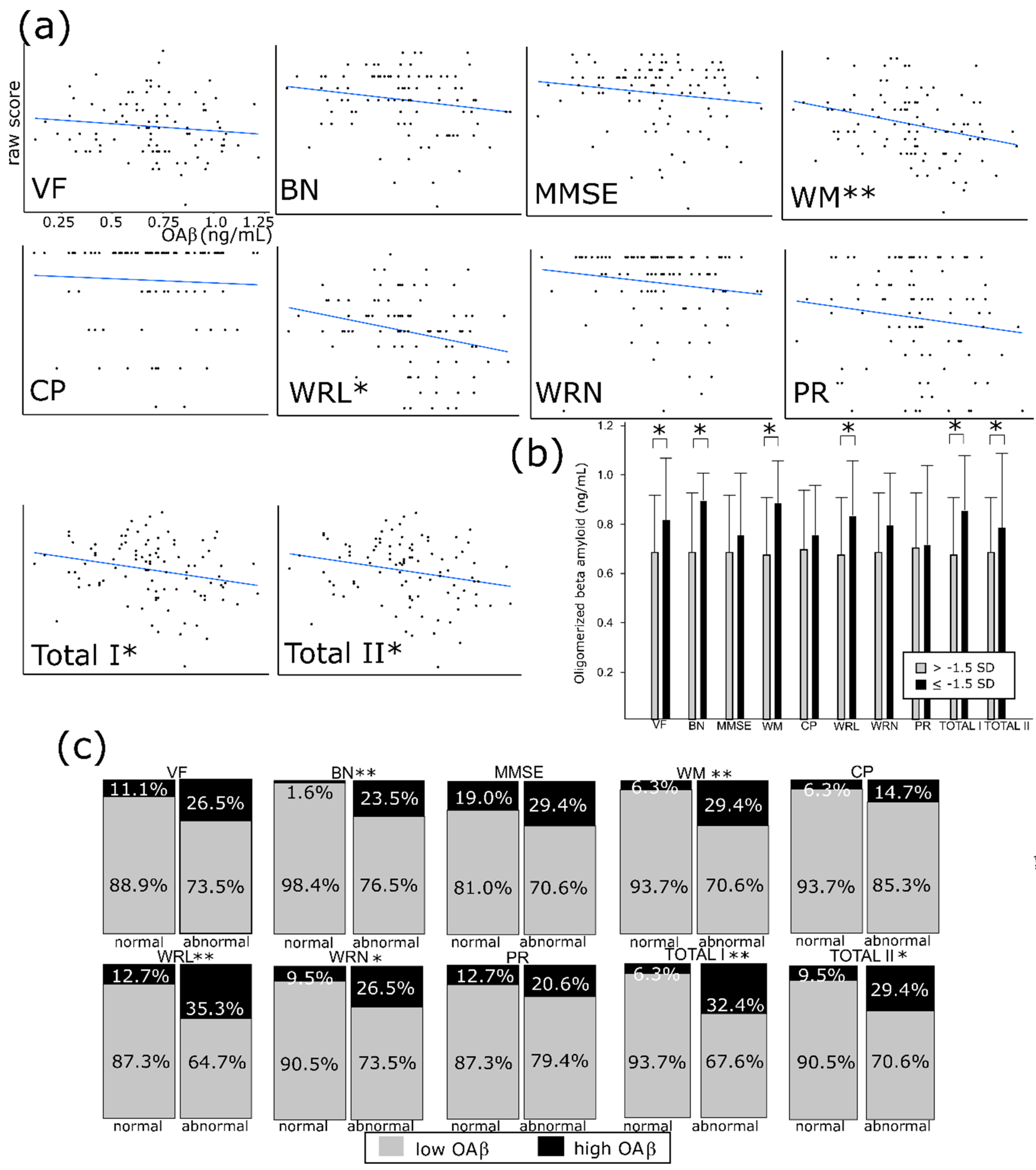
| High OAβ | Low OAβ | p Value | |
|---|---|---|---|
| Age | 72.62 ± 7.00 | 67.67 ± 7.27 | 0.02 |
| Sex (Female %) | 20/34 (58.8) | 38/63 (60.3) | 0.89 |
| Education (yrs) | 14.06 ± 3.26 | 13.30 ±4.05 | 0.34 |
| CDR | 0.24 | ||
| CDR = 0 | 19/34 | 35/63 | |
| CDR = 0.5 | 12/34 | 27/63 | |
| CDR ≥ 1 | 3/34 | 1/63 | |
| GDS | 0.03 | ||
| GDS = 1 | 18/34 | 34/63 | |
| GDS = 2 | 10/34 | 28/63 | |
| GDS ≥ 3 | 6/34 | 1/63 | |
| Apoe4 carrier (%) | 10/32 | 15/58 | 0.59 |
| Hypertension | 12/34 | 29/63 | 0.26 |
| Diabetes | 6/34 | 12/63 | 0.39 |
| Hyperlipidemia | 21/34 | 40/63 | 0.39 |
| Smoking | 0.25 | ||
| Never | 25/34 | 46/63 | |
| Ex | 8/34 | 13/63 | |
| Current | 0/34 | 4/63 | |
| At-risk drinking | 2/25 | 13/58 | 0.21 |
| QIDS-SR(cutoff ≥ 11) | 2/31 | 4/57 | 0.09 |
| CDR = 0 (n = 54) | CDR = 0.5 (n = 39) | |||
|---|---|---|---|---|
| Age | 69.07 ± 7.14 | 68.95 ± 7.90 | ||
| High OAβ (n) | p value | High OAβ (n) | p value | |
| Hypertension | 6/25 | 0.11 | 5/14 | 0.72 |
| Diabetes | 4/12 | 1.00 | 2/6 | 1.00 |
| Hyperlipidemia | 11/35 | 0.43 | 9/25 | 0.48 |
| Problem drinking | 2/9 | 0.65 | 0/6 | 0.30 |
| Smoking | 0.74 | 0.46 | ||
| None | 14/40 | 10/29 | ||
| Ex | 5/13 | 2/7 | ||
| Current | 0/1 | 0/3 | ||
| QIDS-SR (≥moderate) | 2/7 | 0.62 | 2/9 | 0.69 |
| Apoe4 carrier | 6/15 | 0.70 | 2/8 | 1.0 |
| Education (<12 yrs) | 4/10 | 0.73 | 2/8 | 1.0 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, J.-J.; Choi, Y.; Chung, S.; Yoon, D.H.; Choi, S.H.; Kang, S.-M.; Seo, D.; Park, K.-I. Association of Plasma Oligomerized Beta Amyloid with Neurocognitive Battery Using Korean Version of Consortium to Establish a Registry for Alzheimer’s Disease in Health Screening Population. Diagnostics 2020, 10, 237. https://doi.org/10.3390/diagnostics10040237
Lee J-J, Choi Y, Chung S, Yoon DH, Choi SH, Kang S-M, Seo D, Park K-I. Association of Plasma Oligomerized Beta Amyloid with Neurocognitive Battery Using Korean Version of Consortium to Establish a Registry for Alzheimer’s Disease in Health Screening Population. Diagnostics. 2020; 10(4):237. https://doi.org/10.3390/diagnostics10040237
Chicago/Turabian StyleLee, Jung-Ju, Youngki Choi, Soie Chung, Dae Hyun Yoon, Seung Ho Choi, Sung-Min Kang, David Seo, and Kyung-Il Park. 2020. "Association of Plasma Oligomerized Beta Amyloid with Neurocognitive Battery Using Korean Version of Consortium to Establish a Registry for Alzheimer’s Disease in Health Screening Population" Diagnostics 10, no. 4: 237. https://doi.org/10.3390/diagnostics10040237
APA StyleLee, J.-J., Choi, Y., Chung, S., Yoon, D. H., Choi, S. H., Kang, S.-M., Seo, D., & Park, K.-I. (2020). Association of Plasma Oligomerized Beta Amyloid with Neurocognitive Battery Using Korean Version of Consortium to Establish a Registry for Alzheimer’s Disease in Health Screening Population. Diagnostics, 10(4), 237. https://doi.org/10.3390/diagnostics10040237





