Chromatographic Detection of 8-Hydroxy-2′-Deoxyguanosine in Leukocytes of Asbestos Exposed Workers for Assessing Past and Recent Carcinogen Exposures
Abstract
1. Introduction
2. Material and Methods
2.1. Study Population
2.2. DNA Extraction and Purification
2.3. 32P-DNA Postlabeling
2.4. Statistical Analysis
3. Results
3.1. Demographic Variables
3.2. 8-OxodG and Asbestos Workers
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Afaghi, A.; Oryan, S.; Rahzani, K.; Abdollahi, M. Study on genotoxicity, oxidative stress biomarkers and clinical symptoms in workers of an asbestos-cement factory. Excli. J. 2015, 14, 1067–1077. [Google Scholar]
- Knox, J.F.; Holmes, S.; Doll, R.; Hill, I.D. Mortality from lung cancer and other causes among workers in an asbestos textile factory. Br. J. Ind. Med. 1968, 25, 293–303. [Google Scholar] [CrossRef] [PubMed][Green Version]
- IARC. Overall evaluations of carcinogenicity: An updating of IARC Monographs volumes 1 to 42. IARC Monogr. Eval. Carcinog. Risk Chem. Hum. Suppl. 1987, 7, 1–440. [Google Scholar]
- Case, B.W.; Abraham, J.L.; Meeker, G.; Pooley, F.D.; Pinkerton, K.E. Applying definitions of “asbestos” to environmental and “low-dose” exposure levels and health effects, particularly malignant mesothelioma. J. Toxicol. Environ. Health Part B 2011, 14, 3–39. [Google Scholar] [CrossRef]
- Mossman, B.T.; Lippmann, M.; Hesterberg, T.W.; Kelsey, K.T.; Barchowsky, A.; Bonner, J.C. Pulmonary endpoints (lung carcinomas and asbestosis) following inhalation exposure to asbestos. J. Toxicol. Environ. Health Part B 2011, 14, 76–121. [Google Scholar] [CrossRef] [PubMed]
- Toyokuni, S. Iron addiction with ferroptosis-resistance in asbestos-induced mesothelial carcinogenesis: Toward the era of mesothelioma prevention. Free Radic. Biol. Med. 2019, 133, 206–215. [Google Scholar] [CrossRef]
- Park, J.-H.; Gelhaus, S.; Vedantam, S.; Oliva, A.L.; Batra, A.; Blair, I.A.; Troxel, A.B.; Field, J.; Penning, T.M. The pattern of p53 mutations caused by PAH o-quinones is driven by 8-oxo-dGuo formation while the spectrum of mutations is determined by biological selection for dominance. Chem. Res. Toxicol. 2008, 21, 1039–1049. [Google Scholar] [CrossRef]
- Unfried, K.; Schürkes, C.; Abel, J. Distinct Spectrum of Mutations Induced by Crocidolite Asbestos. Cancer Res. 2002, 62, 99. [Google Scholar]
- Loft, S.; Høgh Danielsen, P.; Mikkelsen, L.; Risom, L.; Forchhammer, L.; Møller, P. Biomarkers of oxidative damage to DNA and repair. Biochem. Soc. Trans. 2008, 36, 1071–1076. [Google Scholar] [CrossRef]
- Valavanidis, A.; Vlachogianni, T.; Fiotakis, C. 8-hydroxy-2′ -deoxyguanosine (8-OHdG): A critical biomarker of oxidative stress and carcinogenesis. J. Environ. Sci. Health. Part CEnviron. Carcinog. Ecotoxicol. Rev. 2009, 27, 120–139. [Google Scholar] [CrossRef]
- Brancato, B.; Munnia, A.; Cellai, F.; Ceni, E.; Mello, T.; Bianchi, S.; Catarzi, S.; Risso, G.G.; Galli, A.; Peluso, M.E. 8-Oxo-7, 8-dihydro-2-deoxyguanosine and other lesions along the coding strand of the exon 5 of the tumour suppressor gene P53 in a breast cancer case-control study. DNA Res. Int. J. Rapid Publ. Rep. Genes Genomes 2016, 23, 395–402. [Google Scholar] [CrossRef] [PubMed]
- Shukla, A.; Gulumian, M.; Hei, T.K.; Kamp, D.; Rahman, Q.; Mossman, B.T. Multiple roles of oxidants in the pathogenesis of asbestos-induced diseases. Free Radic. Biol. Med. 2003, 34, 1117–1129. [Google Scholar] [CrossRef]
- Huang, S.X.L.; Jaurand, M.-C.; Kamp, D.W.; Whysner, J.; Hei, T.K. Role of mutagenicity in asbestos fiber-induced carcinogenicity and other diseases. J. Toxicol. Environ. Health Part B Crit. Rev. 2011, 14, 179–245. [Google Scholar] [CrossRef] [PubMed]
- Marini, V.; Michelazzi, L.; Cioe, A.; Fucile, C.; Spigno, F.; Robbiano, L. Exposure to asbestos: Correlation between blood levels of mesothelin and frequency of micronuclei in peripheral blood lymphocytes. Mutat Res. 2011, 721, 114–117. [Google Scholar] [CrossRef]
- Zhao, X.H.; Jia, G.; Liu, Y.Q.; Liu, S.W.; Yan, L.; Jin, Y.; Liu, N. Association between polymorphisms of DNA repair gene XRCC1 and DNA damage in asbestos-exposed workers. Biomed. Environ. Sci. BES 2006, 19, 232–238. [Google Scholar]
- Govercin, M.; Tomatir, A.G.; Evyapan, F.; Acikbas, I.; Coskun, G.; Akdag, B. Elevated micronucleus frequencies in patients with pleural plaque secondary to environmental exposure to asbestos. Genet. Mol. Res. GMR 2014, 13, 598–604. [Google Scholar] [CrossRef]
- Marczynski, B.; Rozynek, P.; Kraus, T.; Schlosser, S.; Raithel, H.J.; Baur, X. Levels of 8-hydroxy-2′-deoxyguanosine in DNA of white blood cells from workers highly exposed to asbestos in Germany. Mutat. Res. 2000, 468, 195–202. [Google Scholar] [CrossRef]
- Tagesson, C.; Chabiuk, D.; Axelson, O.; Baranski, B.; Palus, J.; Wyszynska, K. Increased urinary excretion of the oxidative DNA adduct, 8-hydroxydeoxyguanosine, as a possible early indicator of occupational cancer hazards in the asbestos, rubber, and azo-dye industries. Pol. J. Occup. Med. Environ. Health 1993, 6, 357–368. [Google Scholar]
- Yoshida, R.; Ogawa, Y.; Shioji, I.; Yu, X.; Shibata, E.; Mori, I.; Kubota, H.; Kishida, A.; Hisanaga, N. Urinary 8-oxo-7, 8-dihydro-2′-deoxyguanosine and biopyrrins levels among construction workers with asbestos exposure history. Ind. Health 2001, 39, 186–188. [Google Scholar] [CrossRef]
- Vainio, H.U.; Oksa, P.; Tuomi, T.; Vehmas, T.; Wolff, H.J. Helsinki Criteria update 2014: Asbestos continues to be a challenge for disease prevention and attribution. Epidemiol. Prev. 2016, 40 (Suppl. 1), 15–19. [Google Scholar]
- Betti, M.; Casalone, E.; Ferrante, D.; Aspesi, A.; Morleo, G.; Biasi, A.; Sculco, M.; Mancuso, G.; Guarrera, S.; Righi, L.; et al. Germline mutations in DNA repair genes predispose asbestos-exposed patients to malignant pleural mesothelioma. Cancer Lett. 2017, 405, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Marinaccio, A.; Corfiati, M.; Binazzi, A.; Di Marzio, D.; Scarselli, A.; Ferrante, P.; Bonafede, M.; Verardo, M.; Mirabelli, D.; Gennaro, V.; et al. The epidemiology of malignant mesothelioma in women: Gender differences and modalities of asbestos exposure. Occup. Environ. Med. 2018, 75, 254–262. [Google Scholar] [CrossRef] [PubMed]
- Mohamadkhani, A.; Pourshams, A.; Viti, J.; Cellai, F.; Mortazavi, K.; Sharafkhah, M.; Sotoudeh, M.; MalekZadeh, R.; Boffetta, P.; Peluso, M. Pancreatic cancer patients are prone to the higher oxidative DNA damage of peripheral leukocytes. Asian. Pac. J. Cancer Prev. 2017. [Google Scholar]
- Cristaudo, A.; Foddis, R.; Guglielmi, G. Methodology and results of an experience of medical surveillance of people previously exposed to asbestos in Tuscany. G. Ital. di Med. del Lav. ed Ergon. 2010, 32, 385–388. [Google Scholar]
- Zanoni, T.B.; Hudari, F.; Munnia, A.; Peluso, M.; Godschalk, R.W.; Zanoni, M.V.; den Hartog, G.J.; Bast, A.; Barros, S.B.; Maria-Engler, S.S.; et al. The oxidation of p-phenylenediamine, an ingredient used for permanent hair dyeing purposes, leads to the formation of hydroxyl radicals: Oxidative stress and DNA damage in human immortalized keratinocytes. Toxicol. Lett. 2015, 239, 194–204. [Google Scholar] [CrossRef]
- Godschalk, R.; Remels, A.; Hoogendoorn, C.; van Benthem, J.; Luijten, M.; Duale, N.; Brunborg, G.; Olsen, A.-K.; Bouwman, F.G.; Munnia, A.; et al. Paternal Exposure to Environmental Chemical Stress Affects Male Offspringns Hepatic Mitochondria. Toxicol. Sci. 2018, 162, 241–250. [Google Scholar] [CrossRef]
- Munnia, A.; Saletta, F.; Allione, A.; Piro, S.; Confortini, M.; Matullo, G.; Peluso, M. 32P-Post-labelling method improvements for aromatic compound-related molecular epidemiology studies. Mutagenesis 2007, 22, 381–385. [Google Scholar] [CrossRef]
- Van Houwelingen, H.C.; Arends, L.R.; Stijnen, T. Advanced methods in meta-analysis: Multivariate approach and meta-regression. Stat. Med. 2002, 21, 589–624. [Google Scholar] [CrossRef]
- Izzotti, A.; De Flora, S.; Cartiglia, C.; Are, B.M.; Longobardi, M.; Camoirano, A.; Mura, I.; Dore, M.P.; Scanu, A.M.; Rocca, P.C.; et al. Interplay between Helicobacter pylori and host gene polymorphisms in inducing oxidative DNA damage in the gastric mucosa. Carcinogenesis 2007, 28, 892–898. [Google Scholar] [CrossRef]
- Izzotti, A.; Cartiglia, C.; De Flora, S.; Sacca, S. Methodology for evaluating oxidative DNA damage and metabolic genotypes in human trabecular meshwork. Toxicol. Mech. Methods 2003, 13, 161–168. [Google Scholar] [CrossRef]
- Izzotti, A.; Balansky, R.M.; Dagostini, F.; Bennicelli, C.; Myers, S.R.; Grubbs, C.J.; Lubet, R.A.; Kelloff, G.J.; De Flora, S. Modulation of biomarkers by chemopreventive agents in smoke-exposed rats. Cancer Res. 2001, 61, 2472–2479. [Google Scholar] [PubMed]
- Balansky, R.; Izzotti, A.; D’Agostini, F.; Longobardi, M.; Micale, R.T.; La Maestra, S.; Camoirano, A.; Ganchev, G.; Iltcheva, M.; Steele, V.E.; et al. Assay of lapatinib in murine models of cigarette smoke carcinogenesis. Carcinogenesis 2014, 35, 2300–2307. [Google Scholar] [CrossRef] [PubMed]
- Micale, R.T.; La Maestra, S.; Di Pietro, A.; Visalli, G.; Baluce, B.; Balansky, R.; Steele, V.E.; De Flora, S. Oxidative stress in the lung of mice exposed to cigarette smoke either early in life or in adulthood. Arch. Toxicol. 2013, 87, 915–918. [Google Scholar] [CrossRef] [PubMed]
- Devanaboyina, U.; Gupta, R.C. Sensitive detection of 8-hydroxy-2′deoxyguanosine in DNA by 32P-postlabeling assay and the basal levels in rat tissues. Carcinogenesis 1996, 17, 917–924. [Google Scholar] [CrossRef]
- IARC. Biological agents. Volume 100 B. A review of human carcinogens. Iarc. Monogr. Eval. Carcinog. Risks Hum. 2012, 100, 1–441. [Google Scholar]
- Sorensen, M.; Autrup, H.; Moller, P.; Hertel, O.; Jensen, S.S.; Vinzents, P.; Knudsen, L.E.; Loft, S. Linking exposure to environmental pollutants with biological effects. Mutat. Res. 2003, 544, 255–271. [Google Scholar] [CrossRef]
- Bonassi, S.; Cellai, F.; Munnia, A.; Ugolini, D.; Cristaudo, A.; Neri, M.; Milic, M.; Bonotti, A.; Giese, R.W.; Peluso, M.E. 3-(2-deoxy-β-d-erythro-pentafuranosyl) pyrimido [1, 2-α] purin-10 (3H)-one deoxyguanosine adducts of workers exposed to asbestos fibers. Toxic. Lett. 2017, 270, 1–7. [Google Scholar] [CrossRef]
- Svecova, V.; Topinka, J.; Solansky, I.; Sram, R.J. Personal exposure to volatile organic compounds in the Czech Republic. J. Expo. Sci. Environ. Epidemiol. 2012, 22, 455–460. [Google Scholar] [CrossRef][Green Version]
- Peluso, M.E.; Munnia, A.; Giese, R.W.; Chellini, E.; Ceppi, M.; Capacci, F. Oxidatively damaged DNA in the nasal epithelium of workers occupationally exposed to silica dust in Tuscany region, Italy. Mutagenesis 2015, 30, 519–525. [Google Scholar] [CrossRef] [PubMed]
- Rossnerova, A.; Spatova, M.; Rossner, P.; Solansky, I.; Sram, R.J. The impact of air pollution on the levels of micronuclei measured by automated image analysis. Mutat. Res. 2009, 669, 42–47. [Google Scholar] [CrossRef]
- Topinka, J.; Sevastyanova, O.; Binkova, B.; Chvatalova, I.; Milcova, A.; Lnenickova, Z.; Novakova, Z.; Solansky, I.; Sram, R.J. Biomarkers of air pollution exposure—A study of policemen in Prague. Mutat. Res. 2007, 624, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Toyokuni, S. Mechanisms of asbestos-induced carcinogenesis. Nagoya. J. Med. Sci. 2009, 71, 1–10. [Google Scholar]
- Ceppi, M.; Munnia, A.; Cellai, F.; Bruzzone, M.; Peluso, M.E.M. Linking the generation of DNA adducts to lung cancer. Toxic In Vitro 2017, 390, 160–166. [Google Scholar] [CrossRef] [PubMed]
- Milić, M.; Neri, M.; Ceppi, M.; Bruzzone, M.; Munnia, A.; Ugolini, D.; Cristaudo, A.; Bonotti, A.; Peluso, M.E.; Bonassi, S. DNA damage and genomic instability among workers formerly and currently exposed to asbestos. Scand. J. Work Environ. Health 2018. [Google Scholar]
- Ghio, A.J.; Churg, A.; Roggli, V.L. Ferruginous bodies: Implications in the mechanism of fiber and particle toxicity. Toxicol. Pathol. 2004, 32, 643–649. [Google Scholar] [CrossRef]
- Bernstein, D.; Dunnigan, J.; Hesterberg, T.; Brown, R.; Velasco, J.A.; Barrera, R.; Hoskins, J.; Gibbs, A. Health risk of chrysotile revisited. Crit. Rev. Toxicol. 2013, 43, 154–183. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Liu, T.; Kamp, D.W.; Wang, Y.; He, H.; Zhou, X.; Li, D.; Yang, L.; Zhao, B.; Liu, G. AKT/mTOR and c-Jun N-terminal kinase signaling pathways are required for chrysotile asbestos-induced autophagy. Free Radic. Biol. Med. 2014, 72, 296–307. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Gogol, M.; Gaudenz, K.; Gerton, J.L. Improved transcription and translation with L-leucine stimulation of mTORC1 in Roberts syndrome. BMC Genom. 2016, 17, 25. [Google Scholar] [CrossRef]
- Li, P.; Liu, T.; Kamp, D.W.; Lin, Z.; Wang, Y.; Li, D.; Yang, L.; He, H.; Liu, G. The c-Jun N-terminal kinase signaling pathway mediates chrysotile asbestos-induced alveolar epithelial cell apoptosis. Mol. Med. Rep. 2015, 11, 3626–3634. [Google Scholar] [CrossRef][Green Version]
- Peluso, M.; Munnia, A.; Ceppi, M.; Giese, R.W.; Catelan, D.; Rusconi, F.; Godschalk, R.W.; Biggeri, A. Malondialdehyde-deoxyguanosine and bulky DNA adducts in schoolchildren resident in the proximity of the Sarroch industrial estate on Sardinia Island, Italy. Mutagenesis 2013, 28, 315–321. [Google Scholar] [CrossRef]
- Nie, B.; Gan, W.; Shi, F.; Hu, G.-X.; Chen, L.-G.; Hayakawa, H.; Sekiguchi, M.; Cai, J.-P. Age-dependent accumulation of 8-oxoguanine in the DNA and RNA in various rat tissues. Oxidative Med. Cell. Longev. 2013, 2013, 303181. [Google Scholar] [CrossRef]
- Lodovici, M.; Caldini, S.; Luceri, C.; Bambi, F.; Boddi, V.; Dolara, P. Active and Passive Smoking and Lifestyle Determinants of 8-Oxo-7,8-Dihydro-2′-Deoxyguanosine Levels in Human Leukocyte DNA. Cancer Epidemiol. Prev. Biomark. 2005, 14, 2975. [Google Scholar] [CrossRef]
- Pilger, A.; Radiger, H.W. 8-Hydroxy-2-deoxyguanosine as a marker of oxidative DNA damage related to occupational and environmental exposures. Int. Arch. Occup. Environ. Health 2006, 80, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Risom, L.; Dybdahl, M.; Bornholdt, J.; Vogel, U.; Wallin, H.k.; Møller, P.; Loft, S. Oxidative DNA damage and defence gene expression in the mouse lung after short-term exposure to diesel exhaust particles by inhalation. Carcinogenesis 2003, 24, 1847–1852. [Google Scholar] [CrossRef]
- Jacob, K.D.; Noren Hooten, N.; Trzeciak, A.R.; Evans, M.K. Markers of oxidant stress that are clinically relevant in aging and age-related disease. Mech. Ageing Dev. 2013, 134, 139–157. [Google Scholar] [CrossRef] [PubMed]
- Rundle, A.; Richards, C.; Neslund-Dudas, C.; Tang, D.; Rybicki, B.A. Neighborhood socioeconomic status modifies the association between individual smoking status and PAH-DNA adduct levels in prostate tissue. Environ. Mol. Mutagenesis 2012, 53, 384–391. [Google Scholar] [CrossRef] [PubMed]
- Korkmaz, K.S.; Butuner, B.D.; Roggenbuck, D. Detection of 8-OHdG as a diagnostic biomarker. J. Lab. Precis. Med. 2018, 3. [Google Scholar] [CrossRef]
- Bolognesi, C.; Migliore, L.; Lista, F.; Caroli, S.; Patriarca, M.; De Angelis, R.; Capocaccia, R.; Amadori, S.; Pulliero, A.; Balia, C.; et al. Biological monitoring of Italian soldiers deployed in Iraq. Results of the SIGNUM project. Int. J. Hyg. Environ. Health 2016, 219, 24–32. [Google Scholar] [CrossRef] [PubMed]
- Izzotti, A.; Balansky, R.; Micale, R.T.; Pulliero, A.; La Maestra, S.; De Flora, S. Modulation of smoke-induced DNA and microRNA alterations in mouse lung by licofelone, a triple COX-1, COX-2 and 5-LOX inhibitor. Carcinogenesis 2020, 41, 91–99. [Google Scholar] [CrossRef]
- La Maestra, S.; D’Agostini, F.; Izzotti, A.; Micale, R.T.; Mastracci, L.; Camoirano, A.; Balansky, R.; Trosko, J.E.; Steele, V.E.; De Flora, S. Modulation by aspirin and naproxen of nucleotide alterations and tumors in the lung of mice exposed to environmental cigarette smoke since birth. Carcinogenesis 2015, 36, 1531–1538. [Google Scholar] [CrossRef]
- Peluso, M.; Bollati, V.; Munnia, A.; Srivatanakul, P.; Jedpiyawongse, A.; Sangrajrang, S.; Piro, S.; Ceppi, M.; Bertazzi, P.A.; Boffetta, P.; et al. DNA methylation differences in exposed workers and nearby residents of the Ma Ta Phut industrial estate, Rayong, Thailand. Int. J. Epidemiol. 2012, 41, 1753–1760. [Google Scholar] [CrossRef] [PubMed]
- Peluso, M.E.; Munnia, A.; Bollati, V.; Srivatanakul, P.; Jedpiyawongse, A.; Sangrajrang, S.; Ceppi, M.; Giese, R.W.; Boffetta, P.; Baccarelli, A.A. Aberrant methylation of hypermethylated-in-cancer-1 and exocyclic DNA adducts in tobacco smokers. Toxicol. Sci. Off. J. Soc. Toxicol. 2014, 137, 47–54. [Google Scholar] [CrossRef] [PubMed]
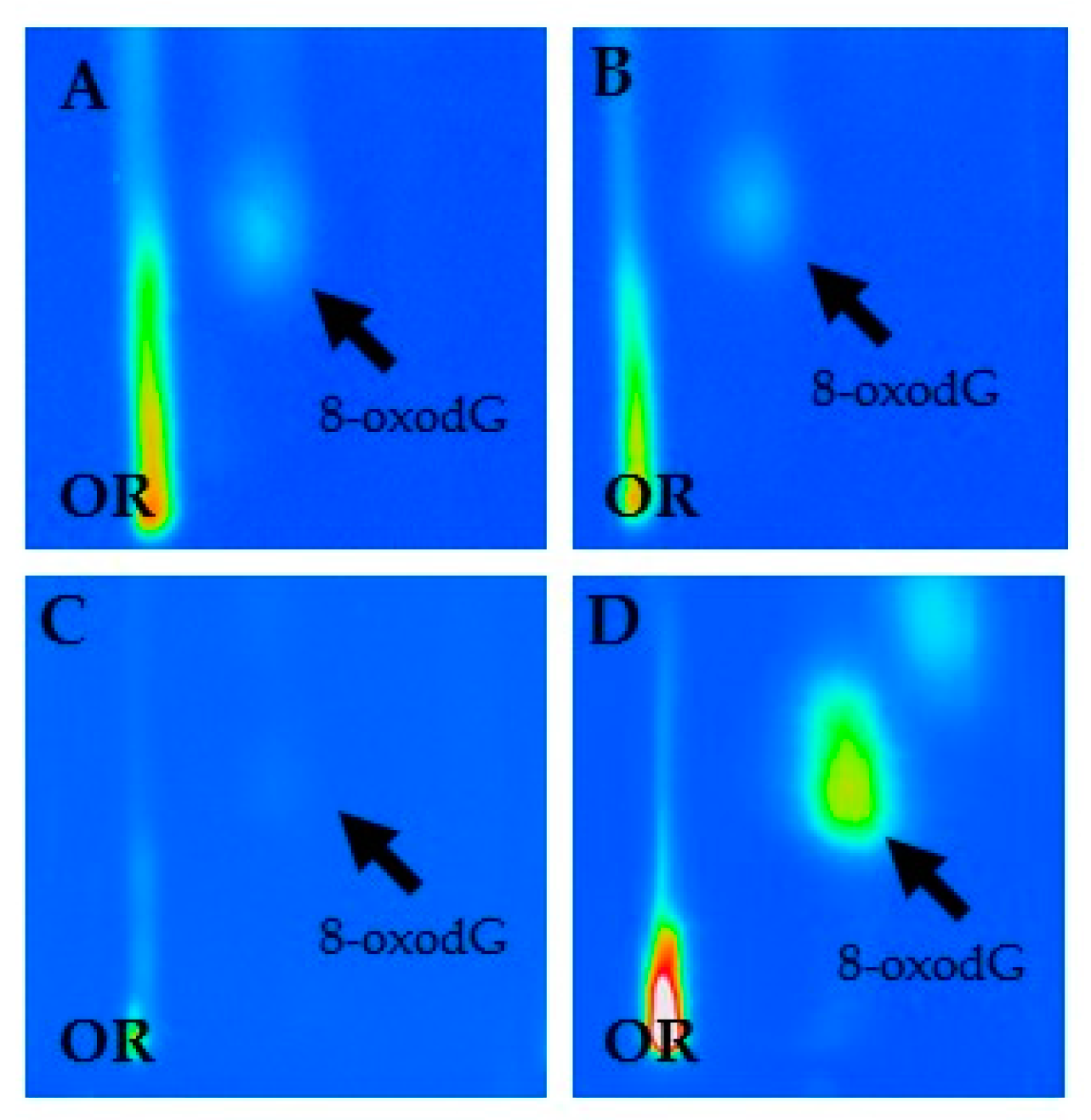
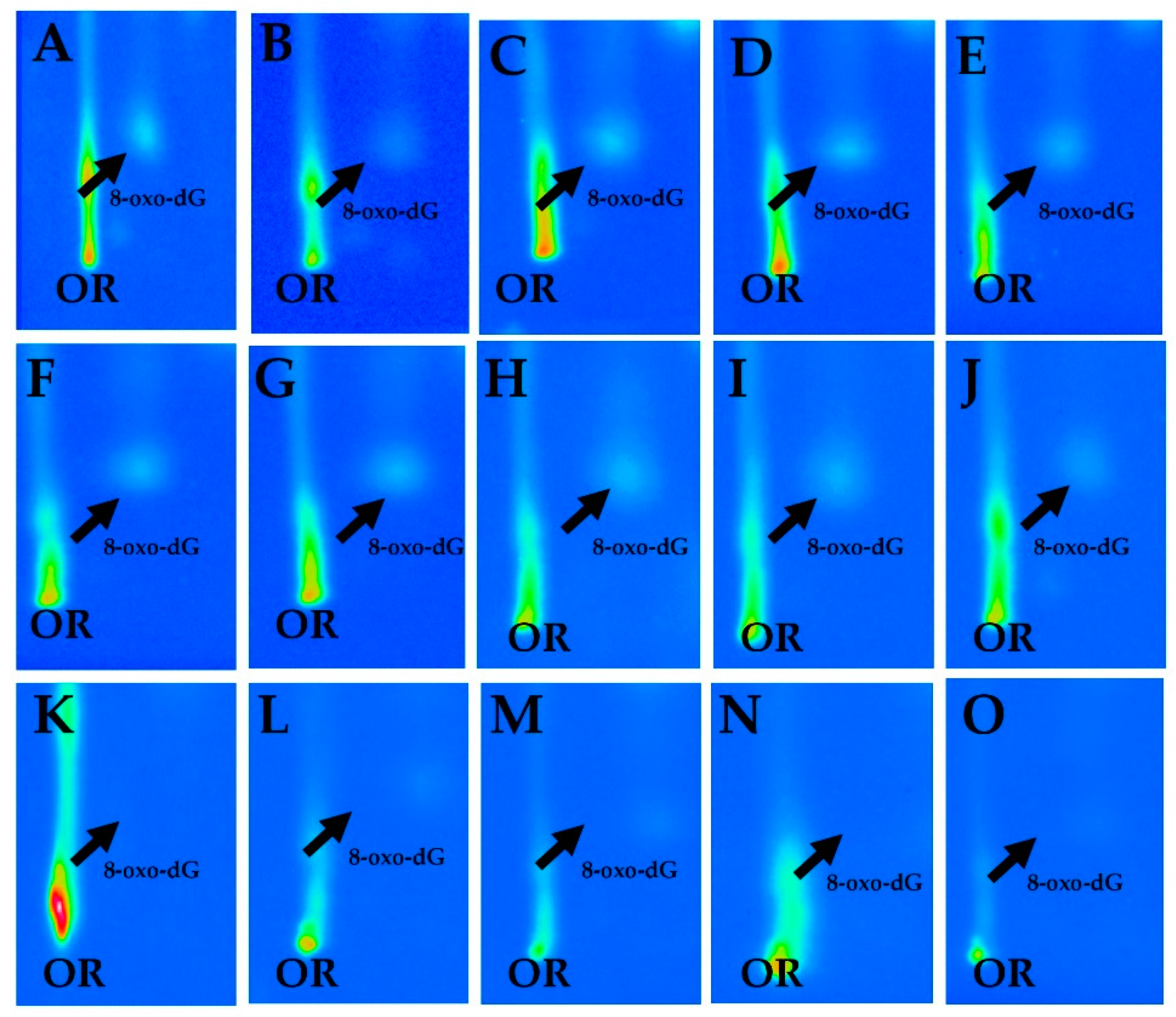
| 8-OxodG per 105 Deoxyguanosine (dG) | ||||
|---|---|---|---|---|
| N a | Mean ± Standard Error (SE) | MR, 95% C.I. | p-Values b | |
| Age (years) | ||||
| ≤52 | 67 | 2.3 ± 0.2 | Reference | |
| 53–58 | 63 | 2.1 ± 0.2 | 1.00 (0.77–1.29) | 0.978 |
| ≥59 | 55 | 2.1 ± 0.2 | 0.89 (0.68–1.17) | 0.408 |
| Educational level | ||||
| Primary education | 86 | 2.3 ± 0.2 | Reference | |
| Secondary education | 72 | 2.3 ± 0.2 | 0.84 (0.67–1.06) | 0.139 |
| University | 27 | 1.3 ± 0.2 | 0.65 (0.48–0.88) | 0.006 |
| Smoking status | ||||
| Non-smokers | 72 | 2.2 ± 0.2 | Reference | |
| Ex-smokers | 66 | 2.0 ± 0.2 | 0.98 (0.67–1.43) | 0.918 |
| Smokers | 46 | 2.4 ± 0.3 | 1.09 (0.74–1.60) | 0.668 |
| 8-OxodG per 105 Deoxyguanosine (dG) | ||||
|---|---|---|---|---|
| N a | Mean ± Standard Error (SE) | MR, 95% C.I. | p-Values b | |
| Asbestos exposure | ||||
| Unexposed controls | 87 | 1.3 ± 0.1 | Reference | |
| Asbestos workers | 98 | 3.0 ± 0.2 | 2.36 (1.99–2.81) | <0.001 |
| Current and past exposure | ||||
| Unexposed controls | 87 | 1.3 ± 0.1 | Reference | |
| Former asbestos workers | 59 | 2.9 ± 0.2 | 2.32 (1.88–2.86) | <0.001 |
| Current asbestos workers | 39 | 3.1 ± 0.3 | 2.43 (1.90–3.12) | <0.001 |
| ≤4 years asbestos workers | 18 | 2.8 ± 0.4 | 2.21 (1.64–2.99) | <0.001 |
| 5–9 years asbestos workers | 21 | 2.9 ± 0.3 | 2.40 (1.79–3.23) | <0.001 |
| ≥10 years asbestos workers | 16 | 3.5 ± 0.5 | 2.94 (2.11–4.11) | <0.001 |
| Occupational history | ||||
| ≤4 years | 18 | 2.8 ± 0.4 | Reference | |
| 5–9 years | 21 | 2.9 ± 0.3 | 1.09 (0.73–1.64) | 0.672 |
| ≥10 years | 16 | 3.5 ± 0.5 | 1.34 (0.85–2.10) | 0.197 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cellai, F.; Bonassi, S.; Cristaudo, A.; Bonotti, A.; Neri, M.; Ceppi, M.; Bruzzone, M.; Milić, M.; Munnia, A.; Peluso, M. Chromatographic Detection of 8-Hydroxy-2′-Deoxyguanosine in Leukocytes of Asbestos Exposed Workers for Assessing Past and Recent Carcinogen Exposures. Diagnostics 2020, 10, 239. https://doi.org/10.3390/diagnostics10040239
Cellai F, Bonassi S, Cristaudo A, Bonotti A, Neri M, Ceppi M, Bruzzone M, Milić M, Munnia A, Peluso M. Chromatographic Detection of 8-Hydroxy-2′-Deoxyguanosine in Leukocytes of Asbestos Exposed Workers for Assessing Past and Recent Carcinogen Exposures. Diagnostics. 2020; 10(4):239. https://doi.org/10.3390/diagnostics10040239
Chicago/Turabian StyleCellai, Filippo, Stefano Bonassi, Alfonso Cristaudo, Alessandra Bonotti, Monica Neri, Marcello Ceppi, Marco Bruzzone, Mirta Milić, Armelle Munnia, and Marco Peluso. 2020. "Chromatographic Detection of 8-Hydroxy-2′-Deoxyguanosine in Leukocytes of Asbestos Exposed Workers for Assessing Past and Recent Carcinogen Exposures" Diagnostics 10, no. 4: 239. https://doi.org/10.3390/diagnostics10040239
APA StyleCellai, F., Bonassi, S., Cristaudo, A., Bonotti, A., Neri, M., Ceppi, M., Bruzzone, M., Milić, M., Munnia, A., & Peluso, M. (2020). Chromatographic Detection of 8-Hydroxy-2′-Deoxyguanosine in Leukocytes of Asbestos Exposed Workers for Assessing Past and Recent Carcinogen Exposures. Diagnostics, 10(4), 239. https://doi.org/10.3390/diagnostics10040239







