Role of Body-Fluid Biomarkers in Alzheimer’s Disease Diagnosis
Abstract
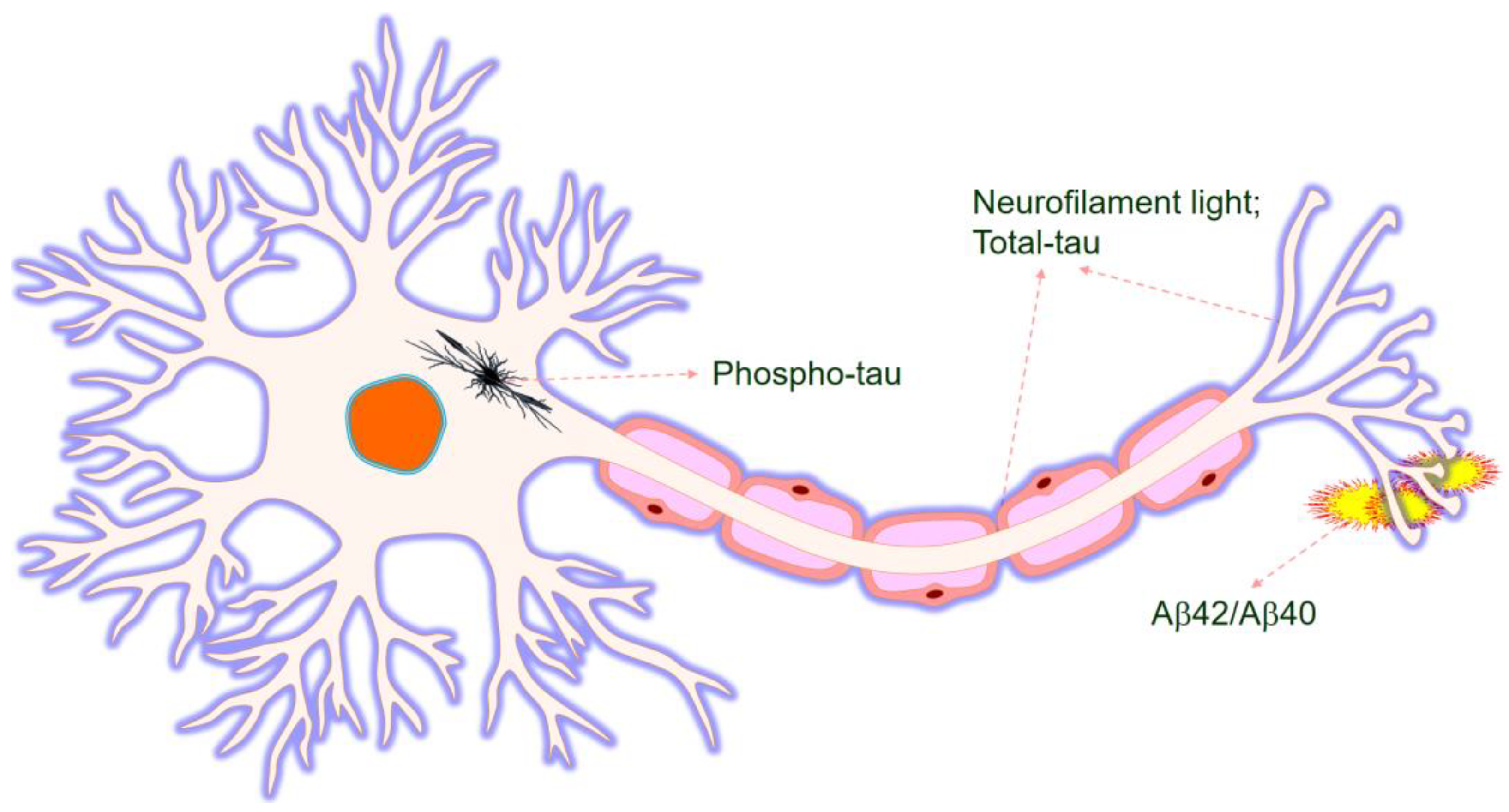
1. Introduction
2. Biomarkers in Alzheimer’s Disease
3. Biomarkers in the Cerebrospinal Fluid
3.1. Cerebrospinal Fluid Aβ
3.2. Cerebrospinal Fluid P-Tau
3.3. Cerebrospinal Fluid T-Tau
4. Biomarkers in Blood
4.1. Plasma Aβ
4.2. Plasma Tau
4.3. Other Biomarkers in Plasma
5. Other Potential Biomarker Sources
6. Conclusions and Future Perspective
Author Contributions
Funding
Conflicts of Interest
References
- Freudenberg-Hua, Y.; Li, W.; Davies, P. The Role of Genetics in Advancing Precision Medicine for Alzheimer’s Disease-A Narrative Review. Front. Med. (Lausanne) 2018, 5, 108. [Google Scholar] [CrossRef] [PubMed]
- Livingston, G.; Sommerlad, A.; Orgeta, V.; Costafreda, S.G.; Huntley, J.; Ames, D.; Ballard, C.; Banerjee, S.; Burns, A.; Cohen-Mansfield, J.; et al. Dementia prevention, intervention, and care. Lancet (London, England) 2017, 390, 2673–2734. [Google Scholar] [CrossRef]
- Prince, M.J.; Wu, F.; Guo, Y.; Robledo, L.M.G.; O’Donnell, M.; Sullivan, R.; Yusuf, S. The burden of disease in older people and implications for health policy and practice. Lancet (London, England) 2015, 385, 549–562. [Google Scholar] [CrossRef]
- Giau, V.V.; Bagyinszky, E.; An, S.S.A.; Kim, S. Clinical genetic strategies for early onset neurodegenerative diseases. Mol. Cell. Toxicol. 2018, 14, 123–142. [Google Scholar] [CrossRef]
- van Giau, V.; An, S.S.A.; Bagyinszky, E.; Kim, S. Gene panels and primers for next generation sequencing studies on neurodegenerative disorders. Mol. Cell. Toxicol. 2015, 11, 89–143. [Google Scholar] [CrossRef]
- Pais, M.; Martinez, L.; Ribeiro, O.; Loureiro, J.; Fernandez, R.; Valiengo, L.; Canineu, P.; Stella, F.; Talib, L.; Radanovic, M.; et al. Early diagnosis and treatment of Alzheimer s disease: New definitions and challenges. Braz. J. Psychiatry 2020. [Google Scholar] [CrossRef]
- Perl, D.P. Neuropathology of Alzheimer’s disease. Mt. Sinai J. Med. 2010, 77, 32–42. [Google Scholar] [CrossRef]
- Sadigh-Eteghad, S.; Sabermarouf, B.; Majdi, A.; Talebi, M.; Farhoudi, M.; Mahmoudi, J. Amyloid-Beta: A Crucial Factor in Alzheimer’s Disease. Med Princ. Pract. 2015, 24, 1–10. [Google Scholar] [CrossRef]
- Chen, G.-f.; Xu, T.-h.; Yan, Y.; Zhou, Y.-r.; Jiang, Y.; Melcher, K.; Xu, H.E. Amyloid beta: Structure, biology and structure-based therapeutic development. Acta Pharmacol. Sinica 2017, 38, 1205–1235. [Google Scholar] [CrossRef]
- Jack, C.R.; Bennett, D.A., Jr.; Blennow, K.; Carrillo, M.C.; Dunn, B.; Haeberlein, S.B.; Holtzman, D.M.; Jagust, W.; Jessen, F.; Karlawish, J.; et al. NIA-AA Research Framework: Toward a biological definition of Alzheimer’s disease. Alzheimer’s Dement. J. Alzheimer’s Assoc. 2018, 14, 535–562. [Google Scholar] [CrossRef]
- Bateman, R.J.; Barthélemy, N.R.; Horie, K. Another step forward in blood-based diagnostics for Alzheimer’s disease. Nat. Med. 2020, 26, 314–316. [Google Scholar] [CrossRef] [PubMed]
- Henriksen, K.; O’Bryant, S.E.; Hampel, H.; Trojanowski, J.Q.; Montine, T.J.; Jeromin, A.; Blennow, K.; Lonneborg, A.; Wyss-Coray, T.; Soares, H.; et al. The future of blood-based biomarkers for Alzheimer’s disease. Alzheimer’s Dement. J. Alzheimer’s Assoc. 2014, 10, 115–131. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, D.; Jelic, V.; Cavallin, L.; Oeksengaard, A.R.; Snaedal, J.; Hogh, P.; Andersen, B.B.; Naik, M.; Engedal, K.; Westman, E.; et al. Electroencephalography Is a Good Complement to Currently Established Dementia Biomarkers. Dement. Geriatr. Cogn. Disord. 2016, 42, 80–92. [Google Scholar] [CrossRef] [PubMed]
- Wurtman, R. Biomarkers in the diagnosis and management of Alzheimer’s disease. Metab. Clin. Exp. 2015, 64, S47–S50. [Google Scholar] [CrossRef] [PubMed]
- Leuzy, A.; Heurling, K.; Ashton, N.J.; Scholl, M.; Zimmer, E.R. In vivo Detection of Alzheimer’s Disease. Yale J. Biol. Med. 2018, 91, 291–300. [Google Scholar]
- Pawlowski, M.; Meuth, S.G.; Duning, T. Cerebrospinal Fluid Biomarkers in Alzheimer’s Disease-From Brain Starch to Bench and Bedside. Diagnostics 2017, 7, E42. [Google Scholar]
- Zetterberg, H.; Rohrer, J.D.; Schott, J.M. Cerebrospinal fluid in the dementias. Handb. Clin. Neurol. 2017, 146, 85–97. [Google Scholar]
- Zetterberg, H. Blood-based biomarkers for Alzheimer’s disease-An update. J. Neurosci. Methods 2019, 319, 2–6. [Google Scholar] [CrossRef]
- Dubois, B.; Feldman, H.H.; Jacova, C.; Cummings, J.L.; Dekosky, S.T.; Barberger-Gateau, P.; Delacourte, A.; Frisoni, G.; Fox, N.C.; Galasko, D.; et al. Revising the definition of Alzheimer’s disease: A new lexicon. Lancet Neurol. 2010, 9, 1118–1127. [Google Scholar] [CrossRef]
- Shankar, G.M.; Li, S.; Mehta, T.H.; Garcia-Munoz, A.; Shepardson, N.E.; Smith, I.; Brett, F.M.; Farrell, M.A.; Rowan, M.J.; Lemere, C.A.; et al. Amyloid-beta protein dimers isolated directly from Alzheimer’s brains impair synaptic plasticity and memory. Nat. Med. 2008, 14, 837–842. [Google Scholar] [CrossRef]
- Lacor, P.N.; Buniel, M.C.; Furlow, P.W.; Clemente, A.S.; Velasco, P.T.; Wood, M.; Viola, K.L.; Klein, W.L. Abeta oligomer-induced aberrations in synapse composition, shape, and density provide a molecular basis for loss of connectivity in Alzheimer’s disease. J. Neurosci. Off. J. Soc. Neurosci. 2007, 27, 796–807. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Jackson, R.J.; Hong, W.; Taylor, W.M.; Corbett, G.T.; Moreno, A.; Liu, W.; Li, S.; Frosch, M.P.; Slutsky, I.; et al. Human Brain-Derived Abeta Oligomers Bind to Synapses and Disrupt Synaptic Activity in a Manner That Requires APP. J. Neurosci. Off. J. Soc. Neurosci. 2017, 37, 11947–11966. [Google Scholar] [CrossRef] [PubMed]
- Calabrese, B.; Shaked, G.M.; Tabarean, I.V.; Braga, J.; Koo, E.H.; Halpain, S. Rapid, concurrent alterations in pre- and postsynaptic structure induced by naturally-secreted amyloid-beta protein. Mol. Cell. Neurosci. 2007, 35, 183–193. [Google Scholar] [CrossRef]
- Koffie, R.M.; Hashimoto, T.; Tai, H.C.; Kay, K.R.; Serrano-Pozo, A.; Joyner, D.; Hou, S.; Kopeikina, K.J.; Frosch, M.P.; Lee, V.M.; et al. Apolipoprotein E4 effects in Alzheimer’s disease are mediated by synaptotoxic oligomeric amyloid-beta. Brain A J. Neurol. 2012, 135, 2155–2168. [Google Scholar] [CrossRef]
- Lue, L.F.; Kuo, Y.M.; Roher, A.E.; Brachova, L.; Shen, Y.; Sue, L.; Beach, T.; Kurth, J.H.; Rydel, R.E.; Rogers, J. Soluble amyloid beta peptide concentration as a predictor of synaptic change in Alzheimer’s disease. Am. J. Pathol. 1999, 155, 853–862. [Google Scholar] [CrossRef]
- Colom-Cadena, M.; Spires-Jones, T.; Zetterberg, H.; Blennow, K.; Caggiano, A.; DeKosky, S.T.; Fillit, H.; Harrison, J.E.; Schneider, L.S.; Scheltens, P.; et al. The Synaptic Health Endpoints Working, The clinical promise of biomarkers of synapse damage or loss in Alzheimer’s disease. Alzheimers Res. Ther. 2020, 12, 21. [Google Scholar] [CrossRef]
- Tapiola, T.; Alafuzoff, I.; Herukka, S.K.; Parkkinen, L.; Hartikainen, P.; Soininen, H.; Pirttila, T. Cerebrospinal fluid {beta}-amyloid 42 and tau proteins as biomarkers of Alzheimer-type pathologic changes in the brain. Arch. Neurol. 2009, 66, 382–389. [Google Scholar] [CrossRef]
- Andreasen, N.; Minthon, L.; Davidsson, P.; Vanmechelen, E.; Vanderstichele, H.; Winblad, B.; Blennow, K. Evaluation of CSF-tau and CSF-Abeta42 as diagnostic markers for Alzheimer disease in clinical practice. Arch. Neurol. 2001, 58, 373–379. [Google Scholar] [CrossRef]
- Blennow, K.; Zetterberg, H.; Minthon, L.; Lannfelt, L.; Strid, S.; Annas, P.; Basun, H.; Andreasen, N. Longitudinal stability of CSF biomarkers in Alzheimer’s disease. Neurosci. Lett. 2007, 419, 18–22. [Google Scholar] [CrossRef]
- Mattsson, N.; Portelius, E.; Rolstad, S.; Gustavsson, M.; Andreasson, U.; Stridsberg, M.; Wallin, A.; Blennow, K.; Zetterberg, H. Longitudinal cerebrospinal fluid biomarkers over four years in mild cognitive impairment. J. Alzheimer’s Dis. JAD 2012, 30, 767–778. [Google Scholar] [CrossRef]
- Zetterberg, H.; Pedersen, M.; Lind, K.; Svensson, M.; Rolstad, S.; Eckerstrom, C.; Syversen, S.; Mattsson, U.B.; Ysander, C.; Mattsson, N.; et al. Intra-individual stability of CSF biomarkers for Alzheimer’s disease over two years. J. Alzheimer’s Dis. JAD 2007, 12, 255–260. [Google Scholar] [CrossRef] [PubMed]
- Andreasen, N.; Minthon, L.; Vanmechelen, E.; Vanderstichele, H.; Davidsson, P.; Winblad, B.; Blennow, K. Cerebrospinal fluid tau and Abeta42 as predictors of development of Alzheimer’s disease in patients with mild cognitive impairment. Neurosci. Lett. 1999, 273, 5–8. [Google Scholar] [CrossRef]
- Thordardottir, S.; Stahlbom, A.K.; Ferreira, D.; Almkvist, O.; Westman, E.; Zetterberg, H.; Eriksdotter, M.; Blennow, K.; Graff, C. Preclinical cerebrospinal fluid and volumetric magnetic resonance imaging biomarkers in Swedish familial Alzheimer’s disease. J. Alzheimer’s Dis. JAD 2015, 43, 1393–1402. [Google Scholar] [CrossRef] [PubMed]
- Bateman, R.J.; Xiong, C.; Benzinger, T.L.; Fagan, A.M.; Goate, A.; Fox, N.C.; Marcus, D.S.; Cairns, N.J.; Xie, X.; Blazey, T.M.; et al. Clinical and biomarker changes in dominantly inherited Alzheimer’s disease. N. Engl. J. Med. 2012, 367, 795–804. [Google Scholar] [CrossRef] [PubMed]
- Vos, S.J.; Xiong, C.; Visser, P.J.; Jasielec, M.S.; Hassenstab, J.; Grant, E.A.; Cairns, N.J.; Morris, J.C.; Holtzman, D.M.; Fagan, A.M. Preclinical Alzheimer’s disease and its outcome: A longitudinal cohort study. Lancet Neurol. 2013, 12, 957–965. [Google Scholar] [CrossRef]
- Skoog, I.; Davidsson, P.; Aevarsson, O.; Vanderstichele, H.; Vanmechelen, E.; Blennow, K. Cerebrospinal fluid beta-amyloid 42 is reduced before the onset of sporadic dementia: A population-based study in 85-year-olds. Dement. Geriatr. Cogn. Disord. 2003, 15, 169–176. [Google Scholar] [CrossRef]
- Gustafson, D.R.; Skoog, I.; Rosengren, L.; Zetterberg, H.; Blennow, K. Cerebrospinal fluid beta-amyloid 1–42 concentration may predict cognitive decline in older women. J. Neurol. Neurosurg. Psychiatry 2007, 78, 461–464. [Google Scholar] [CrossRef]
- Ringman, J.M.; Coppola, G.; Elashoff, D.; Rodriguez-Agudelo, Y.; Medina, L.D.; Gylys, K.; Cummings, J.L.; Cole, G.M. Cerebrospinal fluid biomarkers and proximity to diagnosis in preclinical familial Alzheimer’s disease. Dement. Geriatr. Cogn. Disord. 2012, 33, 1–5. [Google Scholar] [CrossRef]
- Dubois, B.; Feldman, H.H.; Jacova, C.; Hampel, H.; Molinuevo, J.L.; Blennow, K.; DeKosky, S.T.; Gauthier, S.; Selkoe, D.; Bateman, R.; et al. Advancing research diagnostic criteria for Alzheimer’s disease: The IWG-2 criteria. Lancet Neurol. 2014, 13, 614–629. [Google Scholar] [CrossRef]
- Buchhave, P.; Minthon, L.; Zetterberg, H.; Wallin, A.K.; Blennow, K.; Hansson, O. Cerebrospinal fluid levels of beta-amyloid 1-42, but not of tau, are fully changed already 5 to 10 years before the onset of Alzheimer dementia. Arch. Gen. Psychiatry 2012, 69, 98–106. [Google Scholar] [CrossRef]
- Shaw, L.M.; Vanderstichele, H.; Knapik-Czajka, M.; Clark, C.M.; Aisen, P.S.; Petersen, R.C.; Blennow, K.; Soares, H.; Simon, A.; Lewczuk, P.; et al. Cerebrospinal fluid biomarker signature in Alzheimer’s disease neuroimaging initiative subjects. Ann. Neurol. 2009, 65, 403–413. [Google Scholar] [CrossRef] [PubMed]
- Visser, P.J.; Verhey, F.; Knol, D.L.; Scheltens, P.; Wahlund, L.-O.; Freund-Levi, Y.; Tsolaki, M.; Minthon, L.; Wallin, Å.K.; Hampel, H.; et al. Prevalence and prognostic value of CSF markers of Alzheimer’s disease pathology in patients with subjective cognitive impairment or mild cognitive impairment in the DESCRIPA study: A prospective cohort study. Lancet Neurol. 2009, 8, 619–627. [Google Scholar] [CrossRef]
- Giau, V.V.; Bagyinszky, E.; An, S.S.A. Potential Fluid Biomarkers for the Diagnosis of Mild Cognitive Impairment. Int. J. Mol. Sci. 2019, 20, E4149. [Google Scholar] [CrossRef] [PubMed]
- Williams, J.H.; Wilcock, G.K.; Seeburger, J.; Dallob, A.; Laterza, O.; Potter, W.; Smith, A.D. Non-linear relationships of cerebrospinal fluid biomarker levels with cognitive function: An observational study. Alzheimers Res. Ther. 2011, 3, 5. [Google Scholar] [CrossRef]
- Janelidze, S.; Zetterberg, H.; Mattsson, N.; Palmqvist, S.; Vanderstichele, H.; Lindberg, O.; van Westen, D.; Stomrud, E.; Minthon, L.; Blennow, K.; et al. CSF Abeta42/Abeta40 and Abeta42/Abeta38 ratios: Better diagnostic markers of Alzheimer disease. Ann. Clin. Transl. Neurol. 2016, 3, 154–165. [Google Scholar] [CrossRef]
- Hansson, O.; Lehmann, S.; Otto, M.; Zetterberg, H.; Lewczuk, P. Advantages and disadvantages of the use of the CSF Amyloid beta (Abeta) 42/40 ratio in the diagnosis of Alzheimer’s Disease. Alzheimers Res. Ther. 2019, 11, 34. [Google Scholar] [CrossRef]
- Martorana, A.; Lorenzo, F.D.; Belli, L.; Sancesario, G.; Toniolo, S.; Sallustio, F.; Sancesario, G.M.; Koch, G. Cerebrospinal Fluid Abeta42 Levels: When Physiological Become Pathological State. CNS Neurosci. Ther. 2015, 21, 921–925. [Google Scholar] [CrossRef]
- Sjogren, M.; Gisslen, M.; Vanmechelen, E.; Blennow, K. Low cerebrospinal fluid beta-amyloid 42 in patients with acute bacterial meningitis and normalization after treatment. Neurosci. Lett. 2001, 314, 33–36. [Google Scholar] [CrossRef]
- Portelius, E.; Mattsson, N.; Pannee, J.; Zetterberg, H.; Gisslen, M.; Vanderstichele, H.; Gkanatsiou, E.; Crespi, G.A.; Parker, M.W.; Miles, L.A.; et al. Ex vivo (18)O-labeling mass spectrometry identifies a peripheral amyloid beta clearance pathway. Mol. Neurodegener. 2017, 12, 18. [Google Scholar] [CrossRef]
- Hampel, H.; Buerger, K.; Zinkowski, R.; Teipel, S.J.; Goernitz, A.; Andreasen, N.; Sjoegren, M.; DeBernardis, J.; Kerkman, D.; Ishiguro, K.; et al. Measurement of phosphorylated tau epitopes in the differential diagnosis of Alzheimer disease: A comparative cerebrospinal fluid study. Arch. Gen. Psychiatry 2004, 61, 95–102. [Google Scholar] [CrossRef]
- Spiegel, J.; Pirraglia, E.; Osorio, R.S.; Glodzik, L.; Li, Y.; Tsui, W.; Louis, L.A.S.; Randall, C.; Butler, T.; Xu, J.; et al. Greater specificity for cerebrospinal fluid P-tau231 over P-tau181 in the differentiation of healthy controls from Alzheimer’s disease. J. Alzheimer’s Dis. JAD 2016, 49, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Fagan, A.M.; Roe, C.M.; Xiong, C.; Mintun, M.A.; Morris, J.C.; Holtzman, D.M. Cerebrospinal fluid tau/beta-amyloid(42) ratio as a prediction of cognitive decline in nondemented older adults. Arch. Neurol. 2007, 64, 343–349. [Google Scholar] [CrossRef] [PubMed]
- Maddalena, A.; Papassotiropoulos, A.; Muller-Tillmanns, B.; Jung, H.H.; Hegi, T.; Nitsch, R.M.; Hock, C. Biochemical diagnosis of Alzheimer disease by measuring the cerebrospinal fluid ratio of phosphorylated tau protein to beta-amyloid peptide42. Arch. Neurol. 2003, 60, 1202–1206. [Google Scholar] [CrossRef]
- Blennow, K.; Hampel, H. CSF markers for incipient Alzheimer’s disease. Lancet. Neurol. 2003, 2, 605–613. [Google Scholar] [CrossRef]
- Blennow, K.; Dubois, B.; Fagan, A.M.; Lewczuk, P.; de Leon, M.J.; Hampel, H. Clinical utility of cerebrospinal fluid biomarkers in the diagnosis of early Alzheimer’s disease. Alzheimer’s Dement. J. Alzheimer’s Assoc. 2015, 11, 58–69. [Google Scholar] [CrossRef]
- Hesse, C.; Rosengren, L.; Andreasen, N.; Davidsson, P.; Vanderstichele, H.; Vanmechelen, E.; Blennow, K. Transient increase in total tau but not phospho-tau in human cerebrospinal fluid after acute stroke. Neurosci. Lett. 2001, 297, 187–190. [Google Scholar] [CrossRef]
- Fagan, A.M.; Shaw, L.M.; Xiong, C.; Vanderstichele, H.; Mintun, M.A.; Trojanowski, J.Q.; Coart, E.; Morris, J.C.; Holtzman, D.M. Comparison of analytical platforms for cerebrospinal fluid measures of β-amyloid 1–42, total tau, and p-tau181 for identifying Alzheimer disease amyloid plaque pathology. Arch. Neurol. 2011, 68, 1137–1144. [Google Scholar] [CrossRef]
- Diniz, B.S.; Junior, J.A.P.; Forlenza, O.V. Do CSF total tau, phosphorylated tau, and beta-amyloid 42 help to predict progression of mild cognitive impairment to Alzheimer’s disease? A systematic review and meta-analysis of the literature. World J. Biol. Psychiatry Off. J. World Fed. Soc. Biol. Psychiatry 2008, 9, 172–182. [Google Scholar] [CrossRef]
- Hansson, O.; Zetterberg, H.; Buchhave, P.; Londos, E.; Blennow, K.; Minthon, L. Association between CSF biomarkers and incipient Alzheimer’s disease in patients with mild cognitive impairment: A follow-up study. Lancet Neurol. 2006, 5, 228–234. [Google Scholar] [CrossRef]
- Wallin, A.K.; Blennow, K.; Andreasen, N.; Minthon, L. CSF biomarkers for Alzheimer’s Disease: Levels of beta-amyloid, tau, phosphorylated tau relate to clinical symptoms and survival. Dement. Geriatr. Cogn. Disord. 2006, 21, 131–138. [Google Scholar] [CrossRef]
- Mattsson, N.; Zetterberg, H.; Hansson, O.; Andreasen, N.; Parnetti, L.; Jonsson, M.; Herukka, S.K.; van der Flier, W.M.; Blankenstein, M.A.; Ewers, M.; et al. CSF biomarkers and incipient Alzheimer disease in patients with mild cognitive impairment. JAMA 2009, 302, 385–393. [Google Scholar] [CrossRef] [PubMed]
- Shea, Y.F.; Chu, L.W.; Zhou, L.; Li, W.M.; Lin, O.Y.; Chan, M.N.; Xu, A.; Wong, R.; Ho, T.Y.; Li, K.; et al. Cerebrospinal fluid biomarkers of Alzheimer’s disease in Chinese patients: A pilot study. Am. J. Alzheimer’s Dis. Dement. 2013, 28, 769–775. [Google Scholar] [CrossRef] [PubMed]
- Park, S.A.; Chae, W.S.; Kim, H.J.; Shin, H.S.; Kim, S.; Im, J.Y.; Ahn, S.I.; Min, K.D.; Yim, S.J.; Ye, B.S.; et al. Cerebrospinal Fluid Biomarkers for the Diagnosis of Alzheimer Disease in South Korea. Alzheimer Dis. Assoc. Disord. 2017, 31, 13–18. [Google Scholar] [CrossRef] [PubMed]
- Yang, E.; Farnum, M.; Lobanov, V.; Schultz, T.; Raghavan, N.; Samtani, M.N.; Novak, G.; Narayan, V.; DiBernardo, A.I.; the Alzheimer’s Disease Neuroimaging. Quantifying the Pathophysiological Timeline of Alzheimer’s Disease. J. Alzheimer’s Dis. 2011, 26, 745–753. [Google Scholar] [CrossRef]
- Graff-Radford, N.R.; Crook, J.E.; Lucas, J.; Boeve, B.F.; Knopman, D.S.; Ivnik, R.J.; Smith, G.E.; Younkin, L.H.; Petersen, R.C.; Younkin, S.G. Association of low plasma Abeta42/Abeta40 ratios with increased imminent risk for mild cognitive impairment and Alzheimer disease. Arch. Neurol. 2007, 64, 354–362. [Google Scholar] [CrossRef]
- Pesaresi, M.; Lovati, C.; Bertora, P.; Mailland, E.; Galimberti, D.; Scarpini, E.; Quadri, P.; Forloni, G.; Mariani, C. Plasma levels of beta-amyloid (1-42) in Alzheimer’s disease and mild cognitive impairment. Neurobiol. Aging 2006, 27, 904–905. [Google Scholar] [CrossRef]
- van Oijen, M.; Hofman, A.; Soares, H.D.; Koudstaal, P.J.; Breteler, M.M. Plasma Abeta(1-40) and Abeta(1-42) and the risk of dementia: A prospective case-cohort study. Lancet. Neurol. 2006, 5, 655–660. [Google Scholar] [CrossRef]
- Zhou, L.; Chan, K.H.; Chu, L.W.; Kwan, J.S.C.; Song, Y.Q.; Chen, L.H.; Ho, P.W.L.; Cheng, O.Y.; Ho, J.W.M.; Lam, K.S.L. Plasma amyloid-β oligomers level is a biomarker for Alzheimer’s disease diagnosis. Biochem. Biophys. Res. Commun. 2012, 423, 697–702. [Google Scholar] [CrossRef]
- Yang, Y.; Giau, V.V.; An, S.S.A.; Kim, S. Plasma Oligomeric Beta Amyloid in Alzheimer’s Disease with History of Agent Orange Exposure. Dement. Neurocogn Disord. 2018, 17, 41–49. [Google Scholar] [CrossRef]
- Bagyinszky, E.; Kang, M.J.; van Giau, V.; Shim, K.; Pyun, J.-M.; Suh, J.; An, S.S.A.; Kim, S. Novel Amyloid Precursor Protein mutation, Val669Leu (“Seoul APP”), in a Korean Early onset Alzheimer’s disease patient. Neurobiol. Aging 2019, 84, 236.e1–236.e7. [Google Scholar] [CrossRef]
- Lambert, J.C.; Schraen-Maschke, S.; Richard, F.; Fievet, N.; Rouaud, O.; Berr, C.; Dartigues, J.F.; Tzourio, C.; Alperovitch, A.; Buee, L.; et al. Association of plasma amyloid beta with risk of dementia: The prospective Three-City Study. Neurology 2009, 73, 847–853. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.J.; Yi, S.; Han, J.-Y.; Park, S.Y.; Jang, J.-W.; Chun, I.K.; Kim, S.E.; Lee, B.S.; Kim, G.J.; Yu, J.S.; et al. Oligomeric forms of amyloid-β protein in plasma as a potential blood-based biomarker for Alzheimer’s disease. Alzheimers Res. Ther. 2017, 9, 98. [Google Scholar] [CrossRef] [PubMed]
- An, S.S.A.; Lee, B.-S.; Yu, J.S.; Lim, K.; Kim, G.J.; Lee, R.; Kim, S.; Kang, S.; Park, Y.H.; Wang, M.J.; et al. Dynamic changes of oligomeric amyloid β levels in plasma induced by spiked synthetic Aβ(42). Alzheimers Res. Ther. 2017, 9, 86. [Google Scholar] [CrossRef] [PubMed]
- Youn, Y.C.; Kang, S.; Suh, J.; Park, Y.H.; Kang, M.J.; Pyun, J.-M.; Choi, S.H.; Jeong, J.H.; Park, K.W.; Lee, H.-W.; et al. Blood amyloid-β oligomerization associated with neurodegeneration of Alzheimer’s disease. Alzheimers Res. Ther. 2019, 11, 40. [Google Scholar] [CrossRef] [PubMed]
- Berman, S.E.; Clark, L.R.; Rivera-Rivera, L.A.; Norton, D.; Racine, A.M.; Rowley, H.A.; Bendlin, B.B.; Blennow, K.; Zetterberg, H.; Carlsson, C.M.; et al. Intracranial Arterial 4D Flow in Individuals with Mild Cognitive Impairment is Associated with Cognitive Performance and Amyloid Positivity. J. Alzheimer’s Dis. JAD 2017, 60, 243–252. [Google Scholar] [CrossRef]
- Bibl, M.; Esselmann, H.; Wiltfang, J. Neurochemical biomarkers in Alzheimer’s disease and related disorders. Ther. Adv. Neurol. Disord. 2012, 5, 335–348. [Google Scholar] [CrossRef]
- Ashton, N.J.; Scholl, M.; Heurling, K.; Gkanatsiou, E.; Portelius, E.; Hoglund, K.; Brinkmalm, G.; Hye, A.; Blennow, K.; Zetterberg, H. Update on biomarkers for amyloid pathology in Alzheimer’s disease. Biomark. Med. 2018, 12, 799–812. [Google Scholar] [CrossRef]
- Verberk, I.M.W.; Slot, R.E.; Verfaillie, S.C.J.; Heijst, H.; Prins, N.D.; van Berckel, B.N.M.; Scheltens, P.; Teunissen, C.E.; van der Flier, W.M. Plasma Amyloid as Prescreener for the Earliest Alzheimer Pathological Changes. Ann. Neurol. 2018, 84, 648–658. [Google Scholar] [CrossRef]
- Janelidze, S.; Stomrud, E.; Palmqvist, S.; Zetterberg, H.; van Westen, D.; Jeromin, A.; Song, L.; Hanlon, D.; Hehir, C.A.T.; Baker, D.; et al. Plasma beta-amyloid in Alzheimer’s disease and vascular disease. Sci. Rep. 2016, 6, 26801. [Google Scholar] [CrossRef]
- Teunissen, C.E.; Chiu, M.J.; Yang, C.C.; Yang, S.Y.; Scheltens, P.; Zetterberg, H.; Blennow, K. Plasma Amyloid-beta (Abeta42) Correlates with Cerebrospinal Fluid Abeta42 in Alzheimer’s Disease. J. Alzheimer’s Dis. JAD 2018, 62, 1857–1863. [Google Scholar] [CrossRef]
- Fan, L.Y.; Tzen, K.Y.; Chen, Y.F.; Chen, T.F.; Lai, Y.M.; Yen, R.F.; Huang, Y.Y.; Shiue, C.Y.; Yang, S.Y.; Chiu, M.J. The Relation Between Brain Amyloid Deposition, Cortical Atrophy, and Plasma Biomarkers in Amnesic Mild Cognitive Impairment and Alzheimer’s Disease. Front. Aging Neurosci. 2018, 10, 175. [Google Scholar] [CrossRef] [PubMed]
- Zetterberg, H.; Mörtberg, E.; Song, L.; Chang, L.; Provuncher, G.K.; Patel, P.P.; Ferrell, E.; Fournier, D.R.; Kan, C.W.; Campbell, T.G.; et al. Hypoxia Due to Cardiac Arrest Induces a Time-Dependent Increase in Serum Amyloid β Levels in Humans. PLoS ONE 2011, 6, e28263. [Google Scholar] [CrossRef] [PubMed]
- Nabers, A.; Perna, L.; Lange, J.; Mons, U.; Schartner, J.; Guldenhaupt, J.; Saum, K.U.; Janelidze, S.; Holleczek, B.; Rujescu, D.; et al. Amyloid blood biomarker detects Alzheimer’s disease. EMBO Mol. Med. 2018, 10, e8763. [Google Scholar] [CrossRef] [PubMed]
- Lue, L.F.; Sabbagh, M.N.; Chiu, M.J.; Jing, N.; Snyder, N.L.; Schmitz, C.; Guerra, A.; Belden, C.M.; Chen, T.F.; Yang, C.C.; et al. Plasma Levels of Abeta42 and Tau Identified Probable Alzheimer’s Dementia: Findings in Two Cohorts. Front. Aging Neurosci. 2017, 9, 226. [Google Scholar] [CrossRef] [PubMed]
- Jensen, M.; Basun, H.; Lannfelt, L. Increased cerebrospinal fluid tau in patients with Alzheimer’s disease. Neurosci. Lett. 1995, 186, 189–191. [Google Scholar] [CrossRef]
- Wallin, A.K.; Blennow, K.; Zetterberg, H.; Londos, E.; Minthon, L.; Hansson, O. CSF biomarkers predict a more malignant outcome in Alzheimer disease. Neurology 2010, 74, 1531–1537. [Google Scholar] [CrossRef]
- Lauridsen, C.; Sando, S.B.; Moller, I.; Berge, G.; Pomary, P.K.; Grontvedt, G.R.; Salvesen, O.; Brathen, G.; White, L.R. Cerebrospinal Fluid Abeta43 Is Reduced in Early-Onset Compared to Late-Onset Alzheimer’s Disease, But Has Similar Diagnostic Accuracy to Abeta42. Front. Aging Neurosci. 2017, 9, 210. [Google Scholar] [CrossRef]
- Cicognola, C.; Brinkmalm, G.; Wahlgren, J.; Portelius, E.; Gobom, J.; Cullen, N.C.; Hansson, O.; Parnetti, L.; Constantinescu, R.; Wildsmith, K.; et al. Novel tau fragments in cerebrospinal fluid: Relation to tangle pathology and cognitive decline in Alzheimer’s disease. Acta Neuropathol. 2019, 137, 279–296. [Google Scholar] [CrossRef]
- Zetterberg, H.; Wilson, D.; Andreasson, U.; Minthon, L.; Blennow, K.; Randall, J.; Hansson, O. Plasma tau levels in Alzheimer’s disease. Alzheimers Res. Ther. 2013, 5, 9. [Google Scholar] [CrossRef]
- Pase, M.P.; Beiser, A.S.; Himali, J.J.; Satizabal, C.L.; Aparicio, H.J.; DeCarli, C.; Chene, G.; Dufouil, C.; Seshadri, S. Assessment of Plasma Total Tau Level as a Predictive Biomarker for Dementia and Related Endophenotypes. JAMA Neurol. 2019, 76, 598–606. [Google Scholar] [CrossRef]
- Mielke, M.M.; Hagen, C.E.; Xu, J.; Chai, X.; Vemuri, P.; Lowe, V.J.; Airey, D.C.; Knopman, D.S.; Roberts, R.O.; Machulda, M.M.; et al. Plasma phospho-tau181 increases with Alzheimer’s disease clinical severity and is associated with tau- and amyloid-positron emission tomography. Alzheimer’s Dement. J. Alzheimer’s Assoc. 2018, 14, 989–997. [Google Scholar] [CrossRef] [PubMed]
- Barthelemy, N.R.; Li, Y.; Joseph-Mathurin, N.; Gordon, B.A.; Hassenstab, J.; Benzinger, T.L.S.; Buckles, V.; Fagan, A.M.; Perrin, R.J.; Goate, A.M.; et al. A soluble phosphorylated tau signature links tau, amyloid and the evolution of stages of dominantly inherited Alzheimer’s disease. Nat. Med. 2020, 26, 398–407. [Google Scholar] [CrossRef] [PubMed]
- Itoh, N.; Arai, H.; Urakami, K.; Ishiguro, K.; Ohno, H.; Hampel, H.; Buerger, K.; Wiltfang, J.; Otto, M.; Kretzschmar, H.; et al. Large-scale, multicenter study of cerebrospinal fluid tau protein phosphorylated at serine 199 for the antemortem diagnosis of Alzheimer’s disease. Ann. Neurol. 2001, 50, 150–156. [Google Scholar] [CrossRef] [PubMed]
- Kohnken, R.; Buerger, K.; Zinkowski, R.; Miller, C.; Kerkman, D.; DeBernardis, J.; Shen, J.; Moller, H.J.; Davies, P.; Hampel, H. Detection of tau phosphorylated at threonine 231 in cerebrospinal fluid of Alzheimer’s disease patients. Neurosci. Lett. 2000, 287, 187–190. [Google Scholar] [CrossRef]
- Buerger, K.; Teipel, S.J.; Zinkowski, R.; Blennow, K.; Arai, H.; Engel, R.; Hofmann-Kiefer, K.; McCulloch, C.; Ptok, U.; Heun, R.; et al. CSF tau protein phosphorylated at threonine 231 correlates with cognitive decline in MCI subjects. Neurology 2002, 59, 627–629. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.M.; Blennow, K.; Andreasen, N.; Laterza, O.; Modur, V.; Olander, J.; Gao, F.; Ohlendorf, M.; Ladenson, J.H. The brain injury biomarker VLP-1 is increased in the cerebrospinal fluid of Alzheimer disease patients. Clin. Chem. 2008, 54, 1617–1623. [Google Scholar] [CrossRef]
- Hol, E.M.; Roelofs, R.F.; Moraal, E.; Sonnemans, M.A.F.; Sluijs, J.A.; Proper, E.A.; de Graan, P.N.E.; Fischer, D.F.; van Leeuwen, F.W. Neuronal expression of GFAP in patients with Alzheimer pathology and identification of novel GFAP splice forms. Mol. Psychiatry 2003, 8, 786–796. [Google Scholar] [CrossRef]
- Bagyinszky, E.; Giau, V.V.; Shim, K.; Suk, K.; An, S.S.A.; Kim, S. Role of inflammatory molecules in the Alzheimer’s disease progression and diagnosis. J. Neurol. Sci. 2017, 376, 242–254. [Google Scholar] [CrossRef]
- Jin, M.; Cao, L.; Dai, Y.-P. Role of Neurofilament Light Chain as a Potential Biomarker for Alzheimer’s Disease: A Correlative Meta-Analysis. Front. Aging Neurosci. 2019, 11, 254. [Google Scholar] [CrossRef]
- Davidsson, P.; Jahn, R.; Bergquist, J.; Ekman, R.; Blennow, K. Synaptotagmin, a synaptic vesicle protein, is present in human cerebrospinal fluid: A new biochemical marker for synaptic pathology in Alzheimer disease? Mol. Chem. Neuropathol. 1996, 27, 195–210. [Google Scholar] [CrossRef]
- Thorsell, A.; Bjerke, M.; Gobom, J.; Brunhage, E.; Vanmechelen, E.; Andreasen, N.; Hansson, O.; Minthon, L.; Zetterberg, H.; Blennow, K. Neurogranin in cerebrospinal fluid as a marker of synaptic degeneration in Alzheimer’s disease. Brain Res. 2010, 1362, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Bereczki, E.; Branca, R.M.; Francis, P.T.; Pereira, J.B.; Baek, J.H.; Hortobagyi, T.; Winblad, B.; Ballard, C.; Lehtio, J.; Aarsland, D. Synaptic markers of cognitive decline in neurodegenerative diseases: A proteomic approach. Brain A J. Neurol. 2018, 141, 582–595. [Google Scholar] [CrossRef] [PubMed]
- Bereczki, E.; Francis, P.T.; Howlett, D.; Pereira, J.B.; Hoglund, K.; Bogstedt, A.; Cedazo-Minguez, A.; Baek, J.H.; Hortobagyi, T.; Attems, J.; et al. Synaptic proteins predict cognitive decline in Alzheimer’s disease and Lewy body dementia. Alzheimer’s Dement. J. Alzheimer’s Assoc. 2016, 12, 1149–1158. [Google Scholar] [CrossRef] [PubMed]
- Sandelius, A.; Portelius, E.; Kallen, A.; Zetterberg, H.; Rot, U.; Olsson, B.; Toledo, J.B.; Shaw, L.M.; Lee, V.M.Y.; Irwin, D.J.; et al. Elevated CSF GAP-43 is Alzheimer’s disease specific and associated with tau and amyloid pathology. Alzheimer’s Dement. J. Alzheimer’s Assoc. 2019, 15, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Brinkmalm, A.; Brinkmalm, G.; Honer, W.G.; Frolich, L.; Hausner, L.; Minthon, L.; Hansson, O.; Wallin, A.; Zetterberg, H.; Blennow, K.; et al. SNAP-25 is a promising novel cerebrospinal fluid biomarker for synapse degeneration in Alzheimer’s disease. Mol. Neurodegener. 2014, 9, 53. [Google Scholar] [CrossRef]
- van Giau, V.; An, S.S.A. Optimization of specific multiplex DNA primers to detect variable CLU genomic lesions in patients with Alzheimer’s disease. BioChip J. 2015, 9, 278–284. [Google Scholar] [CrossRef]
- Foster, E.M.; Dangla-Valls, A.; Lovestone, S.; Ribe, E.M.; Buckley, N.J. Clusterin in Alzheimer’s Disease: Mechanisms, Genetics, and Lessons From Other Pathologies. Front. Neurosci. 2019, 13, 164. [Google Scholar] [CrossRef]
- Elliott, D.A.; Weickert, C.S.; Garner, B. Apolipoproteins in the brain: Implications for neurological and psychiatric disorders. Clin. Lipidol. 2010, 51, 555–573. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, X.; Zhu, B.; Fu, P.; On the behalf of Alzheimer’s Disease Neuroimaging. Association of Clusterin Levels in Cerebrospinal Fluid with Synaptic Degeneration Across the Alzheimer’s Disease Continuum. Neuropsychiatr. Dis. Treat. 2020, 16, 183–190. [Google Scholar] [CrossRef]
- Hsu, J.-L.; Lee, W.-J.; Liao, Y.-C.; Wang, S.-J.; Fuh, J.-L. The clinical significance of plasma clusterin and Aβ in the longitudinal follow-up of patients with Alzheimer’s disease. Alzheimers Res. Ther. 2017, 9, 91. [Google Scholar] [CrossRef]
- Silajdzic, E.; Minthon, L.; Bjorkqvist, M.; Hansson, O. No diagnostic value of plasma clusterin in Alzheimer’s disease. PLoS ONE 2012, 7, e50237. [Google Scholar] [CrossRef] [PubMed]
- Frost, S.; Martins, R.N.; Kanagasingam, Y. Ocular biomarkers for early detection of Alzheimer’s disease. J. Alzheimer’s Dis. JAD 2010, 22, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Frost, S.; Kanagasingam, Y.; Sohrabi, H.; Vignarajan, J.; Bourgeat, P.; Salvado, O.; Villemagne, V.; Rowe, C.C.; Macaulay, S.L.; Szoeke, C.; et al. Retinal vascular biomarkers for early detection and monitoring of Alzheimer’s disease. Transl. Psychiatry 2013, 3, e233. [Google Scholar] [CrossRef] [PubMed]
- Koychev, I.; Galna, B.; Zetterberg, H.; Lawson, J.; Zamboni, G.; Ridha, B.H.; Rowe, J.B.; Thomas, A.; Howard, R.; Malhotra, P.; et al. Abeta42/Abeta40 and Abeta42/Abeta38 Ratios Are Associated with Measures of Gait Variability and Activities of Daily Living in Mild Alzheimer’s Disease: A Pilot Study. J. Alzheimer’s Dis. JAD 2018, 65, 1377–1383. [Google Scholar] [CrossRef] [PubMed]
- Koronyo, Y.; Salumbides, B.C.; Black, K.L.; Koronyo-Hamaoui, M. Alzheimer’s disease in the retina: Imaging retinal abeta plaques for early diagnosis and therapy assessment. Neuro-Degener. Dis. 2012, 10, 285–293. [Google Scholar] [CrossRef] [PubMed]
- Morgia, C.L.; Ross-Cisneros, F.N.; Koronyo, Y.; Hannibal, J.; Gallassi, R.; Cantalupo, G.; Sambati, L.; Pan, B.X.; Tozer, K.R.; Barboni, P.; et al. Melanopsin retinal ganglion cell loss in Alzheimer disease. Ann. Neurol. 2016, 79, 90–109. [Google Scholar] [CrossRef]
- Koronyo-Hamaoui, M.; Koronyo, Y.; Ljubimov, A.V.; Miller, C.A.; Ko, M.K.; Black, K.L.; Schwartz, M.; Farkas, D.L. Identification of amyloid plaques in retinas from Alzheimer’s patients and noninvasive in vivo optical imaging of retinal plaques in a mouse model. NeuroImage 2011, 54 (Suppl. 1), S204–S217. [Google Scholar] [CrossRef]
- Dutescu, R.M.; Li, Q.X.; Crowston, J.; Masters, C.L.; Baird, P.N.; Culvenor, J.G. Amyloid precursor protein processing and retinal pathology in mouse models of Alzheimer’s disease. Graefe’s Arch. Clin. Exp. Ophthalmol. Albrecht von Graefes Arch. fur Klin. und Exp. Ophthalmol. 2009, 247, 1213–1221. [Google Scholar] [CrossRef]
- Frederikse, P.H.; Garland, D.; Zigler, J.S.; Piatigorsky, J., Jr. Oxidative stress increases production of beta-amyloid precursor protein and beta-amyloid (Abeta) in mammalian lenses, and Abeta has toxic effects on lens epithelial cells. J. Biol. Chem. 1996, 271, 10169–10174. [Google Scholar] [CrossRef]
- Goldstein, L.E.; Muffat, J.A.; Cherny, R.A.; Moir, R.D.; Ericsson, M.H.; Huang, X.; Mavros, C.; Coccia, J.A.; Faget, K.Y.; Fitch, K.A.; et al. Cytosolic beta-amyloid deposition and supranuclear cataracts in lenses from people with Alzheimer’s disease. Lancet (London, England) 2003, 361, 1258–1265. [Google Scholar] [CrossRef]
- Moncaster, J.A.; Pineda, R.; Moir, R.D.; Lu, S.; Burton, M.A.; Ghosh, J.G.; Ericsson, M.; Soscia, S.J.; Mocofanescu, A.; Folkerth, R.D.; et al. Alzheimer’s disease amyloid-beta links lens and brain pathology in Down syndrome. PLoS ONE 2010, 5, e10659. [Google Scholar] [CrossRef] [PubMed]
- Gupta, V.K.; Chitranshi, N.; Gupta, V.B.; Golzan, M.; Dheer, Y.; Wall, R.V.; Georgevsky, D.; King, A.E.; Vickers, J.C.; Chung, R.; et al. Amyloid beta accumulation and inner retinal degenerative changes in Alzheimer’s disease transgenic mouse. Neurosci. Lett. 2016, 623, 52–56. [Google Scholar] [CrossRef] [PubMed]
- van Wijngaarden, P.; Hadoux, X.; Alwan, M.; Keel, S.; Dirani, M. Emerging ocular biomarkers of Alzheimer disease. Clin. Exp. Ophthalmol. 2017, 45, 54–61. [Google Scholar] [CrossRef] [PubMed]
- van Giau, V.; An, S.S. Emergence of exosomal miRNAs as a diagnostic biomarker for Alzheimer’s disease. J. Neurol. Sci. 2016, 360, 141–152. [Google Scholar] [CrossRef]
- Lee, J.Y.; Jin, H.K.; Bae, J.-S. Sphingolipids in neuroinflammation: A potential target for diagnosis and therapy. BMB Rep. 2020, 53, 28–34. [Google Scholar] [CrossRef]
- Milà-Alomà, M.; Suárez-Calvet, M.; Molinuevo, J.L. Latest advances in cerebrospinal fluid and blood biomarkers of Alzheimer’s disease. Ther. Adv. Neurol. Disord. 2019. [Google Scholar] [CrossRef]
- Ma, Q.L.; Teng, E.; Zuo, X.; Jones, M.; Teter, B.; Zhao, E.Y.; Zhu, C.; Bilousova, T.; Gylys, K.H.; Apostolova, L.G.; et al. Neuronal pentraxin 1: A synaptic-derived plasma biomarker in Alzheimer’s disease. Neurobiol. Dis. 2018, 114, 120–128. [Google Scholar] [CrossRef]
- Cervellati, C.; Trentini, A.; Rosta, V.; Passaro, A.; Bosi, C.; Sanz, J.M.; Bonazzi, S.; Pacifico, S.; Seripa, D.; Valacchi, G.; et al. Serum beta-secretase 1 (BACE1) activity as candidate biomarker for late-onset Alzheimer’s disease. GeroScience 2020, 42, 159–167. [Google Scholar] [CrossRef]
- Crunkhorn, S. Identification of blood-based biomarkers. Nat. Rev. Drug Discov. 2018, 17, 166. [Google Scholar] [CrossRef]
- Giau, V.V.; Bagyinszky, E.; An, S.S.; Kim, S.Y. Role of apolipoprotein E in neurodegenerative diseases. Neuropsychiatr. Dis. Treat. 2015, 11, 1723–1737. [Google Scholar] [CrossRef]
- Menendez-Gonzalez, M.; Gasparovic, C. Albumin Exchange in Alzheimer’s Disease: Might CSF Be an Alternative Route to Plasma? Front. Neurol. 2019, 10, 1036. [Google Scholar] [CrossRef] [PubMed]
- Twohig, D.; Nielsen, H.M. α-synuclein in the pathophysiology of Alzheimer’s disease. Mol. Neurodegener. 2019, 14, 23. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; North, B.J.; Zhang, T.; Dai, X.; Tao, K.; Guo, J.; Wei, W. The emerging roles of protein homeostasis-governing pathways in Alzheimer’s disease. Aging Cell 2018, 17, e12801. [Google Scholar] [CrossRef] [PubMed]
- Labbadia, J.; Morimoto, R.I. The biology of proteostasis in aging and disease. Annu. Rev. Biochem. 2015, 84, 435–464. [Google Scholar] [CrossRef]
- Tarawneh, R.; D’Angelo, G.; Macy, E.; Xiong, C.; Carter, D.; Cairns, N.J.; Fagan, A.M.; Head, D.; Mintun, M.A.; Ladenson, J.H.; et al. Visinin-like protein-1: Diagnostic and prognostic biomarker in Alzheimer disease. Ann. Neurol. 2011, 70, 274–285. [Google Scholar] [CrossRef]
- Schindler, S.E.; Li, Y.; Todd, K.W.; Herries, E.M.; Henson, R.L.; Gray, J.D.; Wang, G.; Graham, D.L.; Shaw, L.M.; Trojanowski, J.Q.; et al. Emerging cerebrospinal fluid biomarkers in autosomal dominant Alzheimer’s disease. Alzheimer’s Dement. 2019, 15, 655–665. [Google Scholar] [CrossRef]
- Jack, C.R.; Knopman, D.S., Jr.; Weigand, S.D.; Wiste, H.J.; Vemuri, P.; Lowe, V.; Kantarci, K.; Gunter, J.L.; Senjem, M.L.; Ivnik, R.J.; et al. An operational approach to National Institute on Aging-Alzheimer’s Association criteria for preclinical Alzheimer disease. Ann. Neurol. 2012, 71, 765–775. [Google Scholar] [CrossRef]
- Dani, M.; Brooks, D.J.; Edison, P. Suspected non-Alzheimer’s pathology - Is it non-Alzheimer’s or non-amyloid? Ageing Res. Rev. 2017, 36, 20–31. [Google Scholar] [CrossRef]

| Sample Size | Biomarker(s) | Cut-Off | Sensitivity | Specificity | Reference |
|---|---|---|---|---|---|
| 21 AD | ↓ Aβ42 | Aβ42: < 427 ng/L | 86% | 88% | [60] |
| ↑ t-tau | t-tau: < 445 ng/L | 86% | 88% | ||
| 24 normals | ↑ p-tau | p-tau: < 74 ng/L | 60% | 88% | |
| 180 MCI | ↓ Aβ42 ↓ p-tau181 | t-tau: > 50 ng/L | 95% | 83% | [59] |
| 57 MCI-AD | ↑ t-tau | p-tau181: > 60 ng/L | |||
| 137 MCI | ↓ Aβ42 | Aβ42: ≤ 0.64 ng/mL | 93% | 53% | [59] |
| ↓ Aβ42/Aβ40 ratio | Aβ42–Aβ40: ≤ 0.95 | 87% | 78% | ||
| 529 AD | ↓ Aβ42 | Aβ42: ≤ 482 ng/l | 79% | 65% | [61] |
| 304 normal | ↑ t-tau | t-tau: ≥ 320 ng/l | 84% | 47% | |
| 271 AD | ↑ p-tau | p-tau: ≥ 52 ng/l | 86% | 56% | |
| 24 AD | Aβ42 | t-tau: > 325.7 pg/mL | 83% | 91% | [62] |
| 76 AD | ↑ t-tau | Aβ42: < 481 pg/mL | 94% | 87% | [63] |
| 47 dementia | ↑ p-tau | t-tau: > 326 pg/mL | 84% | 96% | |
| p-tau: > 57 pg/mL | 72% | 90% | |||
| t-tau/Aβ42: > 0.55 | 99% | 95% | |||
| p-tau/Aβ42: > 0.10 | 96% | 96% | |||
| t-tau/Aβ42: > 0.08l | 93% | 70% |
| Biomarker | Measuring | Method for Measurement | Monitoring | Stage of Development |
|---|---|---|---|---|
| Aβ40 amyloid and total Aβ42 amyloid, free, bound, free/bound, truncated, sAPPα | Blood/plasma | Amyloid | Diagnostic, prognostic, predictive | Clinical trials |
| Fatty acid binding protein 3 | CSF | Neuronal damage | Diagnostic | Clinical trials |
| Circulatory microRNAs | Blood/plasma | Cell signaling | Unknown | Preclinical |
| Multi-parameter diagnostic blood test | Blood/plasma | Unknown | Diagnostic | Clinical trials |
| Ceramides | Blood/plasma | Inflammation | Diagnostic | Clinical trials |
| Neocortical β-amyloid burden | Blood/plasma | Amyloid | Susceptibility/risk | Clinical trials |
| Blood brain barrier | Blood/plasma, imaging, CSF | Vasculature | Diagnostic, monitoring | Clinical trials |
| Blood biomarker for mtDNA Damage | Blood/plasma | Genetic variation/DNA | Diagnostic, monitoring | Clinical trials |
| Neurogranin | CSF | Neuronal damage | Diagnostic, susceptibility/risk | Clinical trials |
| Neuronal pentraxin 1 | Blood/plasma | Neuronal damage | Diagnostic | Clinical trials |
| BACE1 | Blood/plasma | Amyloid | Diagnostic, susceptibility/risk | Clinical trials |
| Neuronal pentraxin 2 | CSF | Inflammation | Diagnostic | Clinical trials |
| APP 669–711/Aβ 1–42 | Blood/plasma | Amyloid | Prognostic | Preclinical |
| APOE4 | Blood/plasma, other bodily fluids | Genetic variation/DNA | Susceptibility/risk | In use (FDA approved) |
| Neuron specific enolase | CSF | Neuronal damage | Susceptibility/risk, predictive | Clinical trials |
| Albumin ratio | Blood/plasma, CSF | Amyloid | Diagnostic | Clinical trials |
| Aβ42/Aβ40 (Plasma) | Blood/plasma | Amyloid | Pharmacodynamic/response, susceptibility/risk, safety | Clinical trials |
| Aβ1–42/Aβ1–40 (CSF) | CSF | Amyloid | Diagnostic, prognostic | Clinical trials |
| Aβ42 (salivary) | Other bodily fluids | Amyloid | Diagnostic, prognostic | Clinical trials |
| Aβ42 (blood) | Blood/plasma | Amyloid | Diagnostic | Clinical trials |
| Aβ42 (CSF) | CSF | Amyloid | Diagnostic, prognostic, susceptibility/risk | Clinical trials |
| Aβ1–17 (Aβ17) | Blood/plasma | Amyloid | Diagnostic | Preclinical |
| Plasma lipoproteome | Blood/plasma | Neuronal damage | Diagnostic | Clinical trials |
| α-synuclein | CSF | Amyloid | Diagnostic | Clinical trials |
| Proteostasis-related biomarkers | CSF | Amyloid, inflammation, neuronal damage, Tau | Diagnostic, monitoring, susceptibility/risk | Clinical trials |
| Tau in the biological fluids | Blood/plasma, CSF | Tau | Diagnostic, monitoring | Clinical trials |
| TNF-α (plasma) | Blood/plasma | Inflammation | Diagnostic | Clinical trials |
| Vascular cell adhesion molecule 1 | Blood/plasma | Neuronal damage | Diagnostic, monitoring, prognostic | Clinical trials |
| Visinin-like protein 1 | CSF | Neuronal damage | Pharmacodynamic/response | Clinical trials |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nguyen, T.T.; Ta, Q.T.H.; Nguyen, T.K.O.; Nguyen, T.T.D.; Vo, V.G. Role of Body-Fluid Biomarkers in Alzheimer’s Disease Diagnosis. Diagnostics 2020, 10, 326. https://doi.org/10.3390/diagnostics10050326
Nguyen TT, Ta QTH, Nguyen TKO, Nguyen TTD, Vo VG. Role of Body-Fluid Biomarkers in Alzheimer’s Disease Diagnosis. Diagnostics. 2020; 10(5):326. https://doi.org/10.3390/diagnostics10050326
Chicago/Turabian StyleNguyen, Thuy Trang, Qui Thanh Hoai Ta, Thi Kim Oanh Nguyen, Thi Thuy Dung Nguyen, and Van Giau Vo. 2020. "Role of Body-Fluid Biomarkers in Alzheimer’s Disease Diagnosis" Diagnostics 10, no. 5: 326. https://doi.org/10.3390/diagnostics10050326
APA StyleNguyen, T. T., Ta, Q. T. H., Nguyen, T. K. O., Nguyen, T. T. D., & Vo, V. G. (2020). Role of Body-Fluid Biomarkers in Alzheimer’s Disease Diagnosis. Diagnostics, 10(5), 326. https://doi.org/10.3390/diagnostics10050326





