Quantification of Ground Glass Opacities Can Be Useful to Describe Disease Activity in Systemic Sclerosis
Abstract
1. Introduction
2. Materials and Methods
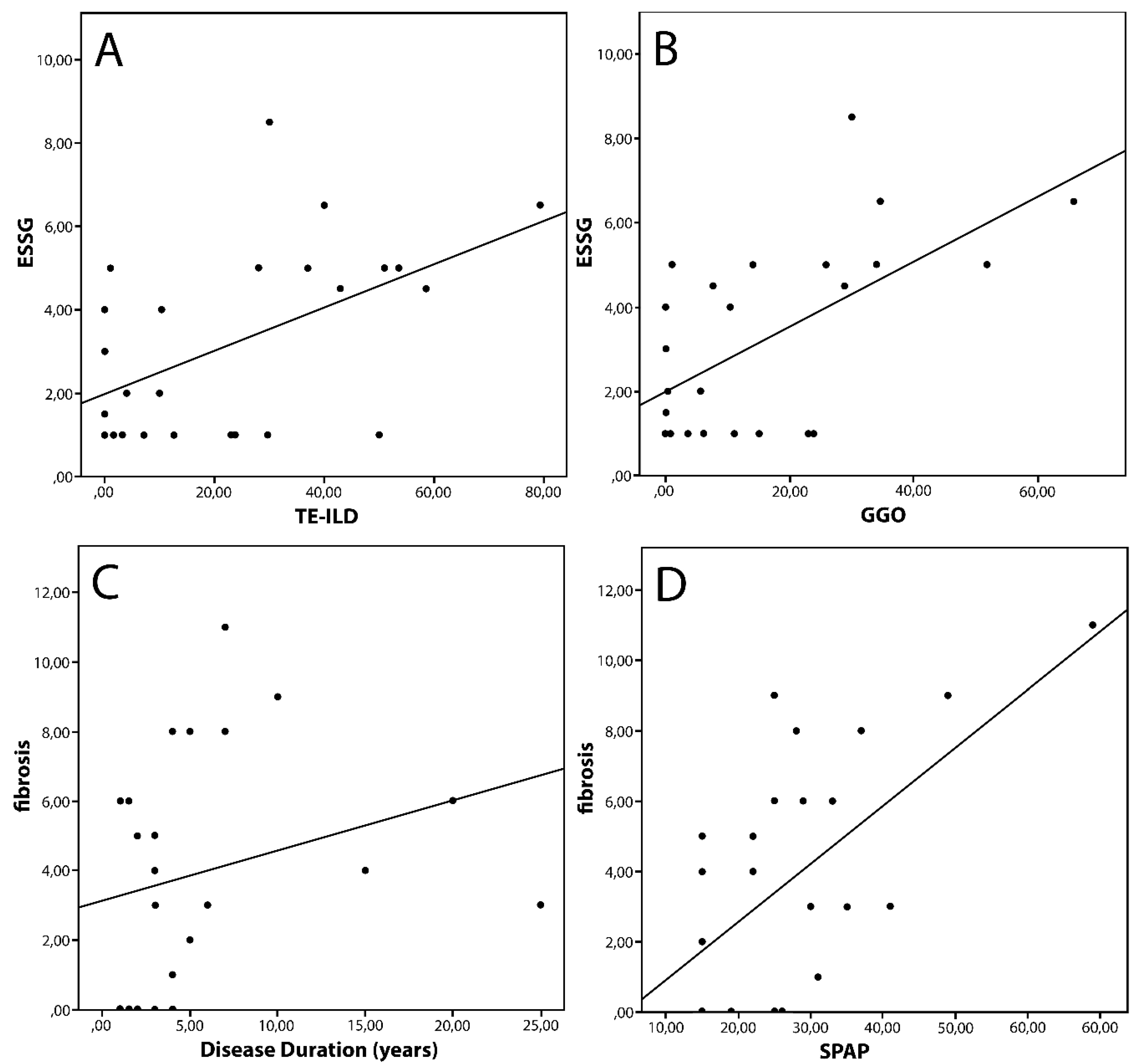
3. Results
4. Discussion
Author Contributions
Acknowledgments
Conflicts of Interest
Abbreviations
| ACR/EULAR | American College of Rheumatology/European League Against Rheumatism |
| Scl70 | Anti-topoisomerase I autoantibodies |
| ACA | Anti-centromeric antibodies |
| dcSSc | Diffuse cutaneous systemic sclerosis |
| DLCO | Diffusion lung for carbon monoxide |
| DA | Disease activity |
| DD | Disease duration |
| ΔCP | Dyspnea |
| ESR | Erythrocyte sedimentation rate |
| GGOs | Ground-glass opacities |
| HRCT | High resolution computed tomography |
| HC | Honeycombing |
| ILD | Interstitial lung disease |
| lcSSc | Limited cutaneous systemic sclerosis |
| mRSS | Modified Rodnan skin score |
| NVC | Nailfold videocapillaroscopy |
| NEMO score | Number of microhemorrhages score |
| NSIP | Nonspecific interstitial pneumonia |
| RP | Raynaud’s phenomenon |
| SSc | Systemic sclerosis |
| SPAP | Systolic pulmonary artery pressure |
| TE-ILD | Total extent of ILD |
| UIP | Usual interstitial pneumonia |
References
- Steen, V.D.; Medsger, T.A. Changes in causes of death in systemic sclerosis, 1972–2002. Ann. Rheum. Dis. 2007, 66, 940–944. [Google Scholar] [CrossRef]
- Varga, J. Systemic sclerosis: an update. Bull. NYU Hosp. Jt. Dis. 2008, 66, 198–202. [Google Scholar]
- Schurawitzki, H.; Stiglbauer, R.; Graninger, W.; Herold, C.; Pölzleitner, D.; Burghuber, O.; Tscholakoff, D. Interstitial lung disease in progressive systemic sclerosis: high-resolution CT versus radiography. Radiology 1990, 176, 755–759. [Google Scholar] [CrossRef] [PubMed]
- Ciancio, N.; Pavone, M.; Torrisi, S.E.; Vancheri, A.; Sambataro, D.; Palmucci, S.; Vancheri, C.; Di Marco, F.; Sambataro, G. Contribution of pulmonary function tests (PFTs) to the diagnosis and follow up of connective tissue diseases. Multidiscip. Respir. Med. 2019, 14, 17. [Google Scholar] [CrossRef]
- Muller, N.L.; Staples, C.A.; Miller, R.R.; Vedal, S.; Thurlbeck, W.M.; Ostrow, D.N. Disease activity in idiopathic pulmonary fibrosis: CT and pathologic correlation. Radiology 1987, 165, 731–734. [Google Scholar] [CrossRef]
- Launay, D.; Remy-Jardin, M.; Michon-Pasturel, U.; Mastora, I.; Hachulla, E.; Lambert, M.; Delannoy, V.; Queyrel, V.; Duhamel, A.; Matran, R.; et al. High resolution computed tomography in fibrosing alveolitis associated with systemic sclerosis. J. Rheumatol. 2006, 33, 1789–1801. [Google Scholar] [PubMed]
- Yabuuchi, H.; Matsuo, Y.; Tsukamoto, H.; Horiuchi, T.; Kamitani, T.; Nagao, M.; Akashi, K.; Honda, H. Evaluation of the extent of ground glass opacity on high resolution TC in patients with interstitial pneumonia associated with systemic sclerosis: comparison between quantitative and qualitative analysis. Clin. Radiol. 2014, 69, 758–764. [Google Scholar] [CrossRef]
- Goh, N.S.; Desai, S.R.; Veeraraghavan, S.; Hansell, D.M.; Copley, S.J.; Maher, T.M.; Corte, T.J.; Sander, C.R.; Ratoff, J.; Devaraj, A.; et al. Interstitial lung disease: a simple staging system. Am. J. Respir. Crit. Care Med. 2008, 177, 1248–1254. [Google Scholar] [CrossRef]
- Desai, S.R.; Veeraraghavan, S.; Hansell, D.M.; Nikolakopolou, A.; Goh, N.S.L.; Nicholson, A.; Colby, T.V.; Denton, C.P.; Black, C.M.; Du Bois, R.M.; et al. CT Features of Lung Disease in Patients with Systemic Sclerosis: Comparison with Idiopathic Pulmonary Fibrosis and Nonspecific Interstitial Pneumonia. Radiology 2004, 232, 560–567. [Google Scholar] [CrossRef]
- Van Den Hoogen, F.; Khanna, D.; Fransen, J.; Johnson, S.R.; Baron, M.; Tyndall, A.; Matucci-Cerinic, M.; Naden, R.P.; Medsger, T.A., Jr.; Carreira, P.E.; et al. 2013 Classification criteria for systemic sclerosis: an American College of Rheumatology/European League agains Rheumatism collaborative initiative. Arthritis Rheum. 2013, 65, 2537–2547. [Google Scholar] [CrossRef]
- Medsger, T.A. Natural history of systemic sclerosis and the assessment of disease activity, severity, functional status, and psychologic well-being. Rheum. Dis. Clin. N. Am. 2003, 29, 255–273. [Google Scholar] [CrossRef]
- Leroy, E.C.; Black, C.; Fleischmajer, R.; Jablonska, S.; Krieg, T.; Medsger, T.A.; Rowell, N.; Wollheim, F. Scleroderma (systemic sclerosis): classification, subsets and pathogenesis. J. Rheumatol. 1988, 15, 202–205. [Google Scholar] [PubMed]
- Valentini, G.; Della Rossa, A.; Bombardieri, S.; Bencivelli, W.; Silman, A.J.; D’Angelo, S.; Cerinic, M.M.; Belch, J.F.; Black, C.M.; Bruhlmann, L.; et al. European multicentre study to define disease activity criteria for systemic sclerosis. II. Identification of disease activity variables and development of preliminary activity indexes. Ann. Rheum. Dis. 2001, 60, 592–598. [Google Scholar] [CrossRef] [PubMed]
- Valentini, G.; Bencivelli, W.; Bombardieri, S.; D’Angelo, S.; Della Rossa, A.; Silman, A.J.; Blach, C.M.; Czirjak, L.; Nielsen, H.; Vlachoyiannopouls, P.G. European multicentre study to define disease activity criteria for systemic sclerosis. III. Assessment of the construct validity of the preliminary activity criteria. Ann. Rheum. Dis. 2003, 62, 901–903. [Google Scholar] [CrossRef]
- Chiarenza, A.; Ultimo, L.E.; Falsaperla, D.; Travali, M.; Foti, P.V.; Torrisi, S.E.; Schisano, M.; Mauro, L.A.; Sambataro, G.; Basile, A.; et al. Chest imaging using signs, symbols, and naturalistic images: a practical guide for radiologists and non-radiologists. Insights Imaging 2019, 10, 114–120. [Google Scholar] [CrossRef]
- Cutolo, M.; Sulli, A.; Smith, V. How to perform and interpret capillaroscopy. Best Pr. Res. Clin. Rheumatol. 2013, 27, 237–248. [Google Scholar] [CrossRef]
- Sambataro, D.; Sambataro, G.; Zaccara, E.; Maglione, W.; Polosa, R.; Afeltra, A.M.; Vitali, C.; Del Papa, N. Nailfold videocapillaroscopy micro-haemorrhage and giant capillary counting a san accurate approach for a steady state definition of disease activity in sistemi sclerosis. Arthritis Res. Ther. 2014, 16, 462. [Google Scholar] [CrossRef][Green Version]
- Andracco, R.; Irace, R.; Zaccara, E.; Vettori, S.; Maglione, W.; Riccardi, A.; Pignataro, F.; Ferrara, R.; Sambataro, D.; Sambataro, G.; et al. The cumulative number of micro-haemorrhages and micro-thromboses in nailfold videocapillaroscopy is a good indicator of disease severity in sistemi sclerosis: a validation study of the NEMO score. Arthritis Res. Ther. 2017, 19, 133. [Google Scholar] [CrossRef]
- Pignataro, F.; Maglione, W.; Minniti, A.; Sambataro, D.; Sambataro, G.; Campanaro, F.; Valentini, G.; Vitali, C.; Del Papa, N. NEMO score in nailfold videocapillaroscopy is a good tool to assess both steady state levels and overtime changes of disease activity in patients with systemic sclerosis: a comparison with the proposed composite indices for this disease status entity. Arthritis Res. 2019, 21, 258. [Google Scholar] [CrossRef]
- Abbas, A.E.; Fortuin, F.D.; Schiller, N.B.; Appleton, C.P.; Moreno, C.A.; Lester, S.J. A simple method for noninvasive estimation of pulmonary vascular resistance. J. Am. Coll. Cardiol. 2003, 41, 1021–1027. [Google Scholar] [CrossRef]
- Miller, M.; Hankinson, J.; Brusasco, V.; Burgos, F.; Casaburi, R.; Coates, A.; Crapo, R.; Enright, P.; Van Der Grinten, C.P.M.; Gustafsson, P.; et al. Standardisation of spirometry. Eur. Respir. J. 2005, 26, 319–338. [Google Scholar] [CrossRef] [PubMed]
- Shand, L.; Lunt, M.; Nihtyanova, S.; Hoseini, M.; Silman, A.; Black, C.M.; Denton, C.P. Relationship between change in skin score and disease outcome in diffuse cutaneous systemic sclerosis: Application of a latent linear trajectory model. Arthritis Rheum. 2007, 56, 2422–2431. [Google Scholar] [CrossRef] [PubMed]
- Nihtyanova, S.; Schreiber, B.E.; Ong, V.H.; Rosenberg, D.; Moinzadeh, P.; Coghlan, J.G.; Wells, A.U.; Denton, C.P. Prediction of Pulmonary Complications and Long-Term Survival in Systemic Sclerosis. Arthritis Rheumatol. 2014, 66, 1625–1635. [Google Scholar] [CrossRef] [PubMed]
- Herzog, E.L.; Mathur, A.; Tager, A.M.; Feghali-Bostwick, C.; Schneider, F.; Varga, J. Review: interstitial lung disease associated with systemic sclerosis and idiopathic pulmonary fibrosis: how similar and distinct? Arthritis Rheumatol. 2014, 66, 1967–1978. [Google Scholar] [CrossRef]
- Ufuk, F.; Demirci, M.; Altinisik, G. Quantitative computed tomography assessment for systemic sclerosis-related interstitial lung disease: comparison of different methods. Eur. Radiol. 2020, 1–12. [Google Scholar] [CrossRef]
- Ariani, A.; Silva, M.; Seletti, V.; Bravi, E.; Saracco, M.; Parisi, S.; De Gennaro, F.; Idolazzi, L.; Caramaschi, P.; Benini, C.; et al. Quantitative chest computed tomography is assocaited with two prediction models of mortality in interstitial lung disease related to systemic sclerosis. Rheumatology 2017, 56, 922–927. [Google Scholar] [CrossRef]
- Ariani, A.; Aiello, M.; Silva, M.; Alfieri, V.; Bonati, E.; Lumetti, F.; Delsante, G.; Sverzellati, N.; Chetta, A. Quantitative CT indexes are significantly associated with exercise oxygen desaturation in interstitial lung disease related to systemic sclerosis. Clin. Respir. J. 2016, 11, 983–989. [Google Scholar] [CrossRef]
- Bocchino, M.; Bruzzese, D.; D’Alto, M.; Argiento, P.; Borgia, A.; Capaccio, A.; Romeo, E.; Russo, B.; Sanduzzi, A.; Valente, T.; et al. Performance of a new quantitative computed tomography index for interstitial lung disease assessment in systemic sclerosis. Sci. Rep. 2019, 9, 9468. [Google Scholar] [CrossRef]
- Kloth, C.; Blum, A.C.; Thaiss, W.M.; Preibsch, H.; Ditt, H.; Grimmer, R.; Fritz, J.; Nikolaou, K.; Bösmüller, H.; Horger, M. Differences in Texture Analysis Parameters Between Active Alveolitis and Lung Fibrosis in Chest CT of Patients with Systemic Sclerosis. Acad. Radiol. 2017, 24, 1596–1603. [Google Scholar] [CrossRef]
- Cottin, V.; Brown, K. Interstitial lung disease associated with systemic sclerosis (SSc-ILD). Respir. Res. 2019, 20, 13. [Google Scholar] [CrossRef]
- Caron, M.; Hoa, S.; Hudson, M.; Schwartzman, K.; Steele, R. Pulmonary function tests as outcomes for systemic sclerosis interstitial lung disease. Eur. Respir. Rev. 2018, 27, 170102. [Google Scholar] [CrossRef] [PubMed]
- Papiris, S.; Kagouridis, K.; Papadaki, G.; Kolilekas, L.; Manali, E.D. Treating CTDs related fibrotic ILDs by immunosuppressants: “facts and faults”. Lung 2013, 192, 221–223. [Google Scholar] [CrossRef] [PubMed]
- Panopoulos, S.; Bournia, V.-K.; Trakada, G.; Giavri, I.; Kostopoulos, C.; Sfikakis, P.P. Mycophenolate Versus Cyclophosphamide for Progressive Interstitial Lung Disease Associated with Systemic Sclerosis: A 2-Year Case Control Study. Lung 2013, 191, 483–489. [Google Scholar] [CrossRef] [PubMed]
- Distler, O.; Highland, K.B.; Gahlemann, M.; Azuma, A.; Fischer, A.; Mayes, M.D.; Raghu, G.; Sauter, W.; Girard, M.; Alves, M.; et al. Nintedanib for Systemic Sclerosis–Associated Interstitial Lung Disease. N. Engl. J. Med. 2019, 380, 2518–2528. [Google Scholar] [CrossRef]
| Whole Cohort | Patients with Diffused Form Systemic Sclerosis (dcSSc) | Patients with Limited Form Systemic Sclerosis (lcSSC) | p | |
|---|---|---|---|---|
| Total patients (gender F/M) | 40 (36/4) | 26 (23/3) | 14 (13/1) | n.s. |
| Age (years) | 54 (47–65) | 51.5 (46–67.5) | 56.5 (51–66.5) | n.s. |
| Disease duration (years) | 5 (2.75–7) | 4 (3–7) | 6 (3–9.75) | n.s. |
| mRSS | 4 (1–8) | 4 (1.75–8) | 3 (0.5–6) | n.s. |
| Scleredema (%) | 65 | 65.3 | 64.2 | n.s. |
| Δ Skin (%) | 30 | 30.7 | 28.4 | n.s. |
| Ulcers (%) | 22.5 | 26.9 | 14.2 | n.s. |
| Δ Vascular (%) | 25 | 19.2 | 35.7 | n.s. |
| Arthritis (%) | 7.5 | 11.5 | 0 | <0.001 |
| DLCO <80 of the predicted (%) | 85 | 88.4 | 78.5 | n.s. |
| Δ cardiopulmonary (%) | 32.5 | 34.6 | 28.6 | n.s. |
| ESR >30 (%) | 40 | 53.8 | 14.3 | 0.01 |
| Hypocomplementemia (%) | 7.5 | 3.8 | 14.3 | n.s. |
| ESSG index | 3 (1–4.5) | 3.5 (1–5) | 3.5 (0.5–4.5) | n.s. |
| SPAP | 25 (15–32) | 25 (20–31) | 20 (15–33.5) | n.s. |
| ILD (overall extent) | 7.3 (1.1–28) | 23.5 (1.6–41.5) | 1.2 (0–2.35) | 0.0008 |
| Quantification of GGOs | 5.5 (0–23) | 10.65 (1–27.3) | 0.3 (0–1.6) | 0.003 |
| Grade of fibrosis | 3 (0–5) | 4 (0–7) | 0.5 (0–3) | 0.007 |
| Whole Cohort of Patients | |||
|---|---|---|---|
| Total Extent ILD | No | Yes | p |
| ΔCP median (n) | 3.6 (27) (0.5–12.6) | 37 (13) (1–51) | 0.01 |
| ESR median (n) | 16.7 (21) (0–16.7) | 29 (19) (1.8–29) | 0.03 |
| GGO | No | Yes | p |
| ΔCP median (n) | 1.2 (27) (0–8.25) | 25.8 (13) (2–34) | 0.02 |
| Fibrosis grade | No | Yes | p |
| ΔCP median (n) | 2 (27) (1–4) | 5 (13) (2–8) | 0.01 |
| Early Systemic Sclerosis Total Extent ILD | No | Yes | p |
| Scleredema median (n) | 0 (7) (0–3) | 13.9 (11) (1.4–37.5) | 0.03 |
| ΔCP median (n) | 1.2 (14) (0–4) | 35 (4) (30–40) | 0.03 |
| GGO | No | Yes | p |
| Scleredema median (n) | 0 (7) (0–1.5) | 13 (11) (1.4–28.7) | 0.01 |
| ΔCP median (n) | 1 (14) (0–6.2) | 32.3 (4) (30–34) | 0.009 |
| Longstanding Systemic Sclerosis Total Extent ILD | No | Yes | p |
| ESR > 30 median (n) | 5 (14) (0.2–15.7) | 37 (9) (10–51) | 0.004 |
| GGO | No | Yes | p |
| ESR > 30 median (n) | 2 (14) (0–3.75) | 8 (9) (3–9) | 0.008 |
| Fibrosis | No | Yes | p |
| ESR > 30 median (n) | 2.14 (14) (0–9.2) | 14 (9) (5.1–28.8) | 0.02 |
| Diffuse Cutaneous Systemic Sclerosis Total Extent ILD | No | Yes | p |
| Scleredema median (n) | 1.6 (9) (0–7.2) | 30 (17) (10.3–50) | 0.009 |
| ΔCP median (n) | 4 (17) (0–12.6) | 42.9 (9) (37–53.5) | <0.0001 |
| GGO | No | Yes | p |
| Scleredema median (n) | 0.68 (9) (0–6) | 15 (17) (7.6–30) | 0.007 |
| ESR > 30 median (n) | 6.2 (11) (2.14–13) | 14 (15) (0.6–32) | 0.04 |
| Fibrosis | No | Yes | p |
| Scleredema median (n) | 2 (9) (0–3) | 5 (17) (3–8) | 0.05 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sambataro, D.; Sambataro, G.; Pignataro, F.; Maglione, W.; Malatino, L.; Vancheri, C.; Colaci, M.; Del Papa, N. Quantification of Ground Glass Opacities Can Be Useful to Describe Disease Activity in Systemic Sclerosis. Diagnostics 2020, 10, 225. https://doi.org/10.3390/diagnostics10040225
Sambataro D, Sambataro G, Pignataro F, Maglione W, Malatino L, Vancheri C, Colaci M, Del Papa N. Quantification of Ground Glass Opacities Can Be Useful to Describe Disease Activity in Systemic Sclerosis. Diagnostics. 2020; 10(4):225. https://doi.org/10.3390/diagnostics10040225
Chicago/Turabian StyleSambataro, Domenico, Gianluca Sambataro, Francesca Pignataro, Wanda Maglione, Lorenzo Malatino, Carlo Vancheri, Michele Colaci, and Nicoletta Del Papa. 2020. "Quantification of Ground Glass Opacities Can Be Useful to Describe Disease Activity in Systemic Sclerosis" Diagnostics 10, no. 4: 225. https://doi.org/10.3390/diagnostics10040225
APA StyleSambataro, D., Sambataro, G., Pignataro, F., Maglione, W., Malatino, L., Vancheri, C., Colaci, M., & Del Papa, N. (2020). Quantification of Ground Glass Opacities Can Be Useful to Describe Disease Activity in Systemic Sclerosis. Diagnostics, 10(4), 225. https://doi.org/10.3390/diagnostics10040225







