Imaging of Adverse Events Related to Checkpoint Inhibitor Therapy
Abstract
1. Introduction
2. Mechanisms Underlying irAEs
3. Grading Severity of irAEs
4. Chronology of irAEs
5. Treatment of Adverse Events
5.1. Treatment of Gastrointestinal Toxicity
5.2. Treatment of Hepatic Toxicity
5.3. Treatment of Pulmonary Toxicity
5.4. Treatment of Thyroid Toxicity
6. Most Relevant Imaging Findings in irAEs
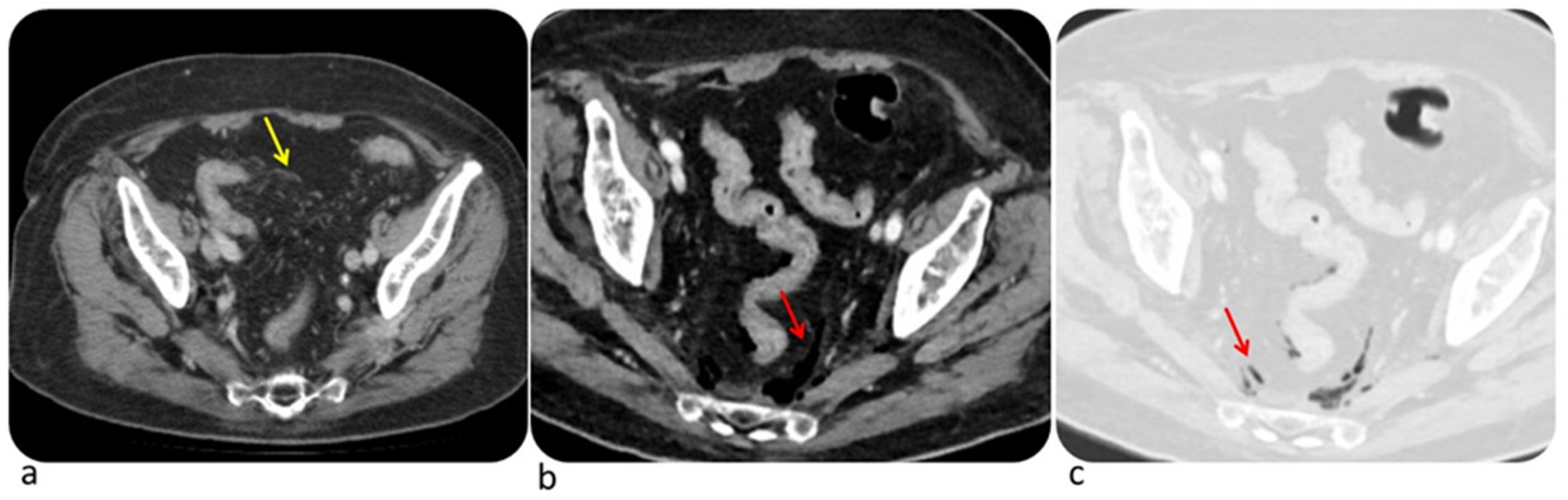
6.1. Colon
6.2. Liver
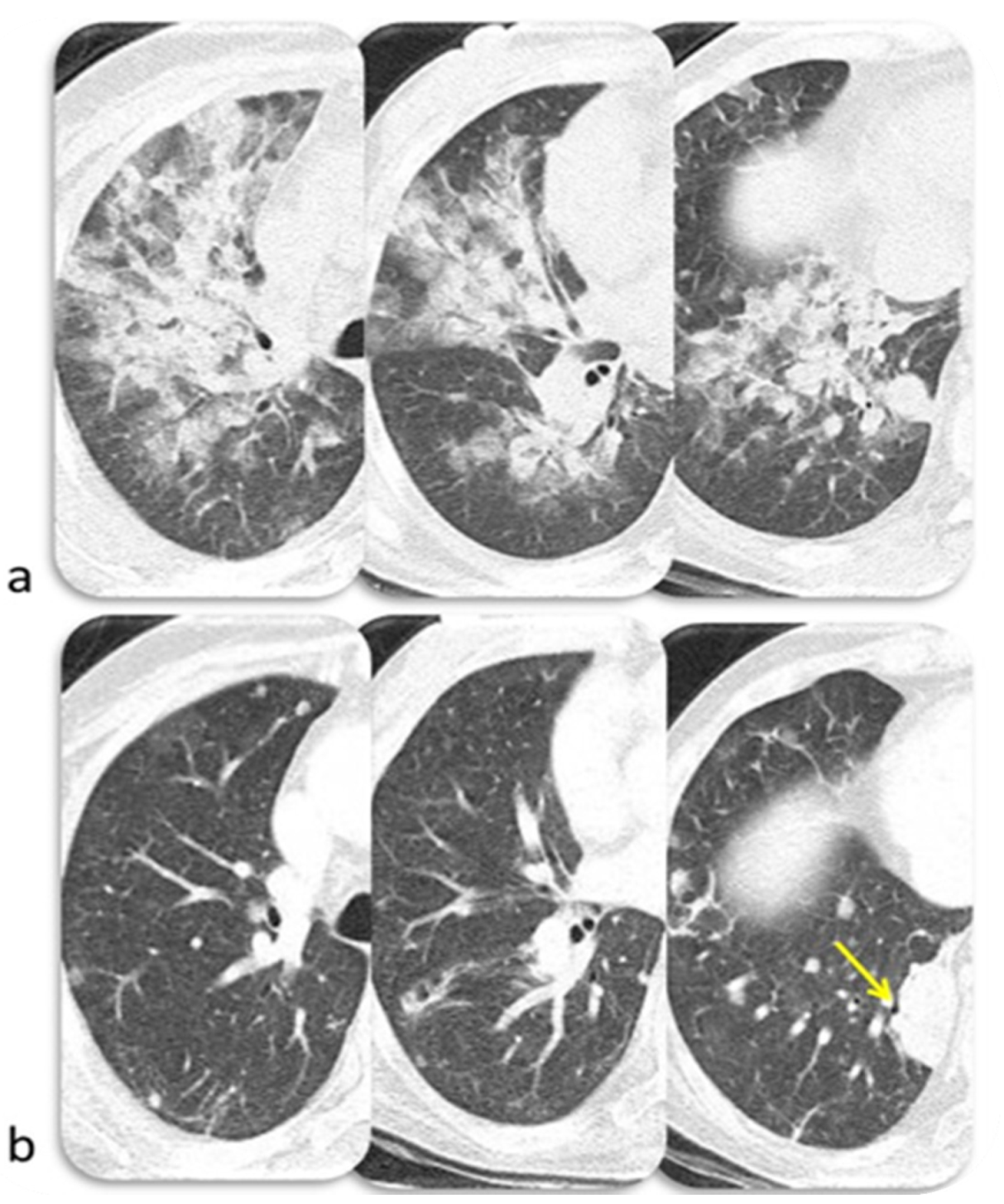
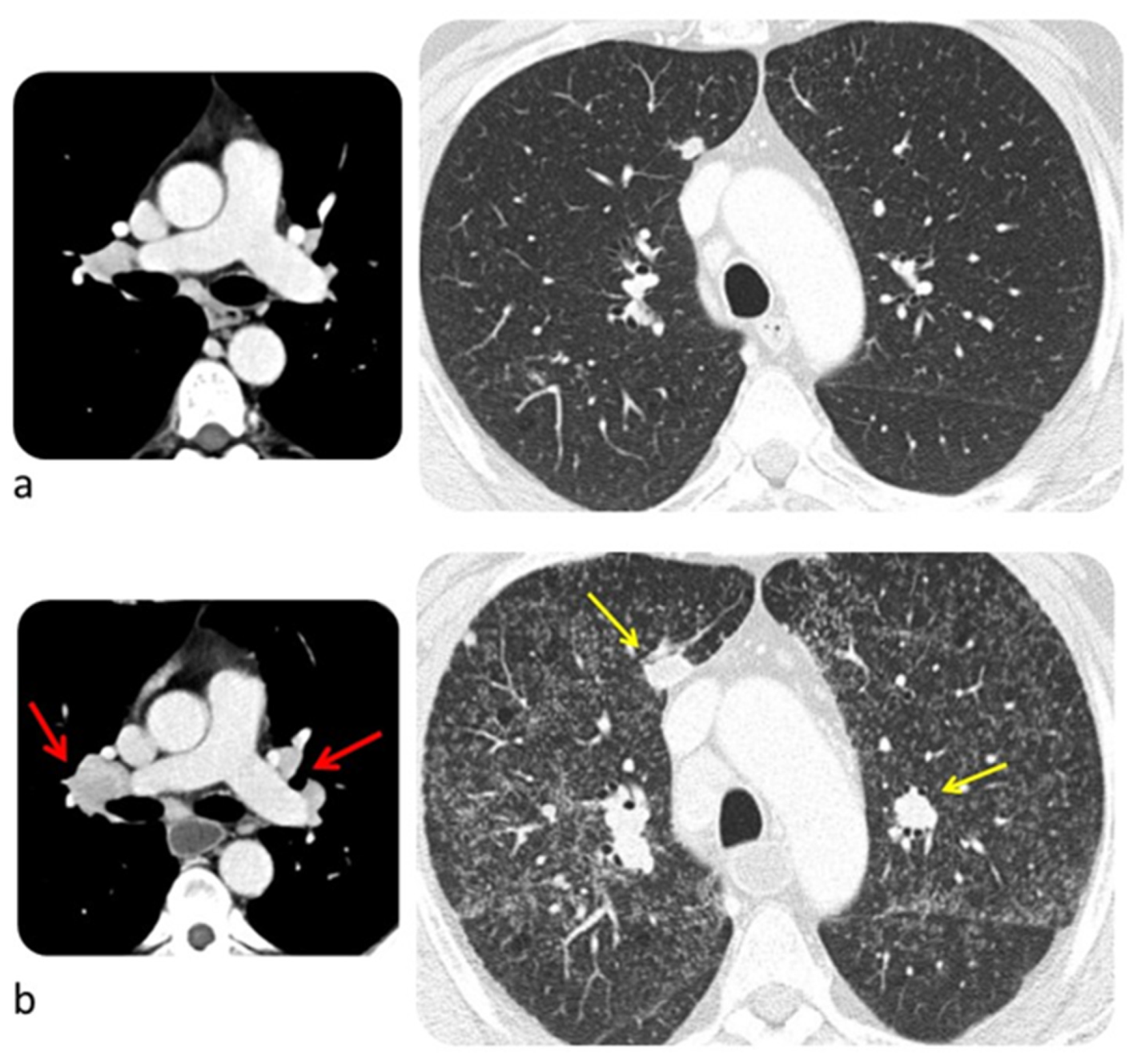
6.3. Lung
6.4. Endocrine System
6.4.1. Pituitary Gland
6.4.2. Thyroid
6.5. Pancreas
7. PET-CT: Confounder or Problem-Solving?
8. Less Frequent irAEs
8.1. Renal Toxicity
8.2. Cardiac Toxicity
8.3. Neurological Toxicity
9. Future Directions and Conclusions
Funding
Conflicts of Interest
Abbreviations
| AIP/ARDS | Acute Interstitial Pneumonia/Acute Respiratory Distress Syndrome |
| ALT | Alanine Transaminase |
| ASCO | American Society of Clinical Oncology |
| AST | Aspartate aminotransferase |
| COP | Cryptogenic Organizing Pneumonia |
| CT | Computed Tomography |
| CTCAE | Common Terminology Criteria for Adverse Events |
| CTLA-4 | Cytotoxic T-Lymphocyte–Associated Antigen 4 |
| EMA | European Medicines Agency |
| ESMO | European Society for Medical Oncology |
| FDG-PET | Fluorodeoxyglucose-Positron Emission Tomography |
| FT4 | Free Thyroxine |
| HP | Hypersensitivity Pneumonitis |
| HRT | Hormone Replacement Therapy |
| ICIs | Immunotherapy with Checkpoint Inhibitors |
| irAEs | immune-related Adverse Events |
| MRI | Magnetic Resonance Imaging |
| NCCN | National Comprehensive Cancer Network |
| NCI-CTCAE | National Cancer Institute - Common Terminology Criteria for Adverse Events |
| NSIP | Non-Specific Interstitial Pneumonia |
| PD-1 | Programmed Death 1 |
| PD-L1 | Programmed Death-Ligand 1 |
| RR | Relative Risk |
| SCAD | Segmental Colitis Associated with Diverticulosis |
| TSH | Thyroid-Stimulating Hormone |
| U.S. FDA | United States Food and Drug Administration |
| US | Ultrasound |
References
- Sosa, A.; Cadena, E.L.; Olive, C.S.; Le Pavec, J.; Collins, M.; Lallart, A.; Cengizalp, G.; Vozy, A.; Laparra, A.; Varga, A.; et al. Clinical assessment of immune-related adverse events. Ther. Adv. Med. Oncol. 2018, 10. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.X.; Kurra, V.; Gainor, J.F.; Sullivan, R.J.; Flaherty, K.T.; Lee, S.I.; Fintelmann, F.J. Immune Checkpoint Inhibitor Cancer Therapy: Spectrum of Imaging Findings. Radiographics 2017, 37, 2132–2144. [Google Scholar] [CrossRef] [PubMed]
- Haanen, J.; Carbonnel, F.; Robert, C.; Kerr, K.M.; Peters, S.; Larkin, J.; Jordan, K. ESMO Guidelines Committee. Management of Toxicities from Immunotherapy: ESMO Clinical Practice Guidelines. Ann. Oncol. 2017, 28, 119–142. [Google Scholar] [CrossRef] [PubMed]
- Braschi-Amirfarzan, M.; Tirumani, S.H.; Hodi, F.S.; Nishino, M. Immune-Checkpoint Inhibitors in the Era of Precision Medicine: What Radiologists Should Know. Korean J. Radiol. 2017, 18, 42–53. [Google Scholar] [CrossRef]
- Kim, K.W.; Ramaiya, N.H.; Krajewski, K.M.; Shinagare, A.B.; Howard, S.A.; Jagannathan, J.P.; Ibrahim, N. Ipilimumab-Associated Colitis: CT Findings. Am. J. Roentgenol. 2013, 200, 468–474. [Google Scholar] [CrossRef]
- MediPaper (MediPr) Medical Communications. Available online: https://medi-paper.com/us-fda-approved-immune-checkpoint-inhibitors-approved-immunotherapies/ (accessed on 14 February 2020).
- Michot, J.M.; Bigenwald, C.; Champiat, S.; Collins, M.; Carbonnel, F.; Postel-Vinay, S.; Berdelou, A.; Varga, A.; Bahleda, R.; Hollebecque, A.; et al. Immune-related adverse events with immune checkpoint blockade: A comprehensive review. Eur. J. Cancer 2016, 54, 139–148. [Google Scholar] [CrossRef]
- Kumar, V.; Chaudhary, N.; Garg, M.; Floudas, C.S.; Soni, P.; Chandra, A.B. Current Diagnosis and Management of Immune Related Adverse Events (irAEs) Induced by Immune Checkpoint Inhibitor Therapy. Front. Pharmacol. 2017, 8, 8–49. [Google Scholar]
- De Velasco, G.; Je, Y.; Bossé, D.; Awad, M.M.; Ott, P.A.; Moreira, R.B.; Schutz, F.; Bellmunt, J.; Sonpavde, G.P.; Hodi, F.S.; et al. Comprehensive Meta-analysis of Key Immune-Related Adverse Events from CTLA-4 and PD-1/PD-L1 Inhibitors in Cancer Patients. Cancer Immunol. Res. 2017, 5, 312–318. [Google Scholar] [CrossRef]
- Day, D.; Hansen, A.R. Immune-Related Adverse Events Associated with Immune Checkpoint Inhibitors. BioDrugs 2016, 30, 571–584. [Google Scholar] [CrossRef]
- Eigentler, T.K.; Hassel, J.C.; Berking, C.; Aberle, J.; Bachmann, O.; Grünwald, V.; Kähler, K.C.; Loquai, C.; Reinmuth, N.; Steins, M.; et al. Diagnosis, monitoring and management of immune-related adverse drug reactions of anti-PD-1 antibody therapy. Cancer Treat. Rev. 2016, 45, 7–18. [Google Scholar] [CrossRef]
- Alessandrino, F.; Sahu, S.; Nishino, M.; Adeni, A.E.; Tirumani, S.H.; Shinagare, A.B.; Awad, M.M. Frequency and imaging features of abdominal immune-related adverse events in metastatic lung cancer patients treated with PD-1 inhibitor. Abdom. Radiol. 2019, 44, 1917–1927. [Google Scholar] [CrossRef] [PubMed]
- Brahmer, J.R.; Lacchetti, C.; Schneider, B.J.; Atkins, M.B.; Brassil, K.J.; Caterino, J.M.; Chau, I.; Ernstoff, M.S.; Gardner, J.M.; Ginex, P.; et al. Management of Immune-Related Adverse Events in Patients Treated With Immune Checkpoint Inhibitor Therapy: American Society of Clinical Oncology Clinical Practice Guideline. J. Clin. Oncol. 2018, 36, 1714–1768. [Google Scholar] [CrossRef] [PubMed]
- El Majzoub, I.; Qdaisat, A.; Thein, K.Z.; Win, M.A.; Han, M.M.; Jacobson, K.; Chaftari, P.S.; Prejean, M.; Reyes-Gibby, C.; Yeung, S.J. Adverse Effects of Immune Checkpoint Therapy in Cancer Patients Visiting the Emergency Department of a Comprehensive Cancer Center. Ann. Emergy Med. 2019, 73, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.Y.; Ye, F.; Zhao, S.; Johnson, D.B. Incidence of immune checkpoint inhibitor-related colitis in solid tumor patients: A systematic review and meta-analysis. Oncoimmunology 2017, 6, 1344805. [Google Scholar] [CrossRef] [PubMed]
- Demlova, R.; Valik, D.; Obermannova, R.; ZdraŽilová-Dubská, L. The safety of therapeutic monoclonal antibodies: Implications for cancer therapy including immuno-checkpoint inhibitors. Physiol. Res. 2016, 65, 455–462. [Google Scholar] [CrossRef] [PubMed]
- European Medicines Agency. Available online: https://www.ema.europa.eu/en/documents/product-information/yervoy-epar-product-information_en.pdf (accessed on 14 February 2020).
- Santini, F.C.; Rizvi, H.; Plodkowski, A.J.; Ni, A.; Lacouture, M.E.; Gambarin-Gelwan, M.; Wilkins, O.; Panora, E.; Halpenny, D.F.; Long, N.M.; et al. Safety and Efficacy of Re-treating with Immunotherapy after Immune-Related Adverse Events in Patients with NSCLC. Cancer Immunol. Res. 2018, 6, 1093–1099. [Google Scholar] [CrossRef]
- Simonaggio, A.; Michot, J.M.; Voisin, A.L.; Le Pavec, J.; Collins, M.; Lallart, A.; Cengizalp, G.; Vozy, A.; Laparra, A.; Varga, A.; et al. Evaluation of Readministration of Immune Checkpoint Inhibitors After Immune-Related Adverse Events in Patients With Cancer. JAMA Oncol. 2019, 5, 1310–1317. [Google Scholar] [CrossRef]
- Pollack, M.H.; Betof, A.; Dearden, H.; Rapazzo, K.; Valentine, I.; Brohl, A.S.; Ancell, K.K.; Long, G.V.; Menzies, A.M.; Eroglu, Z.; et al. Safety of resuming anti-PD-1 in patients with immune-related adverse events (irAEs) during combined anti-CTLA-4 and anti-PD1 in metastatic melanoma. Ann. Oncol. 2018, 29, 250–255. [Google Scholar] [CrossRef]
- Abu-Sbeih, H.; Ali, F.S.; Wang, X.; Mallepally, N.; Chen, E.; Altan, M.; Bresalier, R.S.; Charabaty, A.; Dadu, R.; Jazaeri, A.; et al. Early introduction of selective immunosuppressive therapy associated with favorable clinical outcomes in patients with immune checkpoint inhibitor-induced colitis. J. Immunother. Cancer 2019, 7, 93. [Google Scholar] [CrossRef]
- Wang, Y.; Abu-Sbeih, H.; Mao, E.; Ali, N.; Qiao, W.; Trinh, V.A.; Zobniw, C.; Johnson, D.H.; Samdani, R.; Lum, P.; et al. Endoscopic and Histologic Features of Immune Checkpoint Inhibitor-Related Colitis. Inflamm. Bowel Dis. 2018, 24, 1695–1705. [Google Scholar] [CrossRef]
- National Cancer Institute. Available online: https://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/CTCAE_v5_Quick_Reference_8.5x11.pdf (accessed on 14 February 2020).
- Cramer, P.; Bresalier, R.S. Gastrointestinal and Hepatic Complications of Immune Checkpoint Inhibitors. Curr. Gastroenterol. Rep. 2017, 19, 3. [Google Scholar] [CrossRef] [PubMed]
- Alessandrino, F.; Tirumani, S.H.; Krajewski, K.M.; Shinagarea, A.B.; Jagannathana, J.P.; Ramaiyaa, N.H.; Di Salvo, D.N. Imaging of hepatic toxicity of systemic therapy in a tertiary cancer centre: Chemotherapy, haematopoietic stem cell transplantation, molecular targeted therapies, and immune checkpoint inhibitors. Clin. Radiol. 2017, 72, 521–533. [Google Scholar] [CrossRef] [PubMed]
- National Comprehensive Cancer Network. Available online: https://www.nccn.org/store/login/login.aspx?ReturnURL=https://www.nccn.org/professionals/physician_gls/pdf/immunotherapy.pdf (accessed on 14 February 2020).
- Tanaka, R.; Fujisawa, Y.; Sae, I.; Maruyama, H.; Ito, S.; Hasegawa, N.; Sekine, I.; Fujimoto, M. Severe hepatitis arising from ipilimumab administration, following melanoma treatment with nivolumab. Jpn. J. Clin. Oncol. 2017, 47, 175–178. [Google Scholar] [CrossRef] [PubMed]
- Doherty, G.J.; Duckworth, A.M.; Davies, S.E.; Mells, G.F.; Brais, R.; Harden, S.V.; Parkinson, C.A.; Corrie, P.G. Severe steroid-resistant anti-PD1 T-cell checkpoint inhibitor-induced hepatotoxicity driven by biliary injury. ESMO Open 2017, 2, e000268. [Google Scholar] [CrossRef]
- Iwamoto, K.; Ishitsuka, Y.; Tanaka, R.; Sekine, I.; Fujimoto, M. Azathioprine combination therapy for steroid-refractory hepatic immune system-related adverse events. Eur. J. Dermatol. 2017, 27, 301–303. [Google Scholar] [CrossRef]
- Lomax, A.J.; McGuire, H.M.; McNeil, C.; Choi, C.J.; Hersey, P.; Karikios, D.; Shannon, K.; van Hal, S.; Carr, U.; Crotty, A.; et al. Immunotherapy-induced sarcoidosis in patients with melanoma treated with PD-1 checkpoint inhibitors: Case series and immunophenotypic analysis. Int. J. Rheum. Dis. 2017, 20, 1277–1285. [Google Scholar] [CrossRef]
- Vogel, W.V.; Guislain, A.; Kvistborg, P.; Schumacher, T.N.; Haanen, J.B.; Blank, C.U. Ipilimumab-induced sarcoidosis in a patient with metastatic melanoma undergoing complete remission. J. Clin. Oncol. 2012, 30, 7–10. [Google Scholar] [CrossRef]
- Andersen, R.; Nørgaard, P.; Al-Jailawi, M.K.; Svane, I.M. Late development of splenic sarcoidosis-like lesions in a patient with metastatic melanoma and long-lasting clinical response to ipilimumab. Oncoimmunology 2014, 3, 954506. [Google Scholar] [CrossRef][Green Version]
- Ueda, T.; Sakagami, T.; Kikuchi, T.; Takada, T. Mycophenolate mofetil as a therapeutic agent for interstitial lung diseases in systemic sclerosis. Respir. Investig. 2018, 56, 14–20. [Google Scholar] [CrossRef]
- Osorio, J.C.; Ni, A.; Chaft, J.E.; Pollina, R.; Kasler, M.K.; Stephens, D.; Rodriguez, C.; Cambridge, L.; Rizvi, H.; Wolchok, J.D.; et al. Antibody-mediated thyroid dysfunction during T-cell checkpoint blockade in patients with non-small-cell lung cancer. Ann. Oncol. 2017, 28, 583–589. [Google Scholar] [CrossRef]
- Wang, Y.; Zhou, S.; Yang, F.; Qi, X.; Wang, X.; Guan, X.; Shen, C.; Duma, N.; Vera Aguilera, J.; Chintakuntlawar, A.; et al. Treatment-Related Adverse Events of PD-1 and PD-L1 Inhibitors in Clinical Trials: A Systematic Review and Meta-analysis. JAMA Oncol. 2019, 5, 1008–1019. [Google Scholar] [CrossRef] [PubMed]
- Barroso-Sousa, R.; Barry, W.T.; Garrido-Castro, A.C.; Hodi, F.S.; Min, L.; Krop, I.E.; Tolaney, S.M. Incidence of Endocrine Dysfunction Following the Use of Different Immune Checkpoint Inhibitor Regimens: A Systematic Review and Meta-analysis. JAMA Oncol. 2018, 4, 173–182. [Google Scholar] [CrossRef] [PubMed]
- Patel, N.S.; Oury, A.; Daniels, G.A.; Bazhenova, L.; Patel, S.P. Incidence of Thyroid Function Test Abnormalities in Patients Receiving Immune-Checkpoint Inhibitors for Cancer Treatment. Oncologist 2018, 23, 1236–1241. [Google Scholar] [CrossRef] [PubMed]
- Garon-Czmil, J.; Petitpain, N.; Rouby, F.; Sassier, M.; Babai, S.; Yelehe-Okouma, M.; Weryha, G.; Klein, M.; Gillet, P. Thyroiditis and immune check point inhibitors: The post-marketing experience using the French National Pharmacovigilance database. Fundam. Clin. Pharmacol. 2019, 33, 241–249. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, E.G.; Rodríguez-Abreu, D. Spanish Group for Cancer Immuno-Biotherapy (GETICA). Immune Checkpoint Inhibitors: Review and Management of Endocrine Adverse Events. Oncologist 2016, 21, 804–816. [Google Scholar] [CrossRef] [PubMed]
- Mekki, A.; Dercle, L.; Lichtenstein, P.H.; Marabelle, A.; Michot, J.M.; Lambotte, O.; Le Pavec, J.; De Martin, E.; Balleyguier, C.; Champiat, S.; et al. Detection of immune-related adverse events by medical imaging in patients treated with anti-programmed cell death 1. Eur. J. Cancer. 2018, 96, 91–104. [Google Scholar] [CrossRef]
- Kwak, J.J.; Tirumani, S.H.; Van den Abbeele, A.D.; Koo, P.J.; Jacene, H.A. Cancer immunotherapy: Imaging assessment of novel treatment response patterns and immune-related adverse events. Radiographics 2015, 35, 424–437. [Google Scholar] [CrossRef]
- Tirumani, S.H.; Ramaiya, N.H.; Keraliya, A.; Bailey, N.D.; Ott, P.A.; Hodi, F.S.; Nishino, M. Radiographic profiling of immune-related adverse events in advanced melanoma patients treated with ipilimumab. Cancer Immunol. Res. 2015, 3, 1185–1192. [Google Scholar] [CrossRef]
- Widmann, G.; Nguyen, V.A.; Plaickner, J.; Jaschke, W. Imaging Features of Toxicities by Immune Checkpoint Inhibitors in Cancer Therapy. Curr. Radiol. Rep. 2017, 5, 59. [Google Scholar] [CrossRef] [PubMed]
- Weber, J.S.; Kähler, K.C.; Hauschild, A. Management of immune-related adverse events and kinetics of response with ipilimumab. J. Clin. Oncol. 2012, 30, 2691–2697. [Google Scholar] [CrossRef]
- Suzman, D.L.; Pelosof, L.; Rosenberg, A.; Avigan, M.I. Hepatotoxicity of immune checkpoint inhibitors: An evolving picture of risk associated with a vital class of immunotherapy agents. Liver Int. 2018, 38, 976–987. [Google Scholar] [CrossRef] [PubMed]
- Sznol, M.; Ferrucci, P.F.; Hogg, D.; Atkins, M.B.; Wolter, P.; Guidoboni, M.; Lebbé, C.; Kirkwood, J.M.; Schachter, J.; Daniels, G.A.; et al. Pooled Analysis Safety Profile of Nivolumab and Ipilimumab Combination Therapy in Patients With Advanced Melanoma. J. Clin. Oncol. 2017, 35, 3815–3822. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.W.; Ramaiya, N.H.; Krajewski, K.M.; Jagannathan, J.P.; Tirumani, S.H.; Srivastava, A.; Ibrahim, N. Ipilimumab associated hepatitis: Imaging and clinicopathologic findings. Investig. New Drugs 2013, 31, 1071–1077. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.Y.; Salem, J.E.; Cohen, J.V.; Chandra, S.; Menzer, C.; Ye, F.; Zhao, S.; Das, S.; Beckermann, K.E.; Ha, L.; et al. Fatal toxic effects associated with immune checkpoint inhibitors: A systematic review and meta-analysis. JAMA Oncol. 2018, 4, 1721–1728. [Google Scholar] [CrossRef] [PubMed]
- Naidoo, J.; Wang, X.; Woo, K.M.; Iyriboz, T.; Halpenny, D.; Cunningham, J.; Chaft, J.E.; Segal, N.H.; Callahan, M.K.; Lesokhin, A.M.; et al. Pneumonitis in Patients Treated With Anti-Programmed Death-1/Programmed Death Ligand 1 Therapy. J. Clin. Oncol. 2017, 35, 709–717. [Google Scholar] [CrossRef]
- Rashdan, S.; Minna, J.D.; Gerber, D.E. Diagnosis and management of pulmonary toxicity associated with cancer immunotherapy. Lancet Respir. Med. 2018, 6, 472–478. [Google Scholar] [CrossRef]
- Nishino, M.; Giobbie-Hurder, A.; Hatabu, H.; Ramaiya, N.H.; Hodi, F.S. Incidence of programmed cell death 1 inhibitor-related pneumonitis in patients with advanced cancer: A systematic review and meta-analysis. JAMA Oncol. 2016, 2, 1607–1616. [Google Scholar] [CrossRef]
- Fehrenbacher, L.; Spira, A.; Ballinger, M.; Kowanetz, M.; Vansteenkiste, J.; Mazieres, J.; Park, K.; Smith, D.; Artal-Cortes, A.; Lewanski, C.; et al. Atezolizumab versus docetaxel for patients with previously treated non-small-cell lung cancer (POPLAR): A multicentre, open-label, phase 2 randomised controlled trial. Lancet 2016, 387, 1837–1846. [Google Scholar] [CrossRef]
- Massard, C.; Gordon, M.S.; Sharma, S.; Rafii, S.; Wainberg, Z.A.; Luke, J.; Curiel, T.J.; Colon-Otero, G.; Hamid, O.; Sanborn, R.E.; et al. Safety and efficacy of durvalumab (MEDI4736), an anti-programmed cell death ligand-1 immune checkpoint inhibitor, in patients with advanced urothelial bladder cancer. J. Clin. Oncol. 2016, 34, 3119–3125. [Google Scholar] [CrossRef]
- Garon, E.B.; Rizvi, N.A.; Hui, R.; Leighl, N.; Balmanoukian, A.S.; Eder, J.P.; Patnaik, A.; Aggarwal, C.; Gubens, M.; Horn, L.; et al. Pembrolizumab for the treatment of non-smallcell lung cancer. N. Engl. J. Med. 2015, 372, 2018–2028. [Google Scholar] [CrossRef]
- Brahmer, J.; Reckamp, K.L.; Baas, P.; Crinò, L.; Eberhardt, W.E.; Poddubskaya, E.; Antonia, S.; Pluzanski, A.; Vokes, E.E.; Holgado, E.; et al. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N. Engl. J. Med. 2015, 373, 123–135. [Google Scholar] [CrossRef] [PubMed]
- Herbst, R.S.; Baas, P.; Kim, D.W.; Felip, E.; Pérez-Gracia, J.L.; Han, J.Y.; Molina, J.; Kim, J.H.; Arvis, C.D.; Ahn, M.J.; et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): A randomized controlled trial. Lancet 2016, 387, 1540–1550. [Google Scholar] [CrossRef]
- Hodi, F.S.; Chesney, J.; Pavlick, A.C.; Robert, C.; Grossmann, K.F.; McDermott, D.F.; Linette, G.P.; Meyer, N.; Giguere, J.K.; Agarwala, S.S.; et al. Combined nivolumab and ipilimumab versus ipilimumab alone in patients with advanced melanoma: 2-year overall survival outcomes in a multicentre, randomised, controlled, phase 2 trial. Lancet Oncol. 2016, 17, 1558–1568. [Google Scholar] [CrossRef]
- Larkin, J.; Chiarion-Sileni, V.; Gonzalez, R.; Grob, J.J.; Cowey, C.L.; Lao, C.D.; Schadendorf, D.; Dummer, R.; Smylie, M.; Rutkowski, P.; et al. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N. Engl. J. Med. 2015, 373, 23–34. [Google Scholar] [CrossRef] [PubMed]
- Nishino, M.; Ramaiya, N.H.; Awad, M.M.; Sholl, L.M.; Maattala, J.A.; Taibi, M.; Hatabu, H.; Ott, P.A.; Armand, P.F.; Hodi, F.S. PD-1 inhibitor-related pneumonitis in advanced cancer patients: Radiographic patterns and clinical course. Clin. Cancer Res. 2016, 22, 6051–6060. [Google Scholar] [CrossRef] [PubMed]
- Hansell, D.M.; Bankier, A.A.; MacMahon, H.; McLoud, T.C.; Müller, N.L.; Remy, J. Fleischner Society: Glossary of terms for thoracic imaging. Radiology 2008, 246, 697–722. [Google Scholar] [CrossRef] [PubMed]
- Travis, W.D.; Costabel, U.; Hansell, D.M.; King, T.E., Jr.; Lynch, D.A.; Nicholson, A.G.; Ryerson, C.J.; Ryu, J.H.; Selman, M.; Wells, A.U.; et al. An official American Thoracic Society/European Respiratory Society statement: Update of the international multidisciplinary classification of the idiopathic interstitial pneumonias. Am. J. Respir. Crit. Care Med. 2013, 188, 733–748. [Google Scholar] [CrossRef]
- Diederich, S. Chest CT for suspected pulmonary complications of oncologic therapies: How I review and report. Cancer Imaging 2016, 11, 7. [Google Scholar] [CrossRef]
- Watanabe, S.; Kimura, H.; Takato, H.; Waseda, Y.; Hara, J.; Sone, T.; Abo, M.; Maeda, S.; Matsushita, T.; Kasahara, K. Severe pneumonitis after nivolumab treatment in a patient with melanoma. Allergol. Int. 2016, 65, 487–489. [Google Scholar] [CrossRef]
- Tetzlaff, M.T.; Nelson, K.C.; Diab, A.; Staerkel, G.A.; Nagarajan, P.; Torres-Cabala, C.A.; Chasen, B.A.; Wargo, J.A.; Prieto, V.G.; Amaria, R.N.; et al. Granulomatous/sarcoid-like lesions associated with checkpoint inhibitors: A marker of therapy response in a subset of melanoma patients. J. Immunother. Cancer 2018, 6, 14. [Google Scholar] [CrossRef]
- Wittenberg, R.; Rossi, S.; Prokop, C.S. Drug-Induced Interstitial Lung Disease in Oncology Patients. In Imaging of Complications and Toxicity following Tumor Therapy, 1st ed.; Kauczor, H.U., Bäuerle, T., Eds.; Springer: Cham, Switzerland, 2015; Volume 4, pp. 130–144. [Google Scholar]
- Nishino, M.; Sholl, L.M.; Awad, M.M.; Hatabu, H.; Armand, P.; Hodi, F.S. Sarcoid-Like Granulomatosis of the Lung Related to Immune-Checkpoint Inhibitors: Distinct Clinical and Imaging Features of a Unique Immune-Related Adverse Event. Cancer Immunol. Res. 2018, 6, 630–635. [Google Scholar] [CrossRef] [PubMed]
- Alessandrino, F.; Shah, H.J.; Ramaiya, N.H. Multimodality imaging of endocrine immune related adverse events: A primer for radiologists. Clin. Imaging 2018, 50, 96–103. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Rahman, O.; El Halawani, H.; Fouad, M. Risk of endocrine complications in cancer patients treated with immune check point inhibitors: A meta-analysis. Future Oncol. 2016, 12, 413–425. [Google Scholar] [CrossRef] [PubMed]
- Robert, C.; Schachter, J.; Long, G.V.; Arance, A.; Grob, J.J.; Mortier, L.; Daud, A.; Carlino, M.S.; McNeil, C.; Lotem, M.; et al. Pembrolizumab versus ipilimumab in advanced melanoma. N. Engl. J. Med. 2015, 372, 2521–2532. [Google Scholar] [CrossRef]
- Chodakiewitz, Y.; Brown, S.; Boxerman, J.L.; Brody, J.M.; Rogg, J.M. Ipilimumab treatment associated pituitary hypophysitis: Clinical presentation and imaging diagnosis. Clin. Neurol. Neurosurg. 2014, 125, 125–130. [Google Scholar] [CrossRef]
- Glezer, A.; Bronstein, M.D. Pituitary autoimmune disease: Nuances in clinical presentation. Endocrine 2012, 42, 74–79. [Google Scholar] [CrossRef]
- Hegerova, L.; Griebeler, M.L.; Reynolds, J.P.; Henry, M.R.; Gharib, H. Metastasis to the Thyroid Gland: Report of a large series from the Mayo Clinic. Am. J. Clin. Oncol. 2015, 38, 338–342. [Google Scholar] [CrossRef]
- Wang, P.F.; Chen, Y.; Song, S.Y.; Wang, T.J.; Ji, W.J.; Li, S.W.; Liu, N.; Yan, C.X. Immune-Related Adverse Events Associated with Anti-PD-1/PD-L1 Treatment for Malignancies: A Meta-Analysis. Front. Pharmacol. 2017, 8, 730. [Google Scholar] [CrossRef]
- Iravani, A.; Hicks, R.J. Pitfalls and Immune-Related Adverse Events. In Atlas of Response to Immunotherapy, 1st ed.; Lopci, E., Fanti, S., Eds.; Springer: Cham, Switzerland, 2020; Volume 10, pp. 101–115. [Google Scholar]
- Nobashi, T.; Baratto, L.; Reddy, S.A.; Srinivas, S.; Toriihara, A.; Hatami, N.; Yohannan, T.K.; Mittra, E. Predicting Response to Immunotherapy by Evaluating Tumors, Lymphoid Cell-Rich Organs, and Immune-Related Adverse Events Using FDG-PET/CT. Clin. Nucl. Med. 2019, 44, 272–279. [Google Scholar] [CrossRef]
- Lang, N.; Dick, J.; Slynko, A.; Schulz, C.; Dimitrakopoulou-Strauss, A.; Sachpekidis, C.; Enk, A.H.; Hassel, J.C. Clinical significance of signs of autoimmune colitis in 18F-fluorodeoxyglucose positron emission tomography-computed tomography of 100 stage-IV melanoma patients. Immunotherapy 2019, 11, 667–676. [Google Scholar] [CrossRef]
- Bronstein, Y.; Ng, C.S.; Hwu, P.; Hwu, W.J. Radiologic manifestations of immune-related adverse events in patients with metastatic melanoma undergoing anti-CTLA-4 antibody therapy. Am. J. Roentgenol. 2011, 197, 992–1000. [Google Scholar] [CrossRef] [PubMed]
- Lyon, A.R.; Yousaf, N.; Battisti, N.M.L.; Moslehi, J.; Larkin, J. Immune checkpoint inhibitors and cardiovascular toxicity. Lancet Oncol. 2018, 19, 447–458. [Google Scholar] [CrossRef]
- Feng, S.; Coward, J.; McCaffrey, E.; Coucher, J.; Kalokerinos, P.; O’Byrne, K. Pembrolizumab-Induced Encephalopathy: A Review of Neurological Toxicities with Immune Checkpoint Inhibitors. J. Thorac. Oncol. 2017, 12, 1626–1635. [Google Scholar] [CrossRef] [PubMed]
- Colen, R.R.; Fujii, T.; Bilen, M.A.; Kotrotsou, A.; Abrol, S.; Hess, K.R.; Hajjar, J.; Suarez-Almazor, M.E.; Alshawa, A.; Hong, D.S.; et al. Radiomics to predict immunotherapy-induced pneumonitis: Proof of concept. Investig. New Drugs 2018, 36, 601–607. [Google Scholar] [CrossRef] [PubMed]
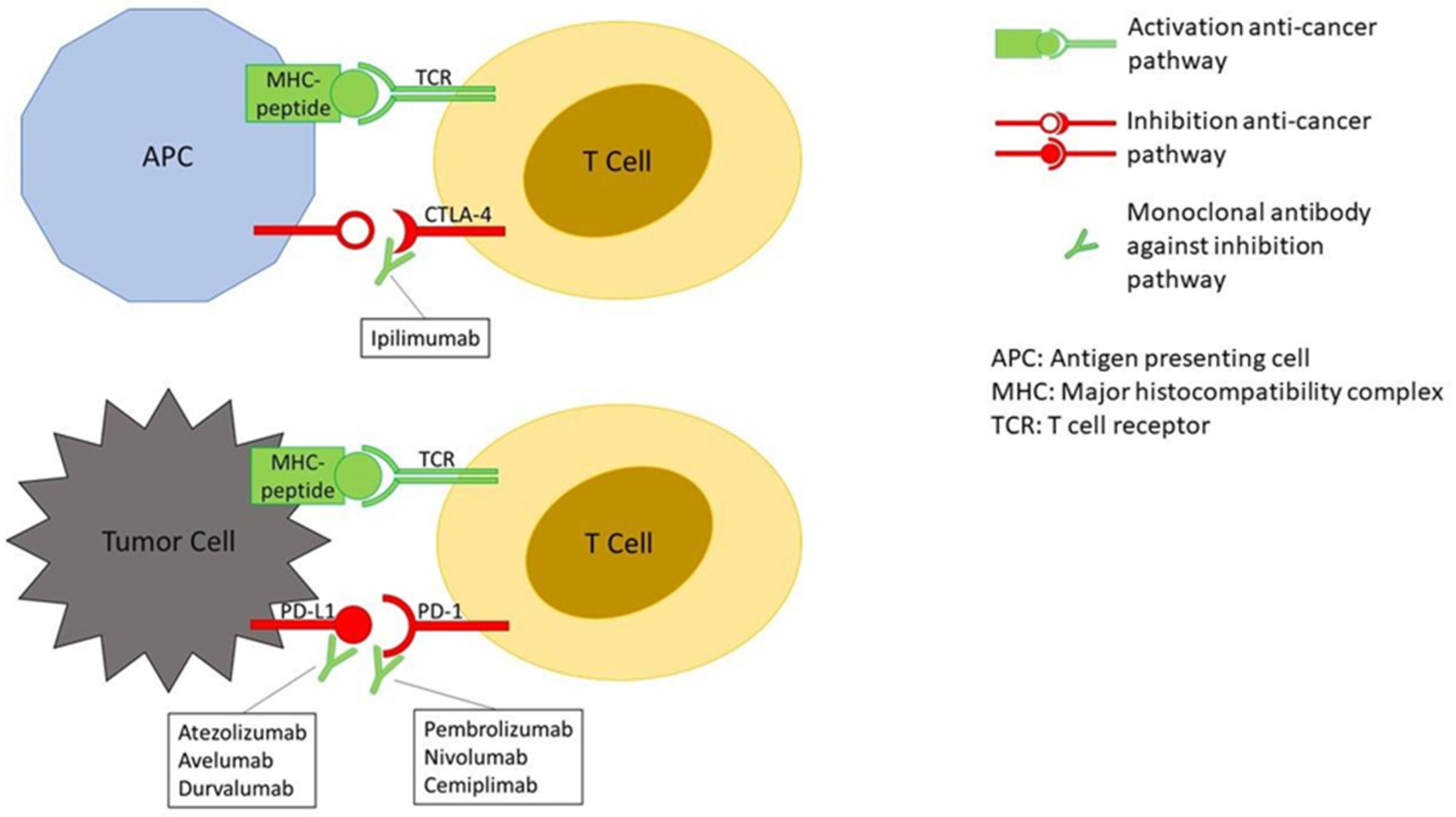




| ICIMechanism of Action | Indication | |
|---|---|---|
| Ipilimumab Anti-CTLA-4 antibody | • Unresectable or Metastatic Melanoma • Adjuvant Treatment of Melanoma • Advanced Renal Cell Carcinoma • Microsatellite Instability-High (MSI-H) or Mismatch Repair Deficient (dMMR) Metastatic Colorectal Cancer | |
| Pembrolizumab Anti-PD-1 antibody |
• Melanoma • Non-Small Cell Lung Cancer • Small Cell Lung Cancer • Head and Neck Squamous Cell Cancer • Classical Hodgkin Lymphoma • Primary Mediastinal Large B-Cell Lymphoma • Urothelial Carcinoma • Microsatellite Instability-High Cancer |
• Gastric Cancer • Esophageal Cancer • Cervical Cancer • Hepatocellular Carcinoma • Merkel Cell Carcinoma • Renal Cell Carcinoma • Endometrial Carcinoma |
| Nivolumab Anti-PD-1 antibody |
• Unresectable or Metastatic Melanoma • Adjuvant Treatment of Melanoma • Metastatic Non-Small Cell Lung Cancer • Small Cell Lung Cancer • Advanced Renal Cell Carcinoma • Classical Hodgkin Lymphoma |
• Squamous Cell Carcinoma of the Head and Neck • Urothelial Carcinoma • Microsatellite Instability-High or Mismatch Repair Deficient Metastatic Colorectal Cancer • Hepatocellular Carcinoma. |
| Ipilimumab + nivolumab | • Unresectable or metastatic melanoma | |
| Atezolizumab Anti-PD-L1 antibody |
• Urothelial Carcinoma • Non-Small Cell Lung Cancer • Locally Advanced or Metastatic Triple-Negative Breast Cancer • Small Cell Lung Cancer | |
| Avelumab Anti-PD-L1 antibody |
• Metastatic Merkel Cell Carcinoma • Locally Advanced or Metastatic Urothelial Carcinoma • Advanced Renal Cell Carcinoma | |
| Durvalumab Anti-PD-L1 antibody |
• Urothelial Carcinoma • Non-Small Cell Lung Cancer | |
| Cemiplimab Anti-PD-1 antibody | • Metastatic Cutaneous Squamous Cell Carcinoma (CSCC) • Locally Advanced CSCC | |
| Diffuse Colitis | Segmental Colitis |
|---|---|
|
|
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vani, V.; Regge, D.; Cappello, G.; Gabelloni, M.; Neri, E. Imaging of Adverse Events Related to Checkpoint Inhibitor Therapy. Diagnostics 2020, 10, 216. https://doi.org/10.3390/diagnostics10040216
Vani V, Regge D, Cappello G, Gabelloni M, Neri E. Imaging of Adverse Events Related to Checkpoint Inhibitor Therapy. Diagnostics. 2020; 10(4):216. https://doi.org/10.3390/diagnostics10040216
Chicago/Turabian StyleVani, Vanina, Daniele Regge, Giovanni Cappello, Michela Gabelloni, and Emanuele Neri. 2020. "Imaging of Adverse Events Related to Checkpoint Inhibitor Therapy" Diagnostics 10, no. 4: 216. https://doi.org/10.3390/diagnostics10040216
APA StyleVani, V., Regge, D., Cappello, G., Gabelloni, M., & Neri, E. (2020). Imaging of Adverse Events Related to Checkpoint Inhibitor Therapy. Diagnostics, 10(4), 216. https://doi.org/10.3390/diagnostics10040216





