Platelet Mitochondrial Respiration, Endogenous Coenzyme Q10 and Oxidative Stress in Patients with Chronic Kidney Disease
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Observed Parameters
2.3. Platelets Preparation
2.4. Platelets Mitochondrial Respiration and Oxidative Phosphorylation
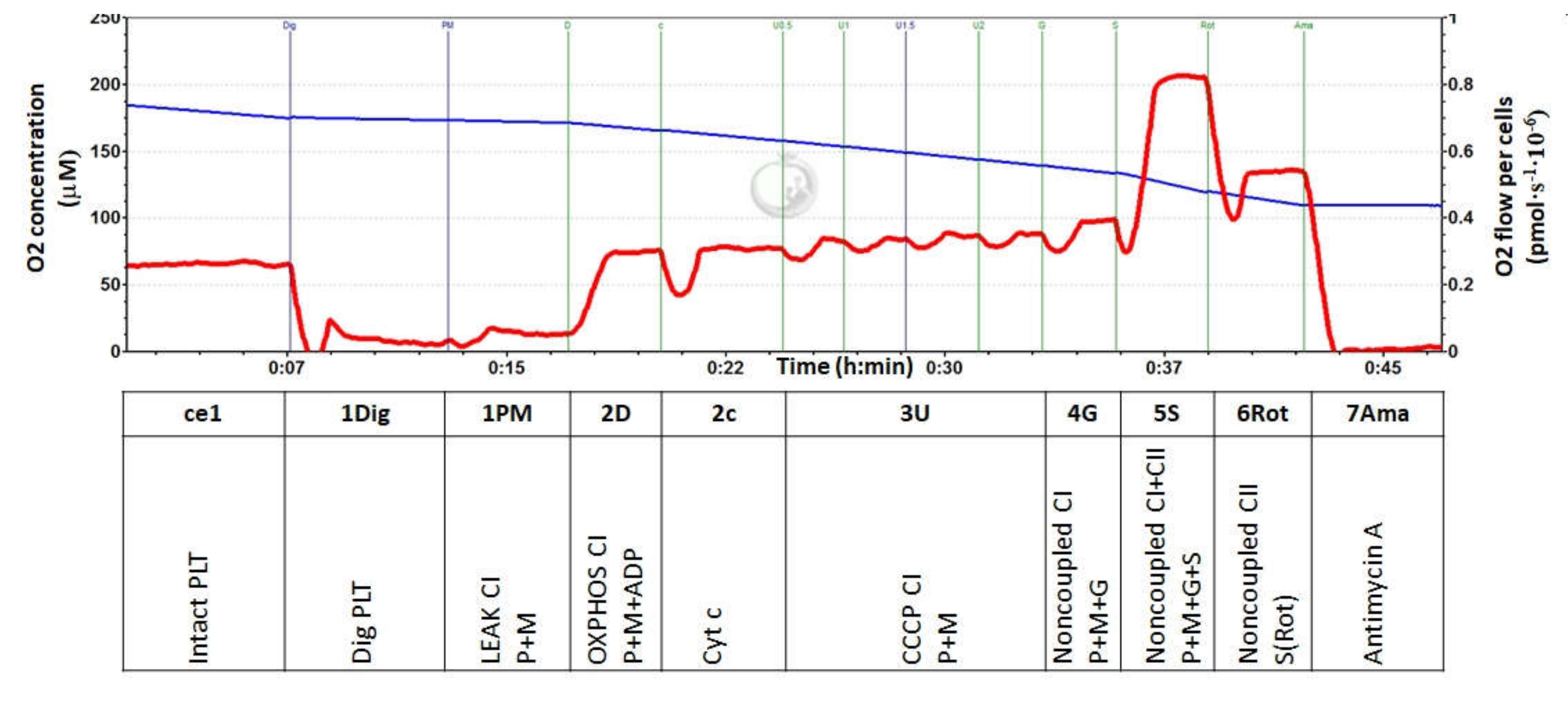
2.5. Experimental Protocol for Platelet Mitochondrial Respiration
2.6. Coenzyme Q10-TOTAL
2.7. Data Analysis
3. Results
3.1. Metabolic Parameters of Healthy Volunteers and Patients with Chronic Kidney Disease
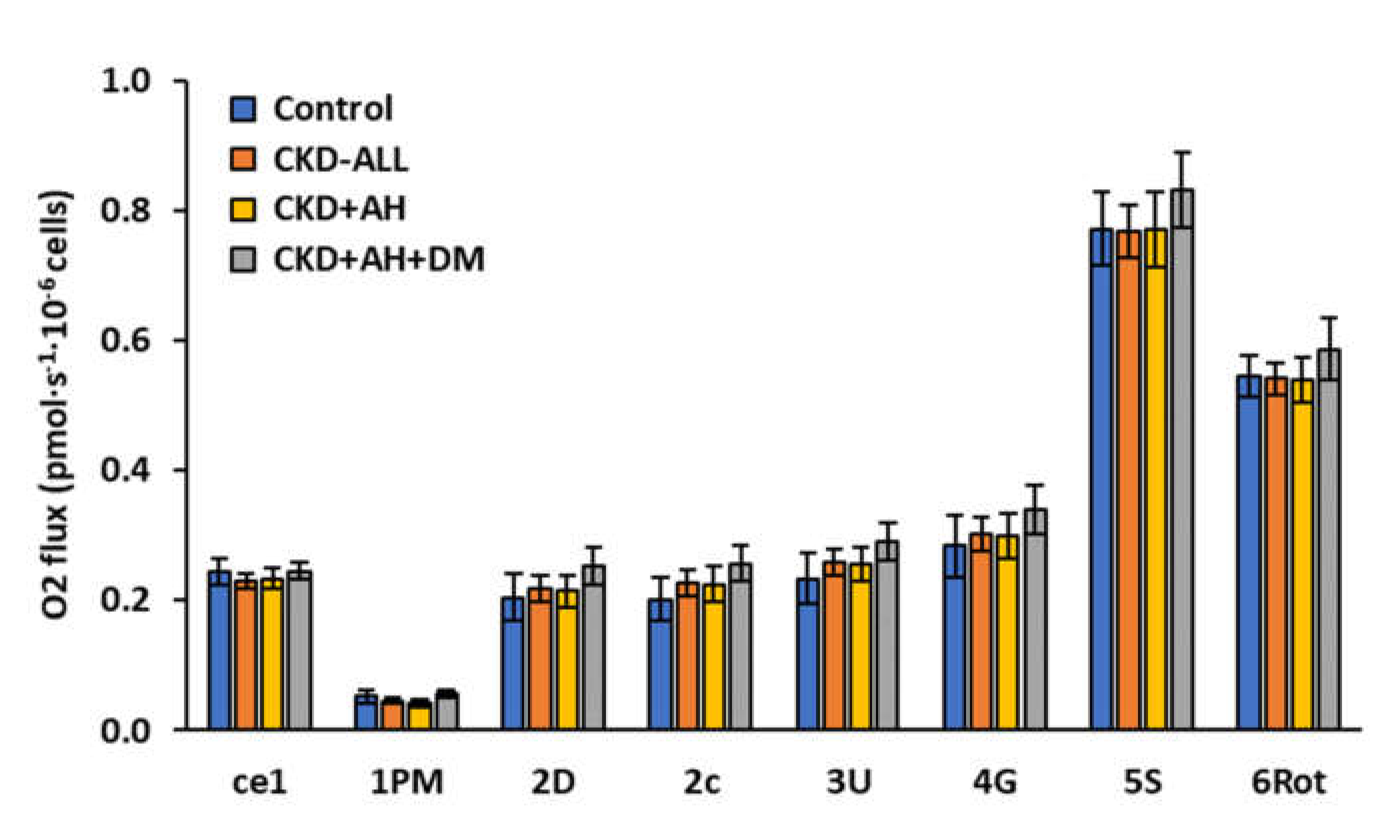
3.2. Platelet Mitochondrial Respiration and Oxidative Phosphorylation in Control Subjects and Groups of CKD Patients
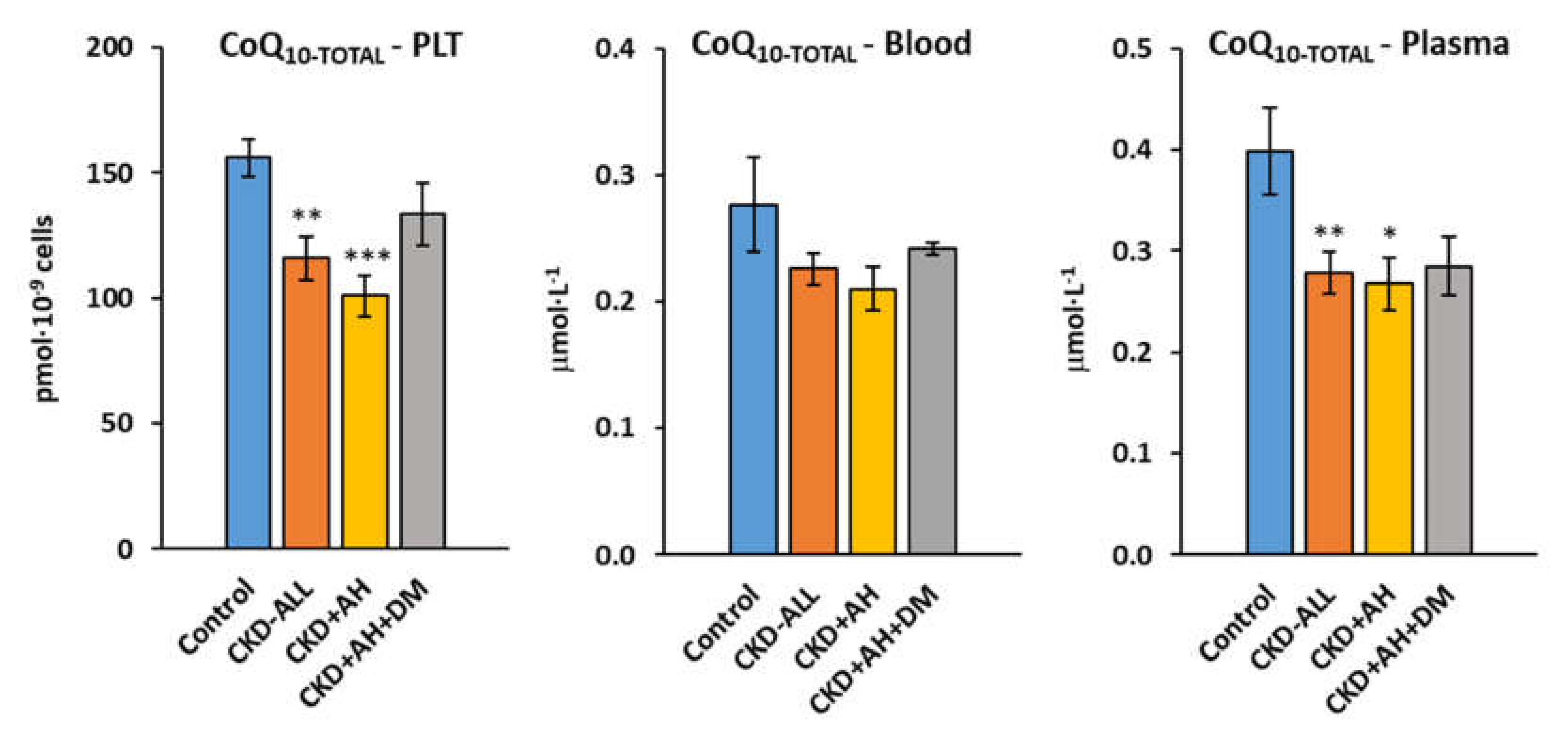
3.3. Endogenous Coenzyme Q10-TOTAL in Platelets, Blood and Plasma in Control Subjects and Groups of CKD Patients
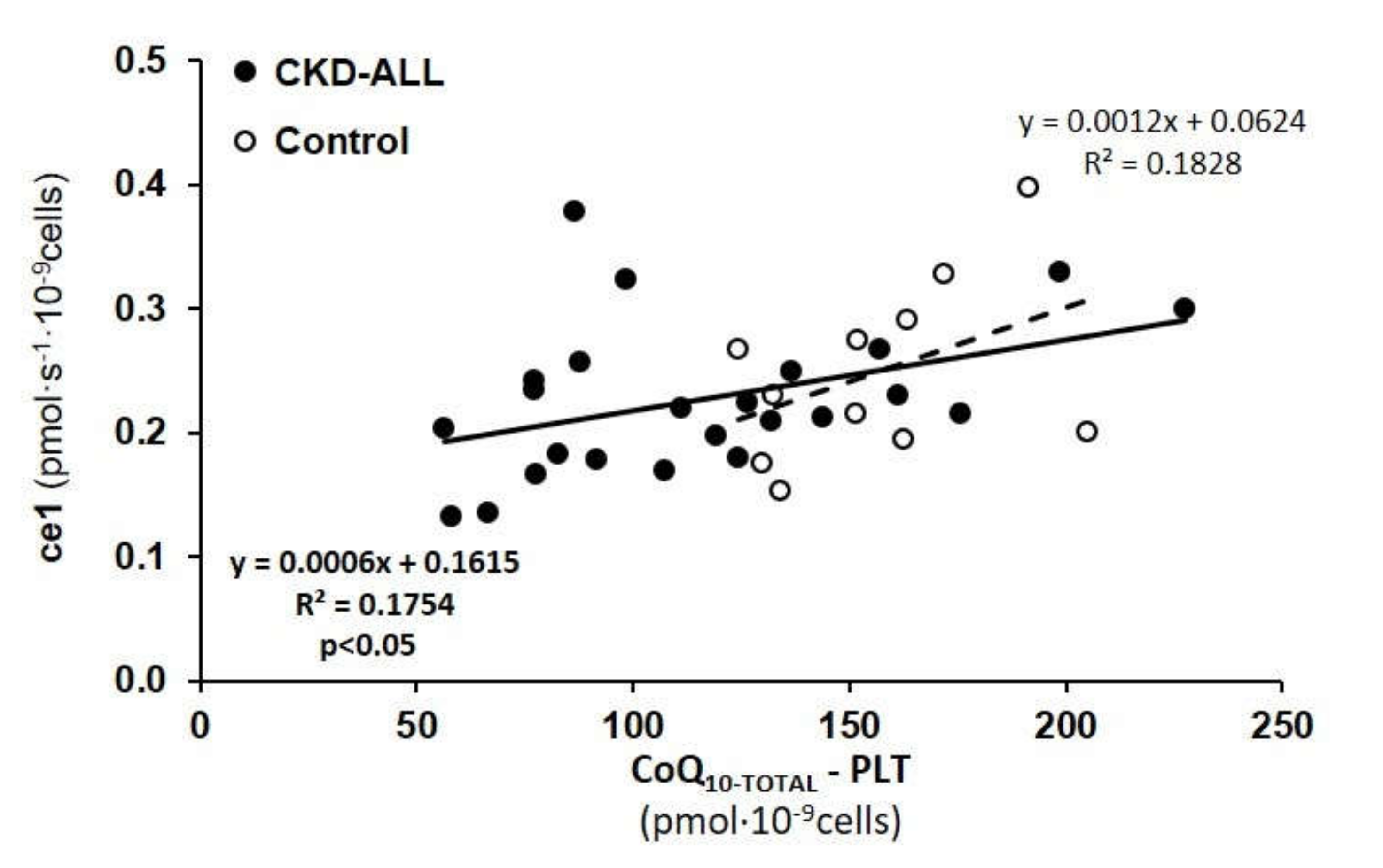
3.4. Correlations between CoQ10-TOTAL in Platelets and Respiration of Intact Platelets in Control Subjects and CKD Patients
3.5. Plasma Lipid Peroxidation of CKD Patients
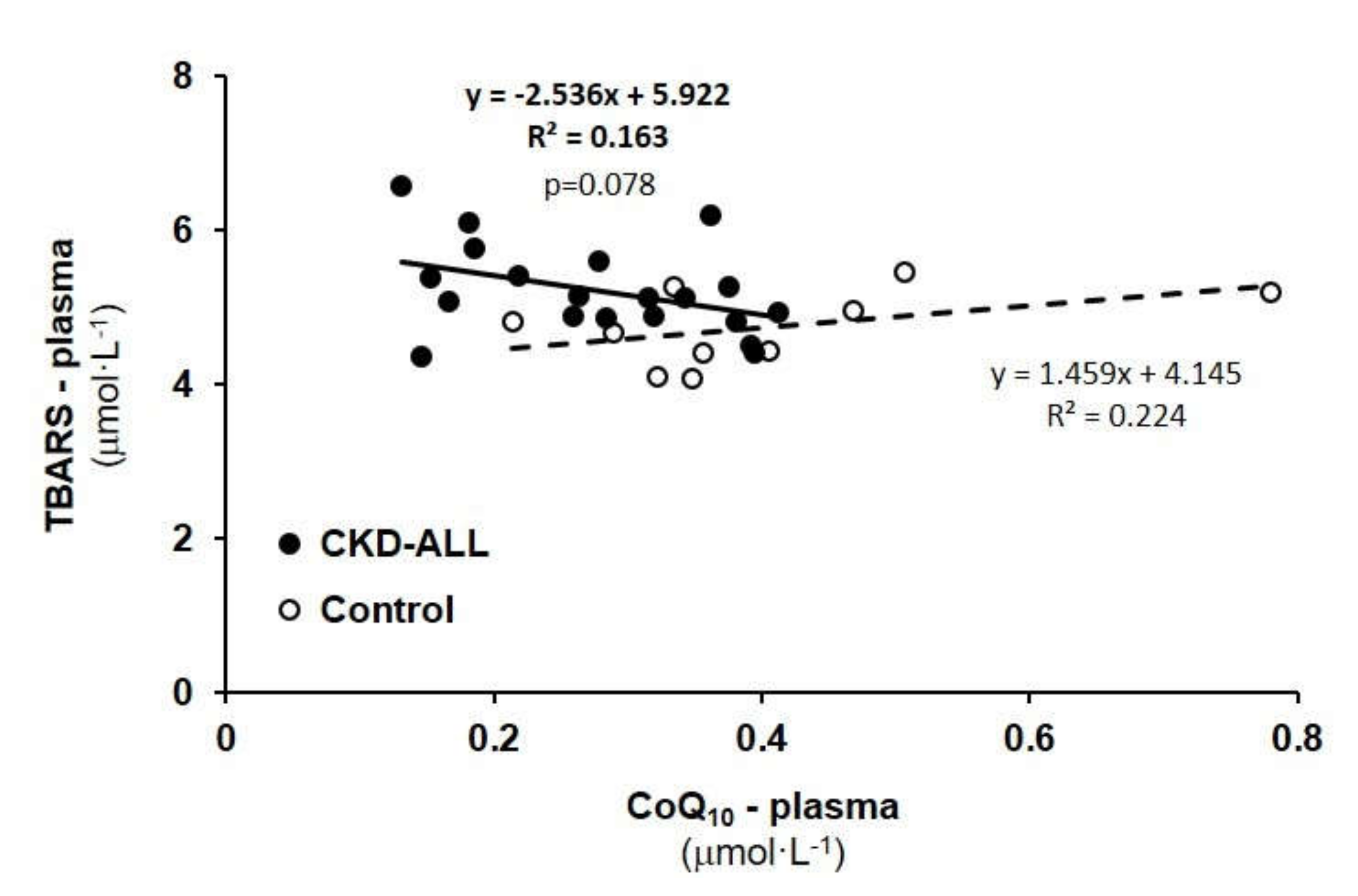
3.6. Correlation Between TBARS and CoQ10-TOTAL in Plasma in Control Subjects and CKD-ALL Patients
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| BMI | body mass index |
| eGFR | estimated glomerular filtration rate |
| CKD | chronic kidney disease |
| NCDs | non-communicable diseases |
| CoQ10 | coenzyme Q10 |
| CoQ10-TOTAL | ubiquinol and ubiquinone |
| HRR | high-resolution respirometry |
| AH | arterial hypertension |
| DM | diabetes mellitus Type 2 |
| PLT | platelets |
| Hgb | hemoglobin |
| CRP | c-reactive protein |
| TAG | triacylglycerols |
| LDL-Chol | low density lipoprotein cholesterol |
| HDL-Chol | high density lipoprotein cholesterol |
| Chol-Total | total cholesterol |
| AST | aspartate aminotransferase |
| ALT | alanine aminotransferase |
| GMT | gama-glutamyltransferase |
| TBARS | thiobarbituric acid reactive substances |
| SUIT | Substrate-Uncoupler-Inhibitor-Titration |
| RP1 | reference protocol 1 |
References
- WHO—World Health Organization. Noncommunicable Diseases, Posed June 1 2018. Available online: http://www.who.int/news-room/fact-sheets/detail/noncommunicable (accessed on 1 September 2018).
- Levey, A.S.; Coresh, J.; Balk, E.; Kausz, A.T.; Levin, A.; Steffes, M.W.; Hogg, R.J.; Perrone, R.D.; Lau, J.; Eknoyan, G. National Kidney Foundation: National Kidney Foundation practice guidelines for chronic kidney disease: Evaluation, classification, and stratification. Ann. Intern. Med. 2003, 139, 137–147. [Google Scholar] [CrossRef] [PubMed]
- Small, D.M.; Gobe, G.C. Oxidative Stress and Antioxidant Therapy in Chronic Kidney and Cardiovascular Disease; Intech: Rijeka, Croatia, 2018; pp. 233–264. Available online: https://books.google.com.hk/books?hl=zh-CN&lr=&id=UGOfDwAAQBAJ&oi=fnd&pg=PA233&ots=vNS3wTKurs&sig=LuJ5QLCKhqNVYJemPeGAajwyQmE&redir_esc=y&hl=zh-CN&sourceid=cndr#v=onepage&q&f=false (accessed on 22 May 2013).
- Small, D.M.; Coombes, J.S.; Bennet, N.; Johnson, N.; Gobe, G.C. Oxidative stress, anti-oxidant therapies and chronic kidney disease. Nephrology 2012, 17, 311–321. [Google Scholar] [CrossRef] [PubMed]
- Che, R.; Yuan, Y.; Huang, S.; Zhang, A. Mitochondrial dysfunction in the pathophysiology of renal diseases. Am. J. Physiol. Renal. Physiol. 2014, 306, F367–F378. [Google Scholar] [CrossRef] [PubMed]
- Granata, S.; Gassa, A.D.; Tomei, P.; Lupo, A.; Zaza, G. Mitochondria: A new therapeutic target in chronic kidney disease. Nutr.Metab. 2015, 12, 49. [Google Scholar] [CrossRef] [PubMed]
- Wei, P.Z.; Szeto, C.C. Mitochondrial dysfunction in diabetic kidney disease. Clin. Chim. Acta. 2019, 496, 108–116. [Google Scholar] [CrossRef]
- Turunen, M.; Swiezewska, E.; Chojnacki, T.; Sindelar, P.; Dallner, G. Regulatory aspects of coenzyme q metabolism. Free Radic. Res. 2002, 36, 437–443. [Google Scholar] [CrossRef]
- Hernandéz-Camacho, J.D.; Bernier, M.; López-Lluch, G.; Navas, P. Coenzyme Q10 supplementation in ageing and disease. Front. Physiol. 2018, 9, 44. [Google Scholar] [CrossRef]
- Alftani, A.; Riou, M.; Charles, A.L.; Meyer, A.; Barning, C.; Andres, E.; Lejay, A.; Talha, S.; Geny, B. Peripheral blood mononuclear cells and platelets mitochondrial dysfunction, oxidative stress, and circulating mtDNA in cardiovascular diseases. J. Clin. Med. 2020, 9, 311. [Google Scholar] [CrossRef]
- Hargreaves, I.; Mantle, D.; Milford, D. Chronic kidney disease and coenzyme Q10 supplementation. J. Kidney Care 2019, 4. [Google Scholar] [CrossRef]
- Dalbhi, S.K.A.; Alquarni, F.A.; Bahatheq, N.M.; Alrasheed, R.S.; Alkhowaiter, R.A.; Alnughaimshi, A.A. Mitochondrial dysfunction and kidney diseases. J. Nephropathol. 2019, 8, e18. [Google Scholar] [CrossRef]
- Granata, S.; Zaza, G.; Simone, S.; Villani, G.; Latorre, D.; Pontrelli, P.; Carella, M.; Schena, F.P.; Grandaliano, G.; Petrosa, G. Mitochondrial dysregulation and oxidative stress in patients with chronic kidney disease. BMC Genom. 2009, 10, 388. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wu, Q.; Fan, Z.; Xie, R.; Wang, Z.; Lu, Y. Platelet mitochondrial dysfunction and the correlation with human disease. Biochem. Soc. Trans. 2017, 45, 1213. [Google Scholar] [CrossRef] [PubMed]
- Melchiner, H.; Jain, K.; Tyagi, T.; Hwa, J. Role of platelet mitochondria: Life in a nucleus-free zone. Front. Cardiovasc. Med. 2019, 6, 153. [Google Scholar] [CrossRef] [PubMed]
- Fišar, Z.; Jirák, R.; Zvěřová, M.; Setnička, V.; Habrtová, L.; Hroudová, J.; Vaníčková, Z.; Raboch, J. Plasma amyloid beta levels and platelet mitochondrial respiration in patients with Alzheimer’s disease. Clin. Biochem. 2019, 72, 71–80. [Google Scholar] [CrossRef]
- Ghose, A.; Taylor, C.M.; Hargreaves, I.; Milford, D.V. Measurement of respiratory chain enzyme activity in human renal biopsy species. J. Clin. Med. 2017, 6, 90. [Google Scholar] [CrossRef]
- Hroudová, J.; Fišar, Z.; Kitzlerová, E.; Zvěřová, M.; Raboch, J. Mitochondrial respiration in blood platelets of depressive patients. Mitochondrion 2013, 13, 795–800. [Google Scholar] [CrossRef]
- Sumbalová, Z.; Droescher, S.; Hiller, E.; Chang, S.; Garcia, L.; Calabria, E.; Volani, C.; Krumschnabel, G.; Gnaiger, E. O2k-Protocols: Isolation of peripheral blood mononuclear cells and platelets from human blood for HRR. Mitochondr. Physiol. Netw. 2018, 17, 1–16. [Google Scholar]
- Pesta, D.; Gnaiger, E. High-resolution respirometry: OXPHOS protocols for human cells and permeabilized fibers from small biopsies of human muscle. Methods Mol. Biol. 2012, 810, 25–58. [Google Scholar]
- Sjövall, F.; Ehinger, J.K.; Marelsson, S.E.; Morota, S.; Frostner, E.A.; Uchino, H.; Lundgren, J.; Arnbjörnsson, E.; Hansson, M.J.; Fellman, V.; et al. Mitochondrial respiration in human viable platelets--methodology and influence of gender, age and storage. Mitochondrion 2013, 13, 7–14. [Google Scholar] [CrossRef]
- Doerrier, C.; Sumbalová, Z.; Krumschnabel, G.; Hiller, E.; Gnaiger, E. SUIT reference protocol for OXPHOS analysis by high-resolution respirometry. Mitochondr. Physiol. Netw. 2016, 21, 1–12. [Google Scholar]
- Lang, J.K.; Gohil, K.; Packer, L. Simultaneous determination of tocopherols, ubiquinols, and ubiquinones in blood, plasma, tissue homogenates, and subcellular fractions. Analyt. Biochem. 1986, 157, 106–116. [Google Scholar] [CrossRef]
- Kucharská, J.; Gvozdjáková, A.; Mizera, S.; Braunová, Z.; Schreinerová, Z.; Schrameková, E.; Pecháň, I.; Fabián, J. Participation of coenzyme Q10 in the rejection development of the transplanted heart. Physiol. Res. 1998, 47, 399–404. [Google Scholar] [PubMed]
- Niklowitz, P.; Menke, T.; Andler, W.; Okun, J.G. Simultaneous analysis of coenzyme Q10 in plasma, erythrocytes and platelets: Comparison of the antioxidant level in blood cells and their enviroment in healthy children and after oral supplementation in adults. Clin. Chim. Acta 2004, 342, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Mosca, F.; Fattorini, D.; Bompadre, S.; Littarru, G.P. Assay of coenzyme Q10 in plasma by a single dilution step. Analyt. Biochem. 2002, 305, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Janero, D.R.; Burghardt, B. Thiobarbituric acid-reactive malondialdehyd formation during suproxide-dependent, iron-catalyzed lipid peroxidation: Influence of peroxidation conditions. Lipids 1989, 24, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Ates, O.; Bilen, H.; Keles, S.; Alp, H.H.; Keles, M.S.; Yildirim, K.; Ondas, O.; Pinar, L.C.; Civelekler, M.; Baykal, O. Plasma coenzyme Q10 levels in type 2 diabetic patients with retinopathy. Int. J. Ophthalmol. 2013, 6, 675–679. [Google Scholar]
- Protti, A.; Fortunato, F.; Artoni, A.; Lecchi, A.; Motta, G.; Mistraletti, G.; Novembrino, C.; Comi, G.P.; Gattinoni, L. Platelet mitochondrial dysfunction in critically ill patients: Comparison between sepsis and cardiogenic shock. Crit. Care 2015, 19, 39. [Google Scholar] [CrossRef]
- Zharikov, S.; Shiva, S. Platelet mitochondrial function: From regulation of thrombosis to biomarker of disease. Biochem. Society Transact 2013, 41, 118–123. [Google Scholar] [CrossRef]
- Vevera, J.; Fisar, Z.; Nekovarova, T.; Vrablík, M.; Zlatohlávek, L.; Hroudová, J.; Singh, N.; Raboch, J.; Valeš, K. Statin-induced changes in mitochondrial respiration in blood platelets in rats and human with dyslipidemia. Physiol. Res. 2016, 65, 777–788. [Google Scholar] [CrossRef]
- Gazdíková, K.; Gvozdjáková, A.; Kucharská, J.; Spustová, V.; Braunová, Z.; Dzúrik, R. Clinical and pharmacologic aspects of mitochondrial nephropathy. Brat. Med. J. 2000, 101, 618–619. [Google Scholar]
- Hsiao, C.P.; Hoppel, C. Analysing mitochondrial function in human peripheral blood mononuclear cells. Analyt. Biochem. 2018, 549, 2–20. [Google Scholar] [CrossRef]
- Heeringa, S.F.; Chernin, G.; Chaki, M.; Zhou, W.; Sloan, A.J.; Ji, Z.; Xie, L.X.; Salviati, L.; Hurd, T.W.; Vega-Warner, V.; et al. COQ6 mutations in human patients produce nephrotic syndrome with sensorineural deafness. J. Clin. Investig. 2011, 12, 2013–20124. [Google Scholar] [CrossRef]
- Menke, T.; Niklowitz, P.; Wiesel, T.; Andler, W. Antioxidant level and redox status of coenzyme Q10 in the plasma and blood cells of children with diabetes mellitus type 1. Pediatr. Diabetes 2008, 9, 540–545. [Google Scholar] [CrossRef] [PubMed]
- Kucharská, J.; Gvozdjáková, A.; Štefek, M.; Sotníková, R.; Sumbalová, Z. Adaptive changes of antioxidant status in development of experimental diabetes. Bratisl. Med. J. 2001, 102, 515–519. [Google Scholar]
- Kuželová, M.; Adamcová, A.; Sumbalová, Z.; Paulíková, I.; Harčárová, A.; Švec, P.; Kucharská, J. The effect of simvastatin on coenzyme Q and antioxidant/oxidant balance in diabetic-hypercholesterolemic rats. Gen. Physiol. Biophys. 2008, 27, 291–298. [Google Scholar] [PubMed]
- Sumbalová, Z.; Gvozdjáková, A.; Kucharská, J.; Rausová, Z.; Vančová, O.; Kuzmiaková, Z.; Kubalová, M.; Uličná, O.; Chládeková, A.; Komlosi, M.; et al. Platelet mitochondrial function, coenzyme Q10, and oxidative stress in patients with chronic kidney diseases. In Proceedings of the 14th Conference on Mitochondrial Physiology MiP2019/MitoEAGLE, Belgrade, Serbia, 13–16 October 2019; pp. 35–36. [Google Scholar]
- Gvozdjáková, A.; Sumbalová, Z.; Kucharská, J.; Chládeková, A.; Rausová, Z.; Vančová, O.; Komlosi, M.; Uličná, O.; Mojto, V. Platelet mitochondrial bioenergetic analysis in patients with nephropathies and non-communicable diseases: A new method. Bratisl. Med. J. 2019, 120, 630–635. [Google Scholar] [CrossRef]
- Gazdíková, K.; Gvozdjáková, A.; Kucharská, J.; Spustová, V.; Braunová, Z.; Dzúrik, R. Oxidative stress and plasma concentration of coenzyme Q10, α-tocopherol, and β-carotene in patients with a mild to moderate decrease of kidney function. Nephron 2001, 88, 285. [Google Scholar] [CrossRef]
| Groups | CONTROL | CKD-ALL | CKD+AH | CKD+AH+DM |
|---|---|---|---|---|
| Table 1a | ||||
| Kidney | ||||
| eGFR (ml s−1.1.73 m2 ) | 1.328 ± 0.069 | 0.578 ± 0.043 *** | 0.574±0.053 *** | 0.583±0.074 *** |
| Creatinine (µmol L−1) | 70.7 ± 5.1 | 194.3 ± 17.0 *** | 198.5 ± 19.6 *** | 187.3 ± 31.3 ** |
| Uric acid (µmol L−1) | 287.4 ± 13.7 | 370.8 ± 25.5 * | 346.8 ± 25.9 | 409.2 ± 47.9 * |
| Table 1b | ||||
| Blood | ||||
| Hgb (g L−1) | 140.5 ± 2.2 | 129.9 ± 4.4 | 132.9 ± 4.8 | 124.7 ± 8.3 |
| CRP (mg L−1) | 1.73 ± 0.28 | 7.23± 1.26 *** | 6.80 ± 1.44 ** | 7.83 ± 2.30 * |
| Glucose (mmol L−1) | 5.78 ± 0.17 | 6.19 ± 0.35 | 5.79 ± 0.24 | 6.87 ± 0.81 |
| Table 1c | ||||
| Lipids | ||||
| TAG (mmol L−1) | 1.16 ± 0.12 | 1.78 ± 0.18 | 1.71 ± 0.21 | 1.92 ± 0.35 |
| LDL-Chol (mmol L−1) | 3.75 ± 0.13 | 3.55 ± 0.21 | 3.50 ± 0.26 | 3.65 ± 0.34 |
| HDL-Chol (mmol L−1) | 1.50 ± 0.07 | 1.31 ± 0.07 | 1.33 ± 0.09 | 1.27 ± 0.10 |
| Chol-Total (mmol L−1) | 5.70 ± 0.15 | 5.5 ± 0.31 | 5.30 ± 0.35 | 6.00 ± 0.56 |
| Table 1d | ||||
| Liver | ||||
| AST (kat L−1) | 0.371 ± 0.028 | 0.405 ± 0.037 | 0.393 ± 0.037 | 0.421 ± 0.070 |
| ALP (kat L−1) | 0.297 ± 0.017 | 0.863 ± 0.148 *** | 0.930 ± 0.203 * | 0.756 ± 0.204 * |
| GMT (kat L−1) | 0.751 ± 0.130 | 0.530 ± 0.066 | 0.505 ± 0.082 | 0.566 ± 0.111 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gvozdjáková, A.; Sumbalová, Z.; Kucharská, J.; Komlósi, M.; Rausová, Z.; Vančová, O.; Számošová, M.; Mojto, V. Platelet Mitochondrial Respiration, Endogenous Coenzyme Q10 and Oxidative Stress in Patients with Chronic Kidney Disease. Diagnostics 2020, 10, 176. https://doi.org/10.3390/diagnostics10030176
Gvozdjáková A, Sumbalová Z, Kucharská J, Komlósi M, Rausová Z, Vančová O, Számošová M, Mojto V. Platelet Mitochondrial Respiration, Endogenous Coenzyme Q10 and Oxidative Stress in Patients with Chronic Kidney Disease. Diagnostics. 2020; 10(3):176. https://doi.org/10.3390/diagnostics10030176
Chicago/Turabian StyleGvozdjáková, Anna, Zuzana Sumbalová, Jarmila Kucharská, Mária Komlósi, Zuzana Rausová, Oľga Vančová, Monika Számošová, and Viliam Mojto. 2020. "Platelet Mitochondrial Respiration, Endogenous Coenzyme Q10 and Oxidative Stress in Patients with Chronic Kidney Disease" Diagnostics 10, no. 3: 176. https://doi.org/10.3390/diagnostics10030176
APA StyleGvozdjáková, A., Sumbalová, Z., Kucharská, J., Komlósi, M., Rausová, Z., Vančová, O., Számošová, M., & Mojto, V. (2020). Platelet Mitochondrial Respiration, Endogenous Coenzyme Q10 and Oxidative Stress in Patients with Chronic Kidney Disease. Diagnostics, 10(3), 176. https://doi.org/10.3390/diagnostics10030176





