Factors Related to Cardiac Troponin T Increase after Participation in a 100 Km Ultra-Marathon
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects and Design
2.2. Methodology
2.3. Study End-Point
2.4. Statistical Analyses
2.5. Ethical Considerations
3. Results
3.1. Baseline Characteristics
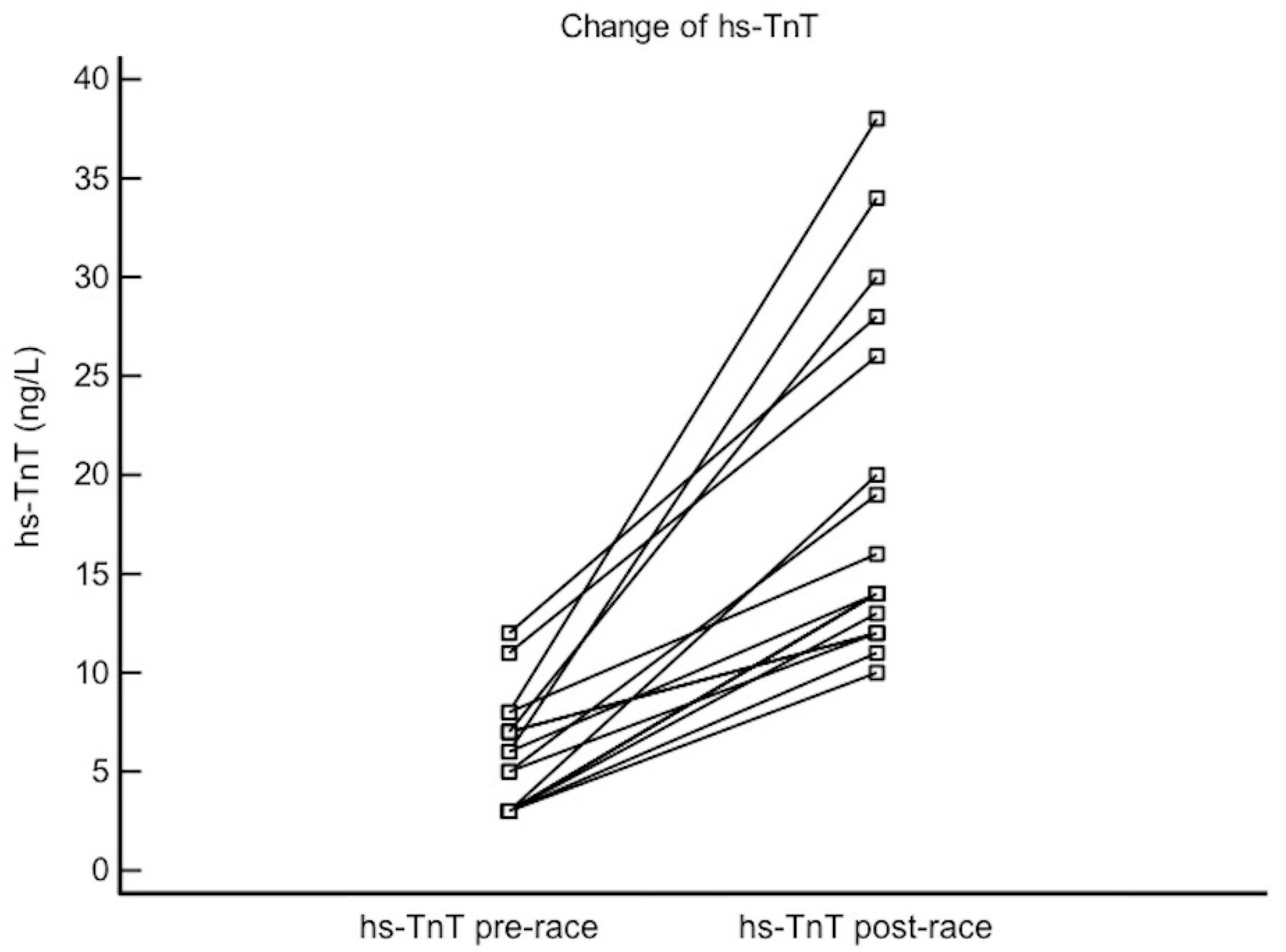
3.2. Pre- Vs. Post-Race Values
3.3. Factors Correlating with Troponin Increase
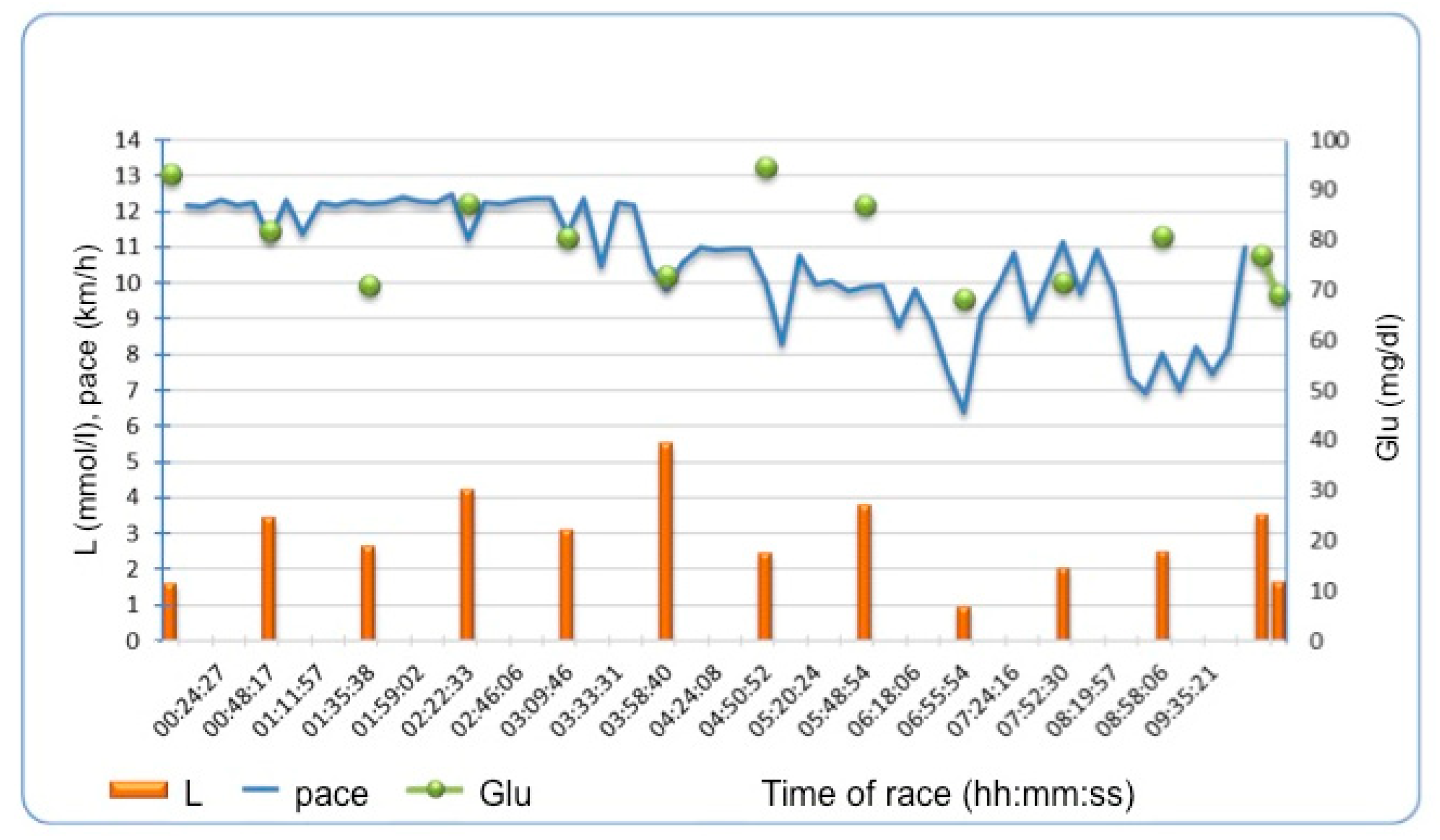
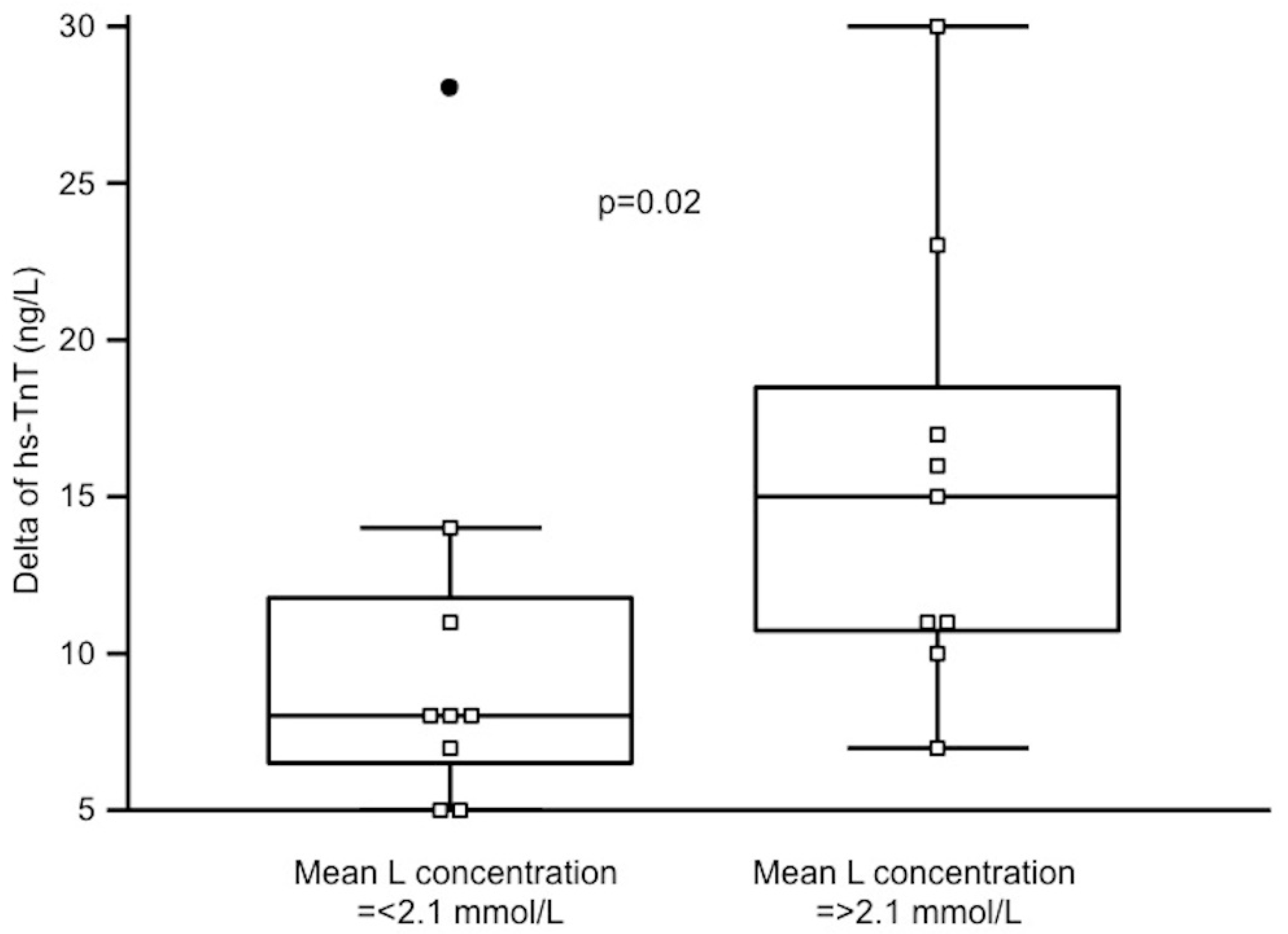
3.4. Periodically Assessed Parameters and Troponin Increase
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Jee, H.; Jin, Y. Effects of prolonged endurance exercise on vascular endothelial and inflammation markers. J. Sports Sci. Med. 2012, 11, 719–726. [Google Scholar] [PubMed]
- Merghani, A.; Malhotra, A.; Sharma, S. The U-shaped relationship between exercise and cardiac morbidity. Trends Cardiovasc. Med. 2016, 26, 232–240. [Google Scholar] [CrossRef] [PubMed]
- Mach, F.; Baigent, C.; Catapano, A.L.; Koskinas, K.C.; Casula, M.; Badimon, L.; Chapman, M.J.; De Backer, G.G.; Delgado, V.; Ference, B.A.; et al. 2019 ESC/EAS Guidelines for the management of dyslipidaemias: Lipid modification to reduce cardiovascular risk. Eur. Heart J. 2019, 41, 111–188. [Google Scholar] [CrossRef] [PubMed]
- Shave, R.; Baggish, A.; George, K.; Wood, M.; Scharhag, J.; Whyte, G. Exercise-induced cardiac troponin elevation: Evidence, mechanisms, and implications. J. Am. Coll Cardiol. 2010, 56, 169–176. [Google Scholar] [CrossRef] [PubMed]
- Roffi, M.; Patrono, C.; Collet, J.P.; Mueller, C.; Valgimigli, M.; Andreotti, F.; Bax, J.J.; Borger, M.A.; Brotons, C.; Chew, D.P.; et al. 2015 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: Task Force for the Management of Acute Coronary Syndromes in Patients Presenting without Persistent ST-Segment Elevation of the European Society of Cardiology (ESC). Eur. Heart J. 2016, 37, 267–315. [Google Scholar]
- Eijsvogels, T.M.; Fernandez, A.B.; Thompson, P.D. Are there deleterious cardiac effects of acute and chronic endurance exercise? Physiol. Rev. 2016, 96, 99–125. [Google Scholar] [CrossRef] [PubMed]
- Stavroulakis, G.A.; George, K.P. Exercise-induced release of troponin. Clin. Cardiol. 2020. [CrossRef]
- Middleton, N.; George, K.; Whyte, G.; Gaze, D.; Collinson, P.; Shave, R. Cardiac troponin T release is stimulated by endurance exercise in healthy humans. J. Am. Coll. Cardiol. 2008, 52, 1813–1814. [Google Scholar] [CrossRef]
- Gaudreault, V.; Tizon-Marcos, H.; Poirier, P.; Pibarot, P.; Gilbert, P.; Amyot, M.; Rodés-Cabau, J.; Després, J.P.; Bertrand, O.; Larose, E. Transient myocardial tissue and function changes during a marathon in less fit marathon runners. Can. J. Cardiol. 2013, 29, 1269–1276. [Google Scholar] [CrossRef]
- Lord, R.N.; Utomi, V.; Oxborough, D.L.; Curry, B.A.; Brown, M.; George, K.P. Left ventricular function and mechanics following prolonged endurance exercise: An update and meta-analysis with insights from novel techniques. Eur. J. Appl. Physiol. 2018, 118, 1291–1299. [Google Scholar] [CrossRef]
- Vessalle, C.; Masotti, S.; Lubrano, V.; Basta, G.; Prontera, C.; Di Cecco, P.; Del Turco, S.; Sabatino, L.; Pingitore, A. Traditional and new candidate cardiac biomarkers assessed before, early, and late after half marathon in trained subjects. Eur. J. Appl. Physiol. 2018, 118, 411–417. [Google Scholar] [CrossRef] [PubMed]
- Martinez, V.; la Garza, M.S.; Grazioli, G.; Bijnens, B.H.; Trape, J.; Garcia, G.; Corzan, P.; Clemente, A.; González, B.; Sitges, M. Cardiac performance after an endurance open water swimming race. Eur. J. Appl. Physiol. 2019, 119, 961–970. [Google Scholar] [CrossRef] [PubMed]
- Pujadas, S.; Doñate, M.; Li, C.H.; Merchan, S.; Cabanillas, A.; Alomar, X.; Pons-Llado, G.; Serra-Grima, R.; Carreras, F. Myocardial remodelling and tissue characterisation by cardiovascular magnetic resonance (CMR) in endurance athletes. Bmj. Open Sport Exerc. Med. 2018, 4, e000422. [Google Scholar] [CrossRef] [PubMed]
- Małek, Ł.A.; Barczuk-Falęcka, M.; Werys, K.; Czajkowska, A.; Mróz, A.; Witek, K.; Burrage, M.; Bakalarski, W.; Nowicki, D.; Roik, D.; et al. Cardiovascular magnetic resonance with parametric mapping in long-term ultra-marathon runners. Eur. J. Radiol. 2019, 117, 89–94. [Google Scholar]
- Małek, Ł.A.; Buciarelli-Ducci, C. Myocardial Fibrosis in Athletes—Current Perspective. Clinical. Cardiol. 2020, in press. [Google Scholar]
- Kyröläinen, H.; Pullinen, T.; Candau, R.; Avela, J.; Huttunen, P.; Komi, P.V. Effects of marathon running on running economy and kinematics. Eur. J. Appl. Physiol. 2000, 82, 297–304. [Google Scholar] [CrossRef]
- Kasapis, C.; Thompson, P.D. The effects of physical activity on serum C-reactive protein and inflammatory markers: A systematic review. J. Am. Coll Cardiol. 2005, 45, 1563–1569. [Google Scholar] [CrossRef]
- Ferguson, B.S.; Rogatzki, M.J.; Goodwin, M.L.; Kane, D.A.; Rightmire, Z.; Gladden, L.B. Lactate metabolism: Historical context, prior misinterpretations, and current understanding. Eur. J. Appl. Physiol. 2018, 118, 691–728. [Google Scholar] [CrossRef]
- Lühker, O.; Berger, M.M.; Pohlmann, A.; Hotz, L.; Gruhlke, T.; Hochreiter, M. Changes in acid-base and ion balance during exercise in normoxia and normobaric hypoxia. Eur. J. Appl. Physiol. 2017, 117, 2251–2261. [Google Scholar] [CrossRef]
- Cooper, D.; Walley, K.; Dodek, P.; Rosenberg, F.; Russel, J.A. Plasma ionized calcium and blood lactate concentrations are inversely associated with human lactic acidosis. Intensive. Care Med. 1992, 18, 286–289. [Google Scholar] [CrossRef]
- Robergs, R.; Ghiasvand, F.; Parker, D. Biochemistry of exercise-induced metabolic acidosis. Am. J. Physiol. Regul. Intgr. Comp. Physiol. 2004, 287, R502–R516. [Google Scholar] [CrossRef] [PubMed]
- Saravia, S.G.; Knebel, F.; Schroeckh, S.; Ziebig, R.; Lun, A.; Weimann, A.; Haberland, A.; Borges, A.C.; Schimke, I. Cardiac troponin T release and inflammation demonstrated in marathon runners. Clin. Lab. 2010, 56, 51–58, Erratum in: Clin Lab. 2011, 57, 273. [Google Scholar] [PubMed]
- Kłapcińska, B.; Waśkiewicz, Z.; Chrapusta, S.J.; Sadowska-Krępa, E.; Czuba, M.; Langfort, J. Metabolic responses toa 48-h ultra-marathon run in middle-aged male amateur runners. Eur. J. Appl. Physiol. 2013, 113, 2781–2793. [Google Scholar] [CrossRef] [PubMed]
- Katsanos, S.; Mavrogenis, A.F.; Kafkas, N.; Sardu, C.; Kamperidis, V.; Katsanou, P.; Farmakis, D.; Parissis, J. Cardiac biomarkers predict 1-year mortality in elderly patients undergoing hip fracture surgery. Orthopedics 2017, 40, e417–e424. [Google Scholar] [CrossRef]
- Sardu, C.; Paolisso, G.; Marfella, R. Molecular mechanisms and therapeutic targets of inflammatory-related Cardiovascular diseases: From molecular mechanisms to therapeutic targets. Curr. Pharm. Des. 2020. [CrossRef]
| Parameter | Ultra-Marathon Runners n = 18 |
|---|---|
| Male sex (%) | 15 (83) |
| Age, yrs (IQR) | 41.5 (36–53) |
| BMI, kg/m2 (IQR) | 24.6 (22.7–25.7) |
| Years of running (IQR) | 4.3 (3.5–6.0) |
| Years of ultra running (IQR) | 2 (0–3) |
| Weekly running distance, km (IQR) | 67.5 (40–85) |
| Number of ultra races completed (IQR) | 2 (0–10) |
| Longest completed race, km (IQR) | 58 (42–80) |
| Pre-Race n = 18 | Post-Race n = 18 | p | |
|---|---|---|---|
| Body mass, kg | 75.0 (69.6–83.3) | 74.2 (69.7–82.7) | <0.0001 |
| TBW, kg | 44.2 (39.8–49.9) | 41.6 (39.2–46.7) | 0.008 |
| TBW, % | 58.1 (54.4–60.1) | 56.6 (54.2–60.1) | 0.32 |
| FAT, kg | 10.9 (7.9–12.5) | 10.5 (7.6–12.2) | 0.46 |
| FAT, % | 13.7 (11.4–17.5) | 14.2 (10.7–18.6) | 0.73 |
| FFM, kg | 65.7 (61.2–72.0) | 64.1 (60.6–70.7) | 0.0001 |
| FFM, % | 86.3 (82.5–88.7) | 85.8 (81.4–89.3) | 0.77 |
| HR, bpm | 54.5 (50–60) | 81.5 (76–93) | <0.0001 |
| SBP, mmHg | 137 (130–146) | 123 (109–133) | 0.0004 |
| DBP, mmHg | 84 (92–91) | 73 (70–78) | <0.0001 |
| CRP, mg/dL | 0.7 (0.43–1.1) | 3.2 (1.9–8.1) | <0.0001 |
| hs-TnT, ng/L | 5 (3–7) | 14 (12–26) | <0.0001 |
| L, mmol/L | 2.0 (1.7–2.4) | 2.2 (1.4–3.5) | 0.22 |
| Glu, mg/dL | 89 (86–95) | 93 (80–100) | 0.83 |
| hs-TnT Change | ||
|---|---|---|
| rho | p | |
| Baseline Parameters | ||
| Age, yrs | 0.04 | 0.86 |
| Weekly distance covered, km | 0.23 | 0.34 |
| Body mass pre, kg | 0.34 | 0.16 |
| TBW pre, kg | 0.17 | 0.49 |
| FFM pre, kg | 0.31 | 0.20 |
| HR pre, bpm | 0.01 | 0.96 |
| SBP pre, mmHg | 0.04 | 0.86 |
| DBP pre, mmHg | 0.01 | 0.99 |
| L, mmol/L | 0.11 | 0.66 |
| Glu, mg/dL | −0.26 | 0.28 |
| Hs-CRP, mg/dL | 0.17 | 0.49 |
| Hs-TnT, mmol/L | 0.23 | 0.32 |
| Race Parameters and Post-Race Changes | ||
| Race time, hours | 0.45 | 0.18 |
| Pace, min/km | 0.12 | 0.62 |
| Water intake during the race, mL | −0.04 | 0.87 |
| Food intake during the race, kcal | 0.07 | 0.76 |
| Delta body mass, kg | 0.08 | 0.74 |
| Delta TBW, kg | 0.31 | 0.20 |
| Delta FFM, kg | 0.29 | 0.23 |
| Delta L, mmol/L | 0.30 | 0.21 |
| Delta Glu, mg/dL | 0.04 | 0.85 |
| Delta hs-CRP, mg/dL | 0.59 | 0.01 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Małek, Ł.A.; Czajkowska, A.; Mróz, A.; Witek, K.; Nowicki, D.; Postuła, M. Factors Related to Cardiac Troponin T Increase after Participation in a 100 Km Ultra-Marathon. Diagnostics 2020, 10, 167. https://doi.org/10.3390/diagnostics10030167
Małek ŁA, Czajkowska A, Mróz A, Witek K, Nowicki D, Postuła M. Factors Related to Cardiac Troponin T Increase after Participation in a 100 Km Ultra-Marathon. Diagnostics. 2020; 10(3):167. https://doi.org/10.3390/diagnostics10030167
Chicago/Turabian StyleMałek, Łukasz A., Anna Czajkowska, Anna Mróz, Katarzyna Witek, Dariusz Nowicki, and Marek Postuła. 2020. "Factors Related to Cardiac Troponin T Increase after Participation in a 100 Km Ultra-Marathon" Diagnostics 10, no. 3: 167. https://doi.org/10.3390/diagnostics10030167
APA StyleMałek, Ł. A., Czajkowska, A., Mróz, A., Witek, K., Nowicki, D., & Postuła, M. (2020). Factors Related to Cardiac Troponin T Increase after Participation in a 100 Km Ultra-Marathon. Diagnostics, 10(3), 167. https://doi.org/10.3390/diagnostics10030167







