The Expansion of Cholangioscopy: Established and Investigational Uses of SpyGlass in Biliary and Pancreatic Disorders
Abstract
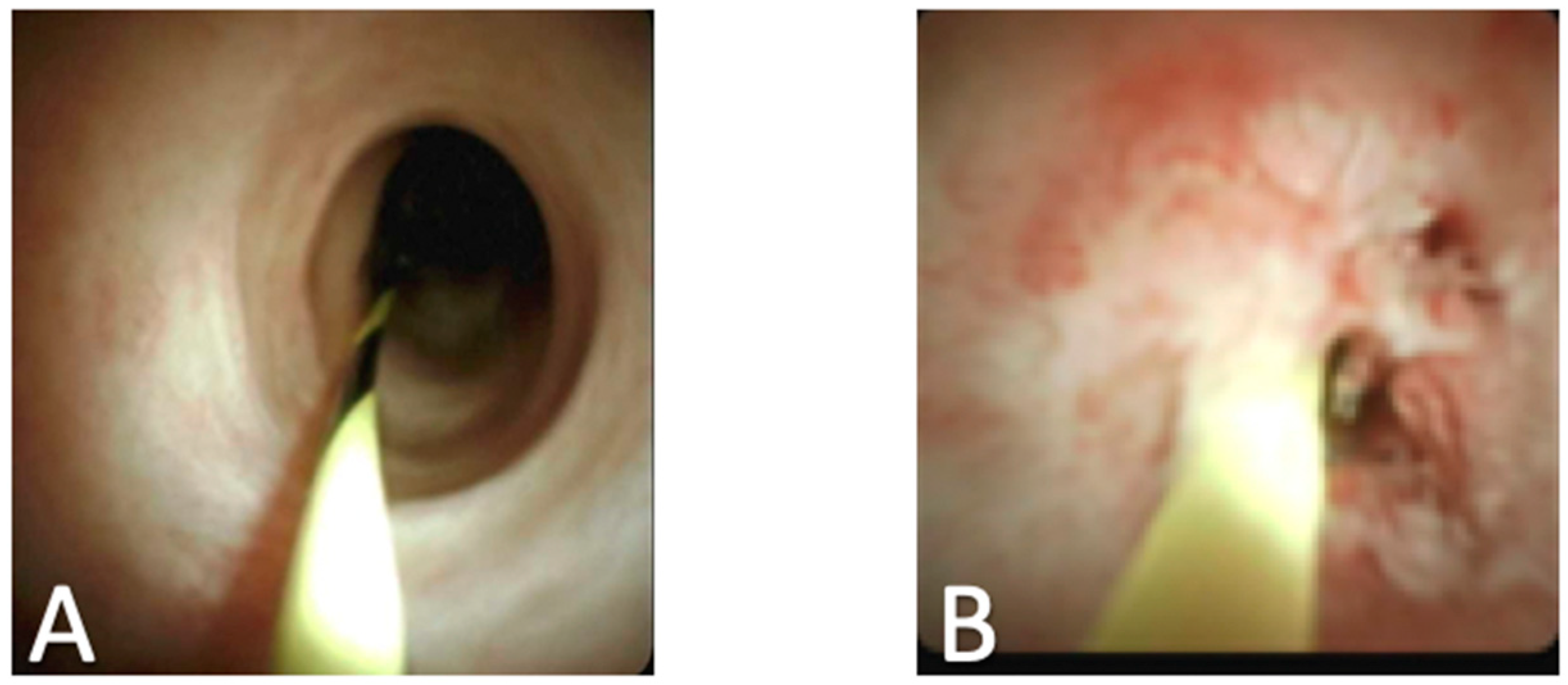
:1. Introduction
2. Biliary Stones
3. Indeterminate Biliary Strictures
4. Cholangioscopy in Primary Sclerosing Cholangitis
5. SpyGlass for Pancreatic Pathologies
6. Expanding Options for Treatment of Post-Transplant Stricture Treatment
7. Management of Cholangiocarcinoma
8. Evaluation of Hemobilia
9. Economic Factors
10. Complications Associated with Cholangioscopy
11. Limitations of Single Operator Cholangioscopy (SOC) and Other Options
12. Conclusions
Funding
Conflicts of Interest
References
- Chen, K.Y.; Pleskow, D.K. SpyGlass single-operator peroral cholangiopancreatoscopy system for the diagnosis and therapy of bile-duct disorders: A clinical feasibility study (with video). Gastrointest. Endosc. 2007, 65, 832–841. [Google Scholar] [CrossRef]
- Navaneethan, U.; Njei, B.; Lourdusamy, V.; Konjeti, R.; Vargo, J.J.; Parsi, M.A. Comparative effectiveness of biliary brush cytology and intraductal biopsy for detection of malignant biliary strictures: A systematic review and meta-analysis. Gastrointest. Endosc. 2015, 81, 168–176. [Google Scholar] [CrossRef] [Green Version]
- Itoi, T.; Sofuni, A.; Itokawa, F.; Tsuchiya, T.; Kurihara, T.; Ishii, K.; Tsuji, S.; Moriyasu, F.; Gotoda, T. Peroral cholangioscopic diagnosis of biliary-tract diseases by using narrow-band imaging (with videos). Gastrointest. Endosc. 2007, 66, 730–736. [Google Scholar] [CrossRef]
- Nakajima, M.; Akasaka, Y.; Fukumoto, K.; Mitsuyoshi, Y.; Kawai, K. Peroral cholangiopancreatosocopy (PCPS) under duodenoscopic guidance. Am. J. Gastroenterol. 1976, 66, 241–247. [Google Scholar]
- Chathadi, V.K.; Chen, Y.K. New kid on the block: Development of a partially disposable system for cholangioscopy. Gastrointest. Endosc. Clin. N. Am. 2009, 19, 545–555. [Google Scholar] [CrossRef]
- Boston Scientific Corporation. SpyGlass DS Direct Visualization System; Boston Scientific Corporation: Boston, USA, 2015. [Google Scholar]
- Dimas, I.D.; Vardas, E.; Papastergiou, V.; Fragaki, M.; Velegraki, M.; Mpitouli, A.; Voudoukis, E.; Theodoropoulou, A.; Giannikaki, E.; Chlouverakis, G.; et al. Comparison of digital versus fiberoptic cholangioscopy in patients requiring evaluation of bile duct disease or treatment of biliary stones. Ann. Gastroenterol. 2019, 32, 199–204. [Google Scholar]
- Moon, J.H.; Terheggen, G.; Choi, H.J.; Neuhaus, H. Peroral cholangioscopy: Diagnostic and therapeutic applications. Gastroenterology 2013, 144, 276–282. [Google Scholar] [CrossRef]
- Yan, S.; Tejaswi, S. Clinical impact of digital cholangioscopy in management of indeterminate biliary strictures and complex biliary stones: A single-center study. Ther. Adv. Gastrointest. Endosc. 2019, 12, 2631774519853160. [Google Scholar] [CrossRef]
- Averbukh, L.D.; Miller, D.; Birk, J.W.; Tadros, M. The utility of single operator cholangioscope (Spyglass) to diagnose and treat radiographically negative biliary stones: A case series and review. J. Dig. Dis. 2019, 20, 262–266. [Google Scholar] [CrossRef]
- Almadi, A.M.; Barkun, J.S.; Barkun, A.N. Management of suspected stones in the common bile duct. CMAJ 2012, 184, 884–892. [Google Scholar] [CrossRef] [Green Version]
- Laleman, W.; Verraes, K.; Van Steenbergen, W.; Cassiman, D.; Nevens, F.; Van der Merwe, S.; Verslype, C. Usefulness of the single-operator cholangioscopy system SpyGlass in biliary disease: A single-center prospective cohort study and aggregated review. Surg. Endosc. 2017, 31, 2223–2232. [Google Scholar] [CrossRef]
- Brewer Gutierrez, O.I.; Bekkali, N.L.H.; Raijman, I.; Sturgess, R.; Sejpal, D.V.; Aridi, H.D.; Sherman, S.; Shah, R.J.; Kwon, R.S.; Buxbaum, J.L.; et al. Efficacy and Safety of Digital Single-Operator Cholangioscopy for Difficult Biliary Stones. Clin. Gastroenterol. Hepatol. 2018, 16, 918–926 e1. [Google Scholar] [CrossRef]
- Maydeo, A.P.; Rerknimitr, R.; Lau, J.Y.; Aljebreen, A.; Niaz, S.K.; Itoi, T.; Ang, T.L.; Reichenberger, J.; Seo, D.W.; Ramchandani, M.K.; et al. Cholangioscopy-guided lithotripsy for difficult bile duct stone clearance in a single session of ERCP: Results from a large multinational registry demonstrate high success rates. Endoscopy 2019, 51, 922–929. [Google Scholar] [CrossRef]
- Wakai, T.; Shirai, Y.; Sakata, J.; Maruyama, T.; Ohashi, T.; Korira, P.V.; Ajioka, Y.; Hatakeyama, K. Clinicopathological features of benign biliary strictures masquerading as biliary malignancy. Am. Surg. 2012, 78, 1388–1391. [Google Scholar]
- Seo, D.W.; Lee, S.K.; Yoo, K.S.; Kang, G.H.; Kim, M.H.; Suh, D.J.; Min, Y.I. Cholangioscopic findings in bile duct tumors. Gastrointest. Endosc. 2000, 52, 630–634. [Google Scholar] [CrossRef]
- Sun, X.; Zhou, Z.; Tian, J.; Wang, Z.; Huang, Q.; Fan, K.; Mao, Y.; Sun, G.; Yang, Y. Is single-operator peroral cholangioscopy a useful tool for the diagnosis of indeterminate biliary lesion? A systematic review and meta-analysis. Gastrointest. Endosc. 2015, 82, 79–87. [Google Scholar] [CrossRef]
- Navaneethan, U.; Hasan, M.K.; Lourdusamy, V.; Njei, B.; Varadarajulu, S.; Hawes, R.H. Single-operator cholangioscopy and targeted biopsies in the diagnosis of indeterminate biliary strictures: A systematic review. Gastrointest. Endosc. 2015, 82, 608–614 e2. [Google Scholar] [CrossRef] [Green Version]
- Prat, F.; Leblanc, S.; Foissac, F.; Ponchon, T.; Laugier, R.; Bichard, P.; Maire, F.; Coumaros, D.; Charachon, A.; Vedrenne, B.; et al. Impact of peroral cholangioscopy on the management of indeterminate biliary conditions: A multicentre prospective trial. Frontline Gastroenterol. 2019, 10, 236–243. [Google Scholar] [CrossRef]
- Ogawa, T.; Ito, K.; Koshita, S.; Kanno, Y.; Masu, K.; Kusunose, H.; Sakai, T.; Murabayashi, T.; Hasegawa, S.; Noda, Y. Usefulness of cholangioscopic-guided mapping biopsy using SpyGlass DS for preoperative evaluation of extrahepatic cholangiocarcinoma: A pilot study. Endosc. Int. Open 2018, 6, E199–E204. [Google Scholar] [CrossRef] [Green Version]
- Baars, J.E.; Keegan, M.; Bonnichsen, M.H.; Aepli, P.; Theyventhiran, R.; Farrell, E.; Kench, J.G.; Saxena, P.; Kaffes, A.J. The ideal technique for processing SpyBite tissue specimens: A prospective, single-blinded, pilot-study of histology and cytology techniques. Endosc. Int. Open 2019, 7, E1241–E1247. [Google Scholar] [CrossRef] [Green Version]
- Bang, J.Y.; Navaneethan, U.; Hasan, M.; Sutton, B.; Hawes, R.; Varadarajulu, S. Optimizing Outcomes of Single-Operator Cholangioscopy-Guided Biopsies Based on a Randomized Trial. Clin. Gastroenterol. Hepatol. 2020, 18, 441–448 e1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bergquist, A.; von Seth, E. Epidemiology of cholangiocarcinoma. Best Pract. Res. Clin. Gastroenterol. 2015, 29, 221–232. [Google Scholar] [CrossRef] [PubMed]
- Charatcharoenwitthaya, P.; Enders, F.B.; Halling, K.C.; Lindor, K.D. Utility of serum tumor markers, imaging, and biliary cytology for detecting cholangiocarcinoma in primary sclerosing cholangitis. Hepatology 2008, 48, 1106–1117. [Google Scholar] [CrossRef]
- Sandha, G.; D’Souza, P.; Halloran, B.; Montano-Loza, A.J. A Cholangioscopy-Based Novel Classification System for the Phenotypic Stratification of Dominant Bile Duct Strictures in Primary Sclerosing Cholangitis-the Edmonton Classification. J. Can. Assoc. Gastroenterol. 2018, 1, 174–180. [Google Scholar] [CrossRef] [PubMed]
- Hara, T.; Yamaguchi, T.; Ishihara, T.; Tsuyuguchi, T.; Kondo, F.; Kato, K.; Asano, T.; Saisho, H. Diagnosis and patient management of intraductal papillary-mucinous tumor of the pancreas by using peroral pancreatoscopy and intraductal ultrasonography. Gastroenterology 2002, 122, 34–43. [Google Scholar] [CrossRef]
- El Hajj, B.B., II.; Wani, S.; Fukami, N.; Attwell, A.R.; Shah, R.J. Role of per-oral pancreatoscopy in the evaluation of suspected pancreatic duct neoplasia: A 13-year U.S. single-center experience. Gastrointest. Endosc. 2017, 85, 737–745. [Google Scholar] [CrossRef] [Green Version]
- Attwell, A.R.; Patel, S.; Kahaleh, M.; Raijman, I.L.; Yen, R.; Shah, R.J. ERCP with per-oral pancreatoscopy-guided laser lithotripsy for calcific chronic pancreatitis: a multicenter U.S. experience. Gastrointest. Endosc. 2015, 82, 311–318. [Google Scholar] [CrossRef]
- Franzini, T.; Sagae, V.M.T.; Guedes, H.G.; Sakai, P.; Waisberg, D.R.; Andraus, W.; D’Albuquerque, L.A.C.; Sethi, A.; de Moura, E.G.H. Cholangioscopy-guided steroid injection for refractory post liver transplant anastomotic strictures: A rescue case series. Ther. Adv. Gastrointest. Endosc. 2019, 12, 2631774519867786. [Google Scholar] [CrossRef]
- Rainer, F.; Blesl, A.; Spindelboeck, W.; Schemmer, P.; Fickert, P.; Schreiber, F. A novel way to avoid reoperation for biliary strictures after liver transplantation: Cholangioscopy-assisted guidewire placement. Endoscopy 2019, 51, E314–E316. [Google Scholar] [CrossRef] [Green Version]
- Bokemeyer, A.; Gross, D.; Brückner, M.; Nowacki, T.; Bettenworth, D.; Schmidt, H.; Heinzow, H.; Kabar, I.; Ullerich, H.; Lenze, F. Digital single-operator cholangioscopy: A useful tool for selective guidewire placements across complex biliary strictures. Surg. Endosc. 2019, 33, 731–737. [Google Scholar] [CrossRef] [PubMed]
- Gunasingam, N.; Craig, P.I. Cholangioscopy-directed radiofrequency ablation of complex biliary cholangiocarcinoma. VideoGIE 2019, 4, 211–213. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Natov, S.N.; Horton, L.C.; Hegde, S.R. Successful endoscopic treatment of an intraductal papillary neoplasm of the bile duct. World J. Gastrointest. Endosc. 2017, 9, 238–242. [Google Scholar] [CrossRef] [PubMed]
- Sum Foong, K.; Lee, A.; Kudakachira, S.; Ramberan, H. Hemobilia from Biliary Angiodysplasia Diagnosed with Cholangioscopy. ACG Case Rep. J. 2016, 3, e132. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Craig, P.I. A case of hemobilia secondary to cancer of the gallbladder confirmed by cholangioscopy and treated with a fully covered self-expanding metal stent. VideoGIE 2018, 3, 381–383. [Google Scholar] [CrossRef] [Green Version]
- Njei, B.; McCarty, T.R.; Varadarajulu, S.; Navaneethan, U. Cost utility of ERCP-based modalities for the diagnosis of cholangiocarcinoma in primary sclerosing cholangitis. Gastrointest. Endosc. 2017, 85, 773–781 e10. [Google Scholar] [CrossRef]
- Deprez, P.H.; Garces Duran, R.; Moreels, T.; Funeri, G.; Demma, F.; Verbeke, L.; Van der Merwe, S.W.; Laleman, W. The economic inpact of using single-operator cholangioscopy for the treatment of difficult bile duct stones and diagnosis of indeterminate bile duct strictures. Endoscopy 2018, 50, 109–118. [Google Scholar]
- Gravito-Soares, M.; Almeida, N. Peroral Cholangiopancreatoscopy: New Advances Bring New Concerns. Port. J. Gastroenterol. 2018, 25, 112–114. [Google Scholar] [CrossRef]
- Korrapati, P.; Ciolino, J.; Wani, S.; Shah, J.; Watson, R.; Muthusamy, V.R.; Klapman, J.; Komanduri, S. The efficacy of peroral cholangioscopy for difficult bile duct stones and indeterminate strictures: A systematic review and meta-analysis. Endosc. Int. Open 2016, 4, E263–E275. [Google Scholar] [CrossRef] [Green Version]
- Sethi, A.; Chen, Y.K.; Austin, G.L.; Brown, W.R.; Brauer, B.C.; Fukami, N.N.; Khan, A.H.; Shah, R.J. ERCP with cholangiopancreatoscopy may be associated with higher rates of complications than ERCP alone: A single-center experience. Gastrointest. Endosc. 2011, 73, 251–256. [Google Scholar] [CrossRef]
- Turowski, F.; Hügle, U.; Dormann, A.; Bechtler, M.; Jakobs, R.; Gottschalk, U.; Nötzel, E.; Hartmann, D.; Lorenz, A.; Kolligs, F.; et al. Diagnostic and therapeutic single-operator cholangiopancreatoscopy with SpyGlassDS: Results of a multicenter retrospective cohort study. Surg. Endosc. 2018, 32, 3981–3988. [Google Scholar] [CrossRef]
- Tringali, A.; Lemmers, A.; Meves, V.; Terheggen, G.; Pohl, J.; Manfredi, G.; Häfner, M.; Costamagna, G.; Devière, J.; Neuhaus, H.; et al. Intraductal biliopancreatic imaging: European Society of Gastrointestinal Endoscopy (ESGE) technology review. Endoscopy 2015, 47, 739–753. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kulpatcharapong, S.; Pittayanon, R.; JKerr, S.; Rerknimitr, R. Diagnostic performance of different cholangioscopes in patients with biliary strictures: A systematic review. Endoscopy 2020, 52, 174–185. [Google Scholar] [CrossRef] [PubMed]
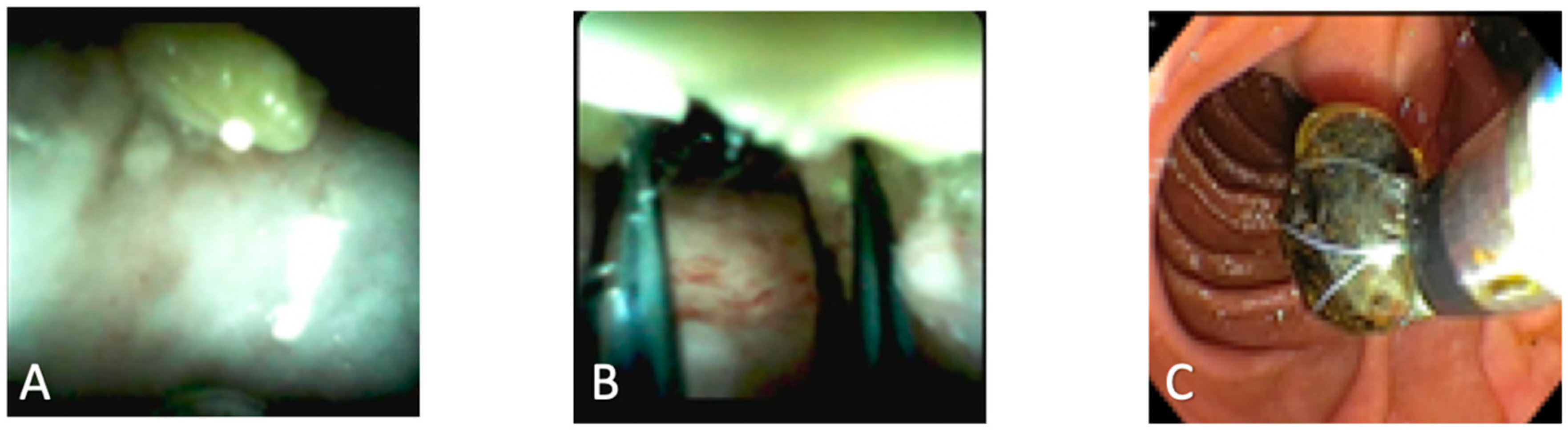
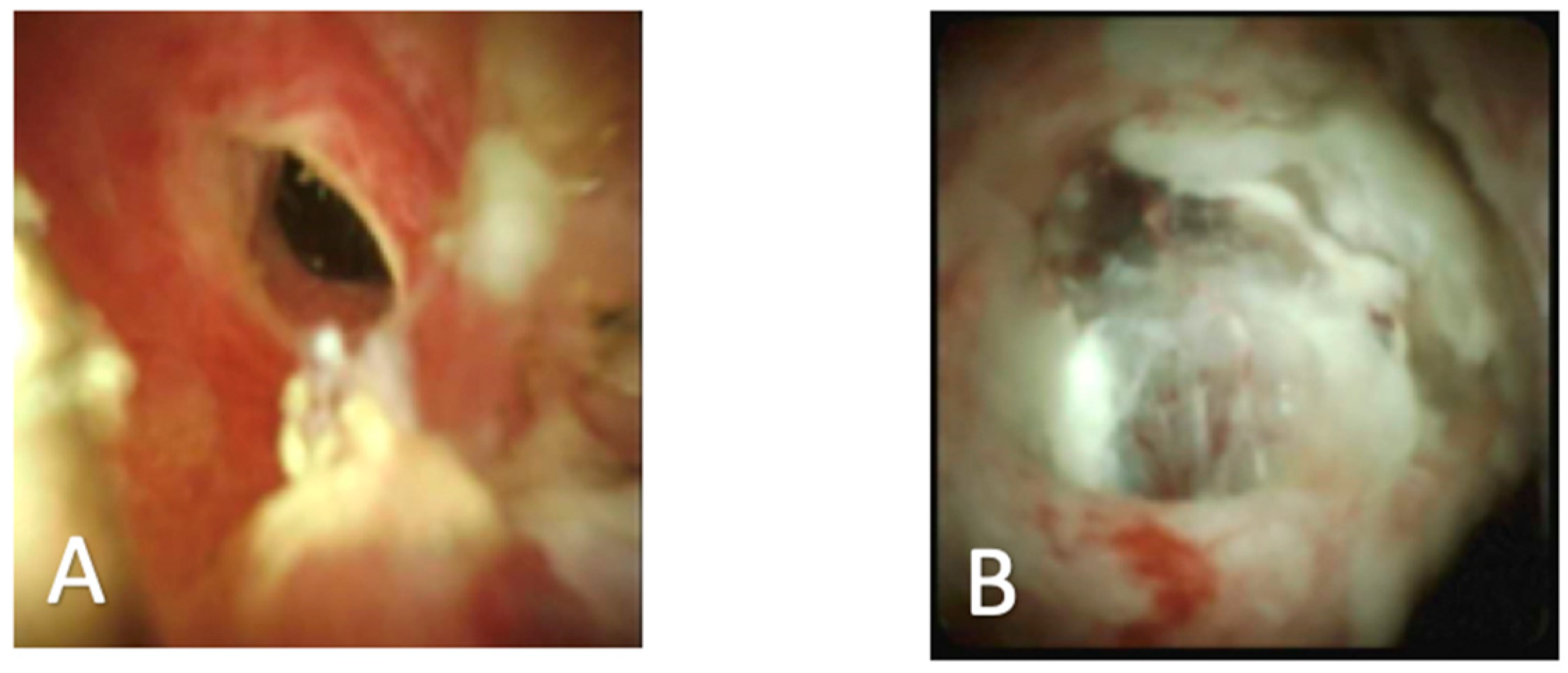
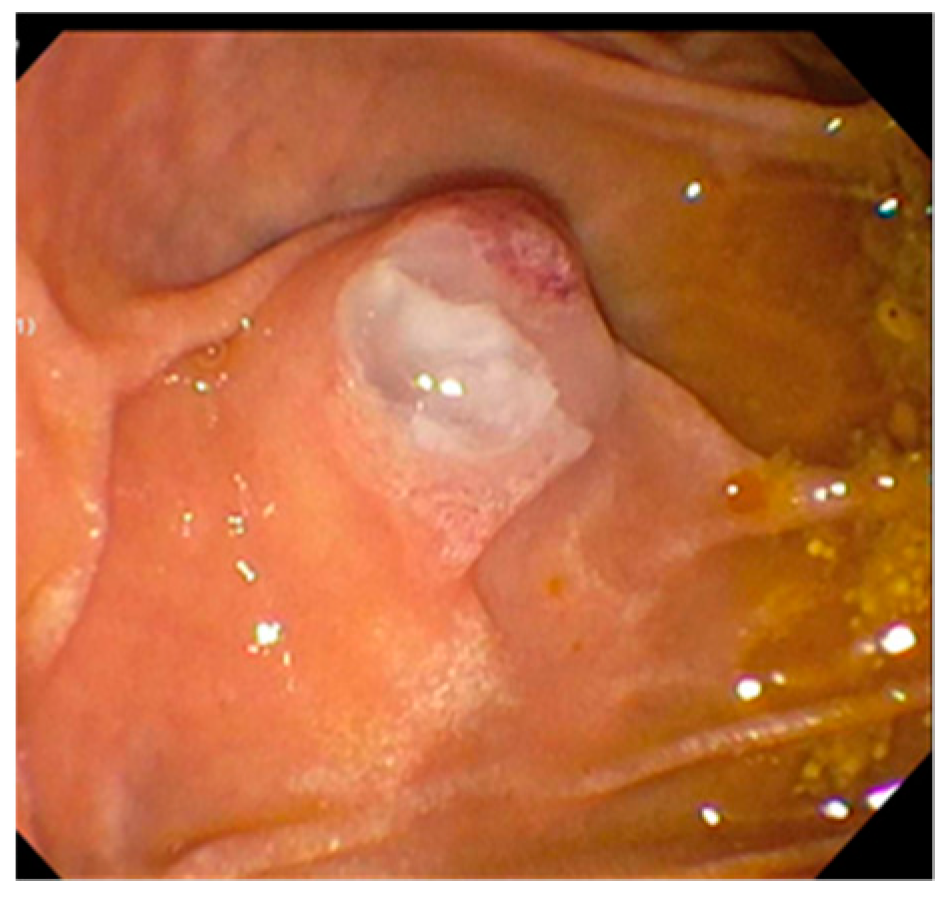
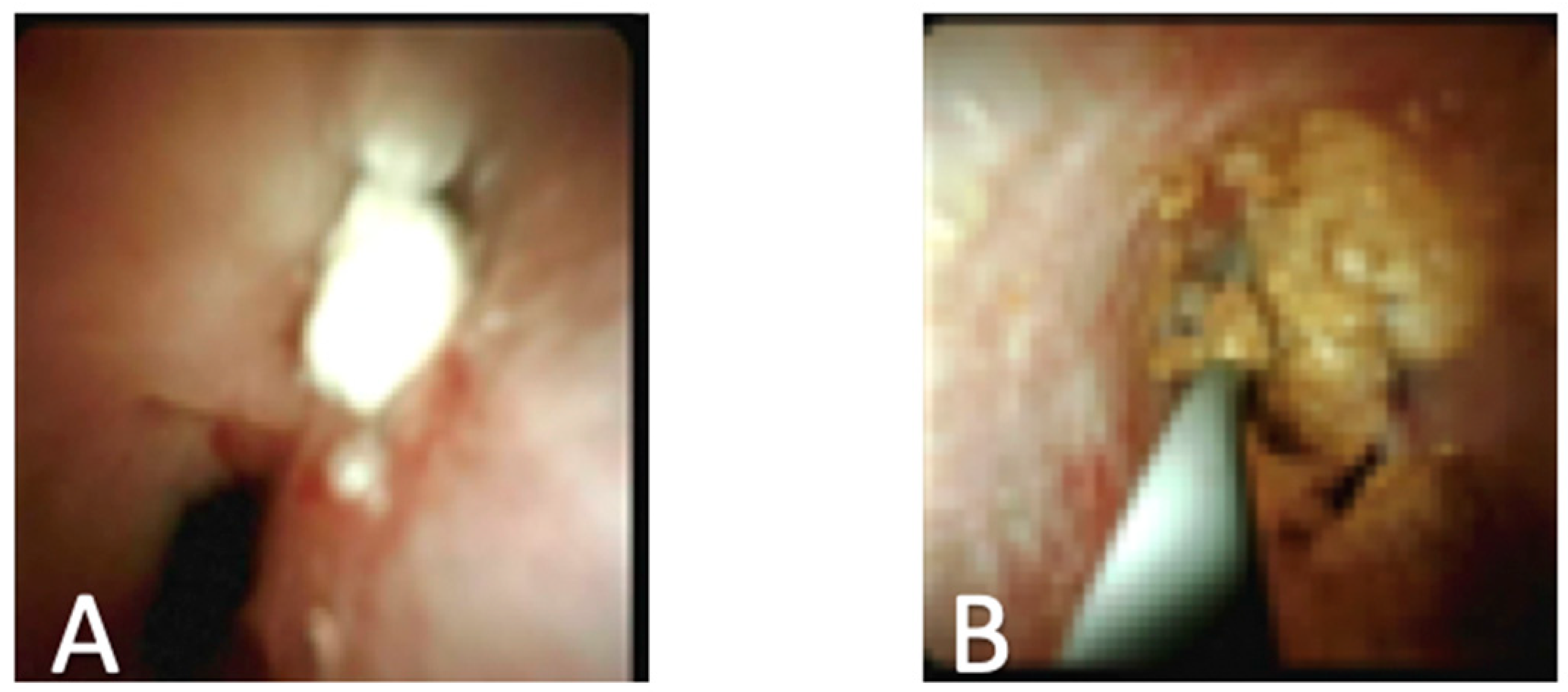
| First Generation SpyGlass (2007) | SpyGlass DS (2015) |
|---|---|
| Single-operator system | Single-operator, single-use system |
| Fiberoptic picture with improved resolution | Digital sensor leading to 4× greater resolution |
| 4-way tip deflection for increased maneuverability | 60% wider field-of-view |
| Dedicated irrigation and accessory channel | Redesigned accessory channel for easier use |
| Diagnostic |
|---|
| Evaluation of strictures |
| Direct visualization of missed stones (from standard cholangiography) |
| Visually guided biopsy |
| Evaluation of hemobilia |
| Therapeutic |
| Visually guided electrohydraulic and laser lithotripsy |
| Visually assisted guidewire placement |
| Guided radiofrequency ablation of ductal tumors |
| Established | Investigational |
|---|---|
| Difficult bile duct stones | Post liver transplant surveillance and stricture treatment |
| Evaluation of hemobilia | |
| Indeterminate biliary stricture | Radiofrequency ablation of tumors |
| Guidewire placement in advanced strictures | |
| Staging of cholangiocarcinoma | |
| Primary Sclerosing Cholangitis (PSC) stricture evaluation |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yodice, M.; Choma, J.; Tadros, M. The Expansion of Cholangioscopy: Established and Investigational Uses of SpyGlass in Biliary and Pancreatic Disorders. Diagnostics 2020, 10, 132. https://doi.org/10.3390/diagnostics10030132
Yodice M, Choma J, Tadros M. The Expansion of Cholangioscopy: Established and Investigational Uses of SpyGlass in Biliary and Pancreatic Disorders. Diagnostics. 2020; 10(3):132. https://doi.org/10.3390/diagnostics10030132
Chicago/Turabian StyleYodice, Michael, Joseph Choma, and Micheal Tadros. 2020. "The Expansion of Cholangioscopy: Established and Investigational Uses of SpyGlass in Biliary and Pancreatic Disorders" Diagnostics 10, no. 3: 132. https://doi.org/10.3390/diagnostics10030132
APA StyleYodice, M., Choma, J., & Tadros, M. (2020). The Expansion of Cholangioscopy: Established and Investigational Uses of SpyGlass in Biliary and Pancreatic Disorders. Diagnostics, 10(3), 132. https://doi.org/10.3390/diagnostics10030132




