A Novel Earwax Method to Measure Acute and Chronic Glucose Levels
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Socio-Demographic, Anthropometric, and Psychological Variables Results
3.2. Baseline and Follow-Up Samples Comparisons
3.3. Time Needed to Analyse Earwax Glucose Concentration vs. Blood Based Estimations
3.4. Estimated Costs Related to Different Sampling Methods for Measuring Chronic Glucose over a Three-Month Period
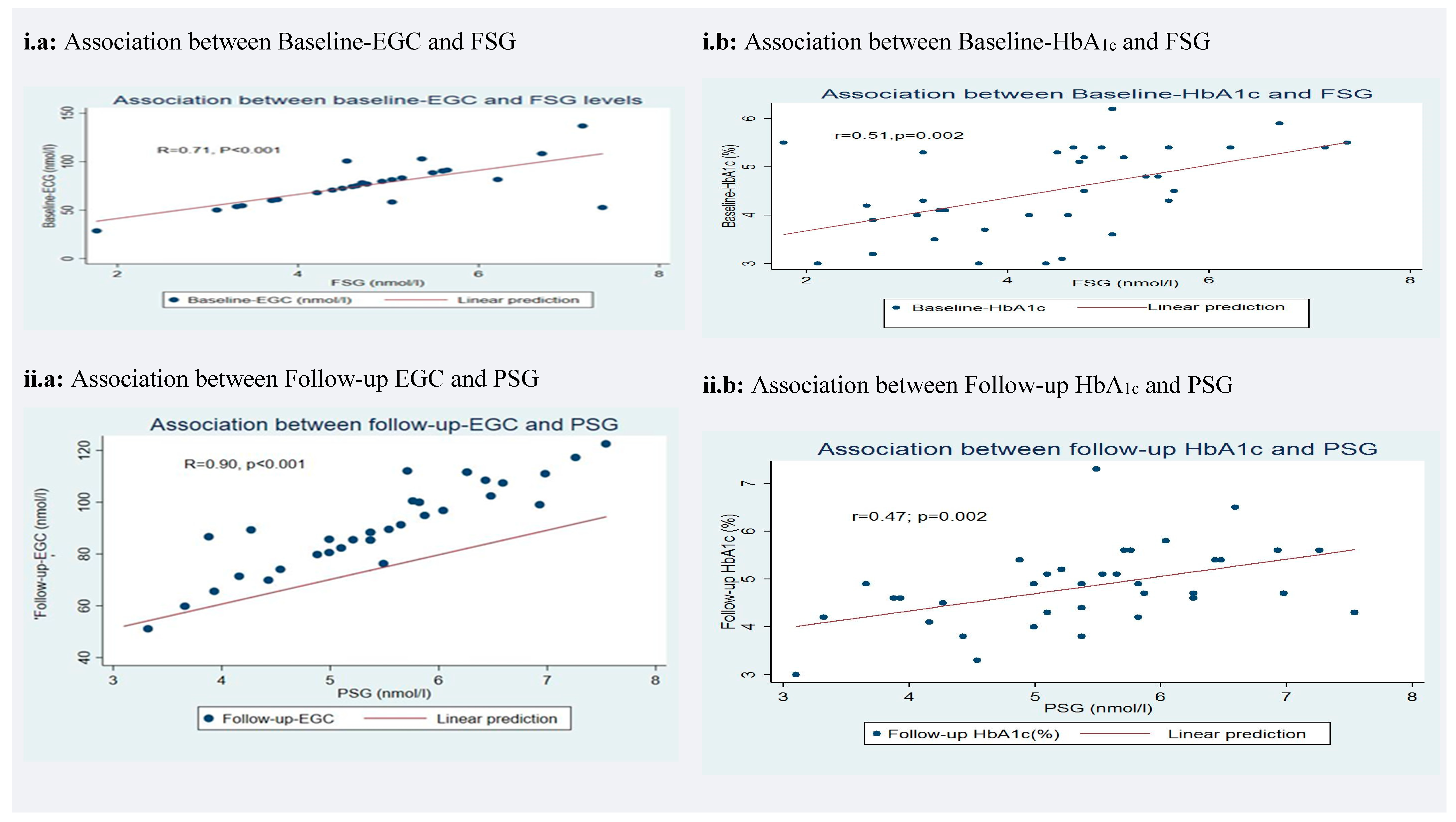
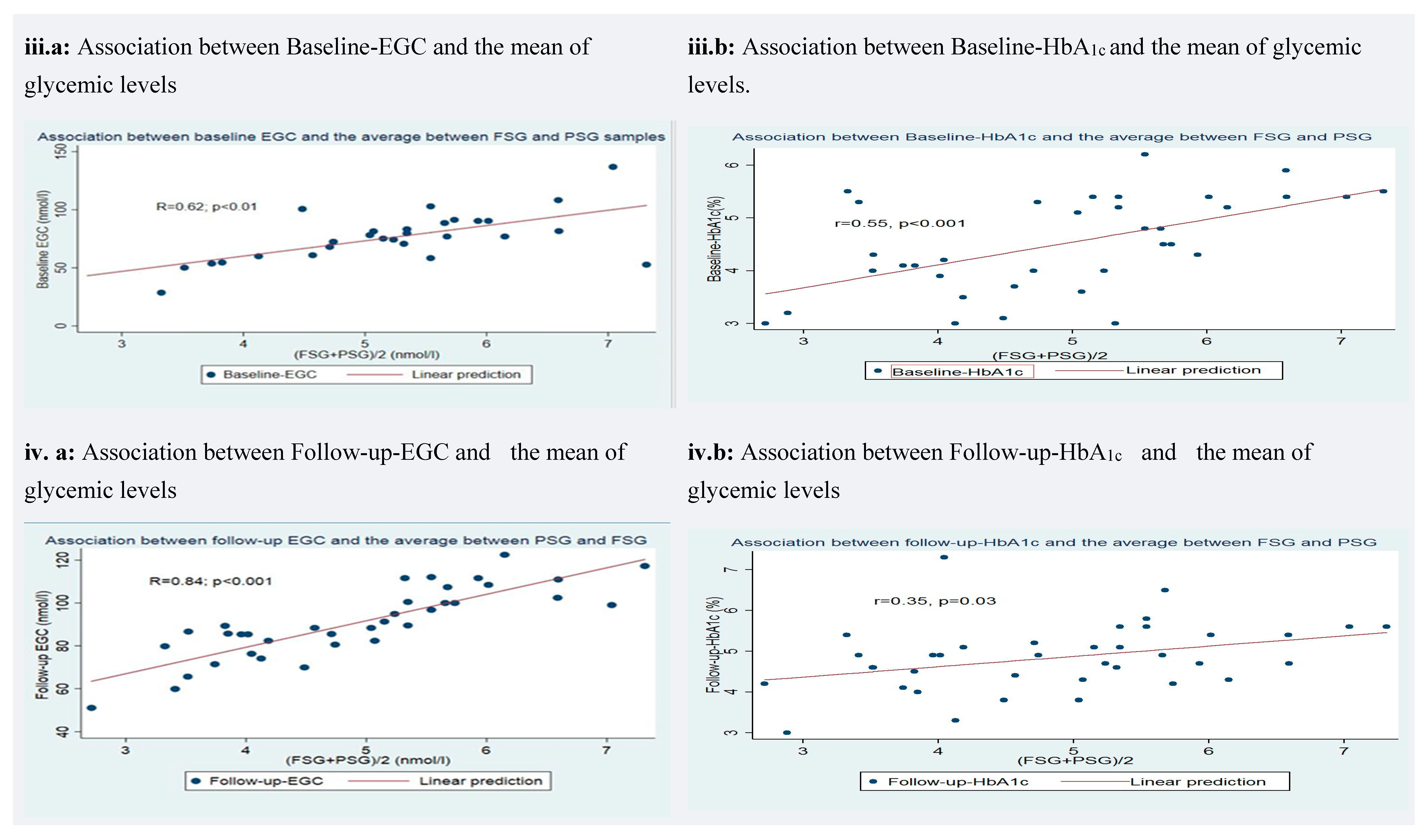
3.5. Correlation of Earwax Glucose Concentration (EGC) and Glycated Hemoglobin (HbA1c) with Glycemic Levels
3.6. Accuracy of Earwax Glucose Concentration (EGC) and Glycated Hemoglobin (HbA1c) in Measuring Chronic Glucose Concentration
3.7. Effect of Covariates on Earwax Glucose Concentration (EGC) and Glycated Hemoglobin (HbA1c) Correlation with Glycemic Levels
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| HbA1c | Glycated Hemoglobin |
| FSG | Fasting Serum Glucose |
| PSG | Postprandial Serum Glucose |
| EGC | Earwax Glycemic Concentration |
| UCN | Universidad Católica del Norte |
| BMI | Body Mass Index |
References
- WHO | World Health Statistics 2018: Monitoring health for the SDGs; WHO: Geneva, Switzerland, 2018.
- Dagogo-Jack, S. Pitfalls in the use of HbA1c as a diagnostic test: The ethnic conundrum. Nat. Rev. Endocrinol. 2010, 6, 589–593. [Google Scholar] [CrossRef]
- Frati, A.C.; Iniestra, F.; Ariza, C.R. Acute Effect of Cigarette Smoking on Glucose Tolerance and Other Cardiovascular Risk Factors. Diabetes Care 1996, 19, 112–118. [Google Scholar] [CrossRef]
- Modan, M.; Halkin, H.; Almog, S.; Lusky, A.; Eshkol, A.; Shefi, M.; Shitrit, A.; Fuchs, Z. Hyperinsulinemia. A link between hypertension obesity and glucose intolerance. J. Clin. Investig. 1985, 75, 809–817. [Google Scholar] [CrossRef]
- Hiller, R.; Podgor, M.J.; Sperduto, R.D.; Nowroozi, L.; Wilson, P.W.; D’Agostino, R.B.; Colton, T. A longitudinal study of body mass index and lens opacities. Ophthalmology 1998, 105, 1244–1250. [Google Scholar] [CrossRef]
- Allen, N.A.; Jacelon, C.; Chipkin, S.R. Feasibility and acceptability of continuous glucose monitoring and accelerometer technology in exercising individuals with type 2 diabetes. J. Clin. Nurs. 2009, 18, 373–383. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Makris, K.; Spanou, L. Is There a Relationship between Mean Blood Glucose and Glycated Hemoglobin? J. Diabetes Sci. Technol. 2011, 5, 1572–1583. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peter, R.; Luzio, S.; Dunseath, G.J.; Pauvaday, V.; Mustafa, N.; Owens, D.R. Relationship between HbA1c and indices of glucose tolerance derived from a standardized meal test in newly diagnosed treatment naive subjects with Type 2 diabetes. Diabet. Med. 2006, 23, 990–995. [Google Scholar] [CrossRef] [PubMed]
- Monnier, L.; Colette, C. Contributions of fasting and postprandial glucose to hemoglobin A1c. Endocr. Pr. 2006, 12, 42–46. [Google Scholar] [CrossRef] [PubMed]
- Rohlfing, C.L.; Wiedmeyer, H.-M.; Little, R.R.; England, J.D.; Tennill, A.; Goldstein, D.E. Defining the Relationship Between Plasma Glucose and HbA1c: Analysis of glucose profiles and HbA1c in the Diabetes Control and Complications Trial. Diabetes Care 2002, 25, 275–278. [Google Scholar] [CrossRef] [Green Version]
- Van’t Riet, E.; Alssema, M.; Rijkelijkhuizen, J.M.; Kostense, P.J.; Nijpels, G.; Dekker, J.M. Relationship Between A1C and Glucose Levels in the General Dutch Population. Diabetes Care 2009, 33, 61–66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leow, M.K.-S. Glycated Hemoglobin (HbA1c): Clinical Applications of a Mathematical Concept. Acta Inform. Med. 2016, 24, 233–238. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goldstein, D.E.; Little, R.R.; Lorenz, R.A.; Malone, J.I.; Nathan, D.; Peterson, C.M.; Sacks, D.B. Tests of glycemia in diabetes. Diabetes Care 2004, 27, 1761–1773. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, J.H.; Kim, G.W.; Lee, M.Y.; Shin, J.Y.; Shin, Y.G.; Koh, S.B.; Chung, C.H. Role of HbA1c in the Screening of Diabetes Mellitus in a Korean Rural Community. Diabetes Metab. J. 2012, 36, 37–42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koenig, R.J.; Peterson, C.M.; Jones, R.L.; Saudek, C.; Lehrman, M.; Cerami, A. Correlation of Glucose Regulation and Hemoglobin AIcin Diabetes Mellitus. N. Engl. J. Med. 1976, 295, 417–420. [Google Scholar] [CrossRef] [PubMed]
- Lipska, K.J.; Warton, E.M.; Huang, E.S.; Moffet, H.H.; Inzucchi, S.E.; Krumholz, H.M.; Karter, A.J. HbA1c and Risk of Severe Hypoglycemia in Type 2 Diabetes. Diabetes Care 2013, 36, 3535–3542. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miller, C.D.; Phillips, L.S.; Ziemer, D.C.; Gallina, D.L.; Cook, C.B.; El-Kebbi, I.M. Hypoglycemia in Patients With Type 2 Diabetes Mellitus. Arch. Intern. Med. 2001, 161, 1653–1659. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laiteerapong, N.; Karter, A.J.; Liu, J.Y.; Moffet, H.H.; Sudore, R.; Schillinger, D.; John, P.M.; Huang, E.S. Correlates of Quality of Life in Older Adults with Diabetes. Diabetes Care 2011, 34, 1749–1753. [Google Scholar] [CrossRef] [Green Version]
- McCoy, R.G.; Van Houten, H.K.; Ziegenfuss, J.Y.; Shah, N.D.; Wermers, R.A.; Smith, S.A.; Gruden, G.; Barutta, F.; Chaturvedi, N.; Schalkwijk, C.; et al. Increased Mortality of Patients with Diabetes Reporting Severe Hypoglycemia. Diabetes Care 2012, 35, 1897–1901. [Google Scholar] [CrossRef] [Green Version]
- Sundaram, R.; Selvaraj, N.; Vijayan, G.; Bobby, Z.; Hamide, A.; Dasse, N.R. Increased plasma malondialdehyde and fructosamine in iron deficiency anemia: Effect of treatment. Biomed. Pharmacother. 2007, 61, 682–685. [Google Scholar] [CrossRef]
- Weatherall, D. The inherited disorders of haemoglobin: An increasingly neglected global health burden. Indian J. Med. Res. 2011, 134, 493–497. [Google Scholar]
- Azami, Y.; Funakoshi, M.; Matsumoto, H.; Ikota, A.; Ito, K.; Okimoto, H.; Shimizu, N.; Tsujimura, F.; Fukuda, H.; Miyagi, C.; et al. Long working hours and skipping breakfast concomitant with late evening meals are associated with suboptimal glycemic control among young male Japanese patients with type 2 diabetes. J. Diabetes Investig. 2018, 10, 73–83. [Google Scholar] [CrossRef] [PubMed]
- Sacks, D.B. A1C Versus Glucose Testing: A Comparison. Diabetes Care 2011, 34, 518–523. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salinas, M.; López-Garrigós, M.; Tormo, C.; Uris, J. Primary care use of laboratory tests in Spain: Measurement through appropriateness indicators. Clin. Lab. 2014, 60, 483–490. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zunić, L. Economic Analysis of Requests for Laboratory Tests in Primary Health Care Centers. Acta Inform. Med. 2012, 20, 21–24. [Google Scholar] [CrossRef]
- Montagna, W. Histology and cytochemistry of human skin. IX. The distribution of non-specific esterases. J. Biophys. Biochem. Cytol. 1955, 1, 13–16. [Google Scholar] [CrossRef] [Green Version]
- Bende, M. Human ceruminous gland innervation. J. Laryngol. Otol. 1981, 95, 11–15. [Google Scholar] [CrossRef]
- Fratini, F.; Cilia, G.; Turchi, B.; Felicioli, A. Beeswax: A minireview of its antimicrobial activity and its application in medicine. Asian Pac. J. Trop. Med. 2016, 9, 839–843. [Google Scholar] [CrossRef]
- Ghanem, N. The Antimicrobial Activity of Some Honey Bee Products and some Saudi Folkloric Plant Extracts. J. King Abdulaziz Univ. 2019, 23, 47–62. [Google Scholar] [CrossRef]
- Stoeckelhuber, M.; Matthias, C.; Andratschke, M.; Stoeckelhuber, B.M.; Koehler, C.; Herzmann, S.; Sulz, A.; Welsch, U. Human ceruminous gland: Ultrastructure and histochemical analysis of antimicrobial and cytoskeletal components. Anat. Rec. Part A: Discov. Mol. Cell. Evol. Biol. 2006, 288, 877–884. [Google Scholar] [CrossRef]
- Lum, C.L.; Jeyanthi, S.; Prepageran, N.; Vadivelu, J.; Raman, R. Antibacterial and antifungal properties of human cerumen. J. Laryngol. Otol. 2009, 123, 375–378. [Google Scholar] [CrossRef]
- Herane-Vives, A.; Ortega, L.; Sandoval, R.; Young, A.H.; Cleare, A.; Espinoza, S.; Hayes, A.; Benöhr, J. Measuring Earwax Cortisol Concentration using a non-stressful sampling method. Heliyon 2020, 6, e05124. [Google Scholar] [CrossRef] [PubMed]
- Shichijo, S.; Masuda, H.; Takeuchi, M. Carbohydrate composition of glycopeptides from the human cerumen. Biochem. Med. 1979, 22, 256–263. [Google Scholar] [CrossRef]
- Masuda, H.; Shichijo, S.; Goya, T.; Takeuchi, M. Isolation and partial characterization of a glycopeptide from cerumen. Kurume Med. J. 1978, 25, 203–205. [Google Scholar] [CrossRef] [Green Version]
- Khasanov, S.A.; Popova, I.A. [Glucose content of ear wax in patients with latent and manifest diabetes mellitus]. Vestnik Otorinolaringol. 1985, 2, 34–36. [Google Scholar]
- Cipriani, C.; Taborelli, G.; Gaddia, G.; Melagrana, A.; Rebora, A.E. Production Rate and Composition of Cerumen. Laryngoscope 1990, 100, 275–276. [Google Scholar] [CrossRef]
- Crandell, C.C.; Roeser, R.J. Incidence of excessive/impacted cerumen in individuals with mental retardation: A longitudinal investigation. Am. J. Ment. Retard. 1993, 97, 568–574. [Google Scholar]
- Pichot, P. [DSM-III: The 3d edition of the Diagnostic and Statistical Manual of Mental Disorders from the American Psychiatric Association]. Rev. Neurol. 1986, 142, 489–499. [Google Scholar]
- Miller, M.A.; Rahe, R.H. Life changes scaling for the 1990s. J. Psychosom. Res. 1997, 43, 279–292. [Google Scholar] [CrossRef]
- Cohen, S.; Kamarck, T.; Mermelstein, R. Perceived Stress Scale. Psychology 1983, 1–3. [Google Scholar] [CrossRef]
- Clegg, A.J.; Loveman, E.; Gospodarevskaya, E.; Harris, P.; Bird, A.; Bryant, J.; Scott, D.A.; Davidson, P.; Little, P.; Coppin, R. The safety and effectiveness of different methods of earwax removal: A systematic review and economic evaluation. Health Technol. Assess. 2010, 14, 1–192. [Google Scholar] [CrossRef] [Green Version]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences, 2nd ed.; Routledge: New York, NY, USA, 2013. [Google Scholar] [CrossRef]
- Dhatariya, K.; Skedgel, C.; Fordham, R. The cost of treating diabetic ketoacidosis in the UK: A national survey of hospital resource use. Diabet. Med. 2017, 34, 1361–1366. [Google Scholar] [CrossRef] [PubMed]
- Ko, S.Q.; Quah, P.; Lahiri, M. The cost of repetitive laboratory testing for chronic disease. Intern. Med. J. 2019, 49, 1168–1170. [Google Scholar] [CrossRef] [PubMed]
- Walker, M.S.; Fonda, S.J.; Salkind, S.; Vigersky, R.A. Advantages and Disadvantages of Realtime Continuous Glucose Monitoring in People with Type 2 Diabetes. US Endocrinol. 2012, 8, 22. [Google Scholar] [CrossRef]
- Klonoff, D.C.; Buckingham, B.; Christiansen, J.S.; Montori, V.M.; Tamborlane, W.V.; Vigersky, R.A.; Wolpert, H. Continuous Glucose Monitoring: An Endocrine Society Clinical Practice Guideline. J. Clin. Endocrinol. Metab. 2011, 96, 2968–2979. [Google Scholar] [CrossRef] [PubMed]
- Arora, T.; Grey, I.; Östlundh, L.; Lam, K.B.H.; Omar, O.M.; Arnone, D. The prevalence of psychological consequences of COVID-19: A systematic review and meta-analysis of observational studies. J. Health Psychol. 2020, 135910532096663. [Google Scholar] [CrossRef]
- Herane-Vives, A.; Sandoval, R.; Ortega, L.; Espinoza, S.; Cleare, A.J.; Hayes, A.; Arnone, D.; Ortuzar, E.; Valdenegro, T.; Aguiló, B.; et al. A Self-Sampling Earwax Device A Novel Earwax Self-Sampling Device: A Feasibility Study. Res. Gate 2020. preprint. [Google Scholar] [CrossRef]
- Uehata, T. Long working hours and occupational stress-related cardiovascular attacks among middle-aged workers in Japan. J. Hum. Ergol. 1991, 20, 147–153. [Google Scholar]
- Herane-Vives, A.; De Angel, V.; Papadopoulos, A.; Wise, T.; Chua, K.-C.; Strawbridge, R.; Castillo, D.; Arnone, D.; Young, A.H.; Cleare, A.J. Short-term and long-term measures of cortisol in saliva and hair in atypical and non-atypical depression. Acta Psychiatr. Scand. 2018, 137, 216–230. [Google Scholar] [CrossRef] [Green Version]
- Avignon, A.; Radauceanu, A.; Monnier, L. Nonfasting plasma glucose is a better marker of diabetic control than fasting plasma glucose in type 2 diabetes. Diabetes Care 1997, 20, 1822–1826. [Google Scholar] [CrossRef]
- Svendsen, P.A.; Lauritzen, T.; Nerup, J. Glycosylated haemoglobin and steady-state mean blood glucose concentration in type 1 (insulin-dependent) diabetes. Diabetologia 1982, 23, 403–405. [Google Scholar] [CrossRef] [Green Version]
- Ozmen, S.; Cil, T.; Atay, A.E.; Tuzcu, A.K.; Bahçeci, M. A simple way to estimate mean plasma glucose and to identify Type 2 diabetic subjects with poor glycaemic control when a standardized HbA1cassay is not available. Diabet. Med. 2006, 23, 1151–1154. [Google Scholar] [CrossRef] [PubMed]
- Koren, R.; Ashwal, E.; Hod, M.; Toledano, Y. Insulin detemir versus glyburide in women with gestational diabetes mellitus. Gynecol. Endocrinol. 2016, 32, 916–919. [Google Scholar] [CrossRef] [PubMed]
- Praet, S.F.; Manders, R.J.; Meex, R.C.R.; Lieverse, A.G.; Stehouwer, C.D.A.; Kuipers, H.; Keizer, H.A.; Van Loon, L.J.C. Glycaemic instability is an underestimated problem in Type II diabetes. Clin. Sci. 2006, 111, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Freckmann, G.; Hagenlocher, S.; Baumstark, A.; Jendrike, N.; Gillen, R.C.; Rössner, K.; Haug, C. Continuous Glucose Profiles in Healthy Subjects under Everyday Life Conditions and after Different Meals. J. Diabetes Sci. Technol. 2007, 1, 695–703. [Google Scholar] [CrossRef] [PubMed]
- Lindor, K.D.; Fleming, C.R.; Burnes, J.U.; Nelson, J.K.; Ilstrup, D.M. A Randomized Prospective Trial Comparing a Defined Formula Diet, Corticosteroids, and a Defined Formula Diet Plus Corticosteroids in Active Crohn’s Disease. Mayo Clin. Proc. 1992, 67, 328–333. [Google Scholar] [CrossRef]
- Franek, F.; Holm, P.; Larsen, F.; Steffansen, B. Interaction between fed gastric media (Ensure Plus®) and different hypromellose based caffeine controlled release tablets: Comparison and mechanistic study of caffeine release in fed and fasted media versus water using the USP dissolution apparatus 3. Int. J. Pharm. 2014, 461, 419–426. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.S. Blood Glucose Measurement: Is Serum Equal to Plasma? Diabetes Metab. J. 2016, 40, 365–366. [Google Scholar] [CrossRef]
- American Diabetes Association. American Diabetes Association Standards of Medical Care in Diabetes—2010. Diabetes Care 2009, 33, S11–S61. [Google Scholar] [CrossRef] [Green Version]
| Variable | Results | |
|---|---|---|
| N Participants Females (N, %) | 37, (20, 54.1) | |
| Age (Years) Mean (SD) | 29.9, (1.4) | |
| Civil status: single (yes), N (%) | 32; (86.5) | |
| Undergraduate or graduates N, (%) | 16; (43.2) | |
| Ethnicity | Mixed race, n (%) | 36, (96.3) |
| White n (%) | 1, (3.7) | |
| Alcohol | (yes) δ, n (%) | 10, (27.0) |
| Units φ mean, (SD) | 1.3; (0.5) | |
| Tobacco (yes), n (%) | 9, (24.3) | |
| Contraceptive pill (yes), n (%) | 9, (52.9) | |
| Medical or psychiatric comorbidity, n (%) | 0, (0) | |
| Medication, n (%) | 0, (0) | |
| Variable | Q1 | Median | Mean, (SD) | Q3 | |
|---|---|---|---|---|---|
| Height (cm) Mean, (SD) | Whole sample | 160 | 167 | 166.7, (1.4) | 173 |
| Female | 157 | 160 | 161.6, (1.8) | 166 | |
| Male | 168 | 173 | 172.7, (1.3) | 176 | |
| Weight (kg) Mean, (SD) | Whole sample | 62 | 72 | 72.5, (2.5) | 78 |
| Female | 57.5 | 65.5 | 64.6, (2.0) | 72 | |
| Male | 72 | 75 | 81.8, (3.9) | 95 | |
| BMI (kg/m2), Mean, (SD) | Whole sample | 23.3 | 24.9 | 25.6, (0.6) | 26.7 |
| Female | 22.8 | 24.6 | 24.2, (0.6) | 25.5 | |
| Male | 24.1 | 25.4 | 27.2, (1.1) | 31.2 | |
| Waist circumference (cm), Mean, (SD) | Whole sample | 77 | 86 | 85.9, (2.4) | 95 |
| Female | 70.5 | 78 | 78.8, (2.3) | 87 | |
| Male | 88 | 93 | 94.4, (3.4) | 102 |
| Questionnaire | Results |
|---|---|
| Perceived Stress Scale (PSS), Mean (SD) | 22.6, (1.1) |
| Life events score (RLCQ), Mean (SD) | 141.2, (20.8) |
| History of severe life events (RLCQ) (last month), N (%) | 10, (27.0) |
| Number of Hassles (last month), Mean (SD) | 16.7, (1.7) |
| Severity index of hassles, Mean (SD) | 22.9, (2.8) |
| Subjects under increased number (>25) of hassles (last month), N (%) | 9; (24.3) |
| Subjects having problems dealing with their hassles (last month), N (%) | 1, (2.7) |
| Assessment | Baseline | Follow-up | p-Value ω | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Time in the morning, H ± SD | 8:41 ± 00:50 | 10:53 ± 00:44 | <0.05 | ||||||
| Sample | EGC (nmol/L) | EGC (nmol/L) | |||||||
| Q1 | Median | Mean (s.d) | Q3 | Q1 | Median | Mean (s.d) | Q3 | ||
| 60.5 | 76.9 | 76.7; (4.0) | 82.5 | 81.5 | 88.9 | 94.7; (2.9) | 101.5 | <0.01 * | |
| Sample | HbA1c ¥ (%) | HbA1c ¥ (%) | |||||||
| Q1 | Median | Mean (s.d) | Q3 | Q1 | Median | Mean (s.d) | Q3 | ||
| 3.9 | 4.5 | 4.5; 0.9 | 5.4 | 4.3 | 4.9 | 4.8; 0.8 | 5.4 | 0.02 * | |
| Sample | FSG (nmol/L) | PSG (nmol/L) | |||||||
| Q1 | Median | Mean (s.d) | Q3 | Q1 | Median | Mean (s.d) | Q3 | ||
| 3.2 | 4.5 | 4.3; (0.3) | 5.2 | 4.9 | 5.4 | 5.4; (0.2) | 6.0 | <0.01 * | |
| Procedure | Time Required (Hours) | |||
|---|---|---|---|---|
| EGC Using the Syringe | Glucose Using Earwax Self-Sampling Device | HbA1c in Serum | Glucose in Serum | |
| Centrifugation of the sample. | 00:00 | 00:00 | 00:20 | 00:20 |
| Pre-extraction drying of the sample with N2. | 08:30 | 00:47 | 00:00 | 00:00 |
| Extraction of the sample using an organic solvent. | 02:10 | 02:10 | 00:00 | 00:00 |
| Post-extraction drying of the sample with N2. | 00:40 | 00:40 | 00:00 | 00:00 |
| Protocol of analysis. | 01:00 | 01:00 | 04:00 | 01:00 |
| Total time | 12:20 | 04:37 | 04:20 | 01:20 |
| Method | Associated Costs | Price (£) | |
|---|---|---|---|
| HbA1c | Healthcare staff Š | 17.04 | |
| Analysis § | 9.07 | ||
| Total | 26.11 | ||
| RT-CGM & | Monitor without insulin pump | 1000.00 | n/a |
| Monitor with insulin pump | n/a | 500.00 | |
| Sensors (3 months) | 360.00 | 360.00 | |
| Total | 1360.00 | 860.00 | |
| Earwax Glucose * | Self-sampling device | 8.07 | |
| Analysis | 30.00 | ||
| Total | 38.07 | ||
| FSG (nmol/L) | PSG (nmol/L) | Mean Glycemia (nmol/L) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| R | R2 | p-Value | R | R2 | p-Value | R | R2 | p-Value | |
| Baseline-EGC (nmol/L) | 0.71 | 0.49 | <0.001 | ||||||
| Baseline-HbA1c (%) | 0.51 | 0.26 | 0.002 | ||||||
| Follow-up-EGC (nmol/L) | 0.90 | 0.81 | <0.001 | ||||||
| Follow-up-EGC (%) | 0.47 | 0.22 | 0.002 | ||||||
| Baseline-EGC (nmol/L) | 0.62 | 0.38 | <0.001 | ||||||
| Baseline-HbA1c (%) | 0.55 | 0.30 | <0.001 | ||||||
| Follow-up-EGC (nmol/L) | 0.84 | 0.71 | <0.001 | ||||||
| Follow-up-HbA1c (%) | 0.35 | 0.12 | 0.03 | ||||||
| Variables | Baseline- EGC (nmol/L) | Follow-up- EGC (nmol/L) | Baseline- HbA1c * (%) | Follow-up- HbA1c (%) | FSG (nmol/L) | PSG (nmol/L) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| β | p-Value | CI | β | p-Value | β | p-Value | CI | β | p-Value | CI | β | p-Value | CI | β | p-Value | CI | ||
| Age | 0.4 | 0.38 | −0.6; 1.4 | 0.3 | 0.29 | −0.3; 1.0 | 0.3 | 0.14 | <−0.1; 0.1 | <0.01 | <0.01 * | <0.1; <0.1 | <0.01 | 0.27 | <−0.1; <0.1 | <0.1 | 0.07 | <−0.1; <0.1 |
| Sex | −5.2 | 0.50 | −22.3; 11.3 | 2.1 | 0.70 | −9.3; 13.5 | −0.2 | 0.43 | −0.9; 0.4 | <−0.1 | 0.86 | −20.5; 16.0 | −0.1 | 0.84 | −1.0; 0.9 | 0.1 | 0.81 | −0.6; 0.8 |
| Graduates or undergraduate ξ | 5.1 | 0.54 | −11.9; 22.1 | 9.1 | 0.10 | −1.9; 20.0 | 0.4 | 0.22 | −0.3; 1.1 | 0.7 | 0.01 * | 0.2; 2.0 | 0.4 | 0.35 | −0.5; 1.4 | 0.7 | 0.04 * | <0.1; 1.4 |
| Alcohol (unit) φ | 4.0 | 0.18 | −1.9; 10.0 | −1.3 | 0.19 | −3.4; 0.7 | <0.1 | 0.73 | −0.1; 0.1 | <−0.1 | 0.70 | −0.1; 0.1 | −0.1 | 0.51 | −0.2; 0.1 | −0.1 | 0.18 | −0.2; <0.1 |
| Tobacco | −9.9 | 0.40 | −33.7; 13.9 | −10.5 | 0.13 | −23.2; 3.1 | −0.5 | 0.19 | −1.2; 0.3 | −0.4 | 0.22 | −1.0; 0.2 | −1.3 | 0.01 * | −2.3; −0.3 | −0.9 | 0.02 * | −1.7; −0.1 |
| BMI (kg/cm2) | −1.3 | 0.17 | −3.3; 0.6 | −0.9 | 0.18 | −2.4; 0.5 | <0.1 | 0.97 | −0.1; 0.1 | 0.1 | 0.61 | −0.1; 0.1 | <−0.1 | 0.74 | −0.1; 0.1 | <−0.1 | 0.43 | −0.1; 0.1. |
| Waist circumference (cm) | −0.24 | 0.36 | −0.8; 0.3 | −0.1 | 0.72 | −0.5; 0.3 | <−0.1 | 0.42 | <−0.1; <0.1 | <−0.1 | 0.36 | <−0.1; <0.1 | <−0.1 | 0.73 | <−0.1; <0.1 | <−0.1 | 0.68 | <−0.1; <0.1 |
| Contraceptive pill | 10.2 | 0.17 | −5.0; 25.4 | 4.2 | 0.53 | −9.7; 18.0 | −0.2 | 0.70 | −1.2; 0.8 | −0.1 | 0.86 | −0.8; 0.7 | 0.74 | 0.13 | −0.2; 1.7 | 0.1 | 0.74 | −0.6; 0.8 |
| PSS | −0.8 | 0.21 | −2.1; 0.5 | <−0.1 | 0.94 | −0.9; 0.8 | <−0.1 | 0.93 | <−0.1; <0.1 | <0.1 | 0.23 | <−0.1; 0.1 | <0.1 | 0.88 | −0.1; 0.1 | <0.1 | 0.57 | <−0.1; 0.1 |
| Number of Hassles | <−0.1 | 0.94 | −0.9; 0.9 | −0.3 | 0.23 | −0.8; 0.2 | <0.1 | 0.49 | <−0.1; <0.1 | <0.1 | 0.36 | <−0.1; <0.1 | <0.1 | 0.84 | <−0.1; <0.1 | <−0.1 | 0.66 | <−0.1; <0.1 |
| Severity of Hassles | <0.1 | 0.98 | −0.5; 0.5 | −0.1 | 0.45 | −0.4; 0.2 | <0.1 | 0.41 | <−0.1; <0.1 | <0.1 | 0.15 | <−0.1; <0.1 | <0.1 | 0.78 | <0.1; <0.1 | <−0.1 | 0.92 | <−0.1; <0.1 |
| RLCQ | <−0.1 | 0.54 | <−0.1; <0.1 | <0.1 | 0.79 | <−0.1; <0.1 | <−0.1 | 0.72 | <−0.1; <0.1 | <−0.1 | 0.09 | <−0.1; <0.1 | <−0.1 | 0.83 | <−0.1; <0.1 | <−0.1 | 0.97 | <−0.1; <0.1 |
| Severe RLCQ | 3.0 | 0.54 | −13.3; 7.2 | <−0.1 | 0.99 | −7.0; 7.0 | <0.1 | 0.81 | −0.3; 0.4 | −0.2 | 0.20 | −0.5; 0.1 | −0.1 | 0.86 | −0.6; 0.5 | <−0.1 | 0.88 | −0.5; 0.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Herane-Vives, A.; Espinoza, S.; Sandoval, R.; Ortega, L.; Alameda, L.; Young, A.H.; Arnone, D.; Hayes, A.; Benöhr, J. A Novel Earwax Method to Measure Acute and Chronic Glucose Levels. Diagnostics 2020, 10, 1069. https://doi.org/10.3390/diagnostics10121069
Herane-Vives A, Espinoza S, Sandoval R, Ortega L, Alameda L, Young AH, Arnone D, Hayes A, Benöhr J. A Novel Earwax Method to Measure Acute and Chronic Glucose Levels. Diagnostics. 2020; 10(12):1069. https://doi.org/10.3390/diagnostics10121069
Chicago/Turabian StyleHerane-Vives, Andrés, Susana Espinoza, Rodrigo Sandoval, Lorena Ortega, Luis Alameda, Allan H. Young, Danilo Arnone, Alexander Hayes, and Jan Benöhr. 2020. "A Novel Earwax Method to Measure Acute and Chronic Glucose Levels" Diagnostics 10, no. 12: 1069. https://doi.org/10.3390/diagnostics10121069
APA StyleHerane-Vives, A., Espinoza, S., Sandoval, R., Ortega, L., Alameda, L., Young, A. H., Arnone, D., Hayes, A., & Benöhr, J. (2020). A Novel Earwax Method to Measure Acute and Chronic Glucose Levels. Diagnostics, 10(12), 1069. https://doi.org/10.3390/diagnostics10121069







