Combination of Systemic Inflammatory Biomarkers in Assessment of Chronic Obstructive Pulmonary Disease: Diagnostic Performance and Identification of Networks and Clusters
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Evaluation of Lung Function
2.3. Blood Sampling and Cytokine Determination
2.4. Statistics
3. Results
3.1. Basic Characteristics and Cytokines’ Concentrations of All Participants
3.2. Association of Cytokines’ Concentrations with the Severity of Airflow Limitation and Symptoms Severity
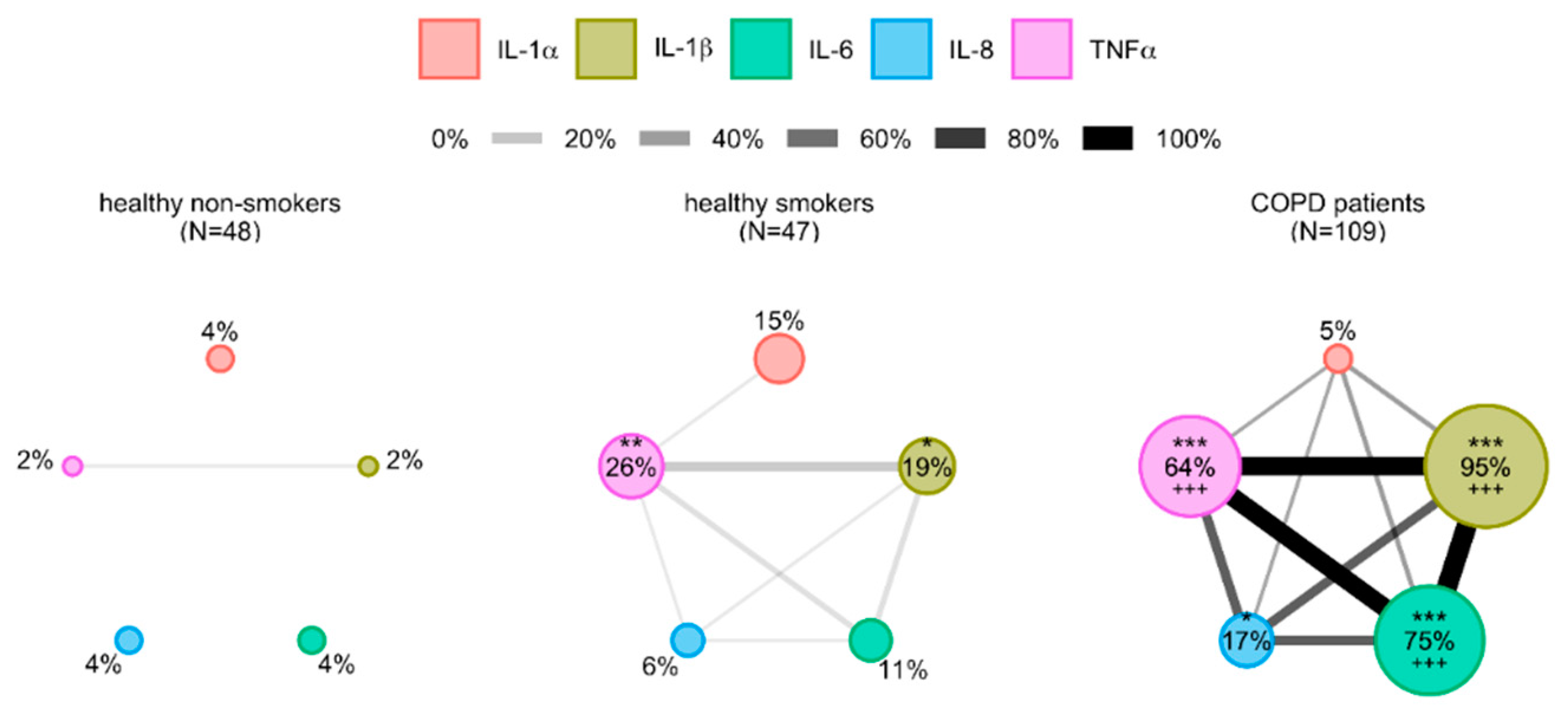
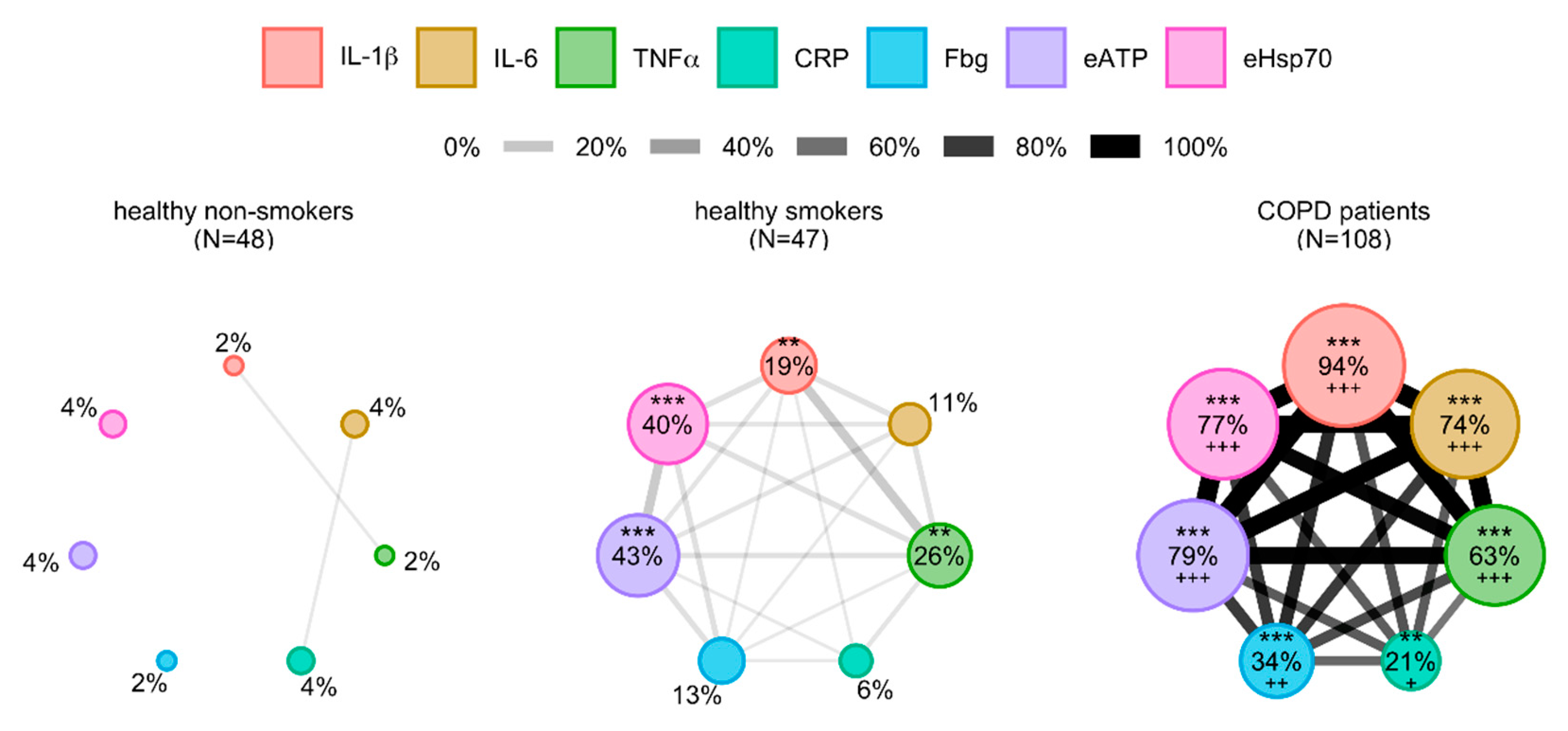
3.3. Cytokines’ Interrelations
3.4. The Potential of Cytokines in Identifying COPD Patients
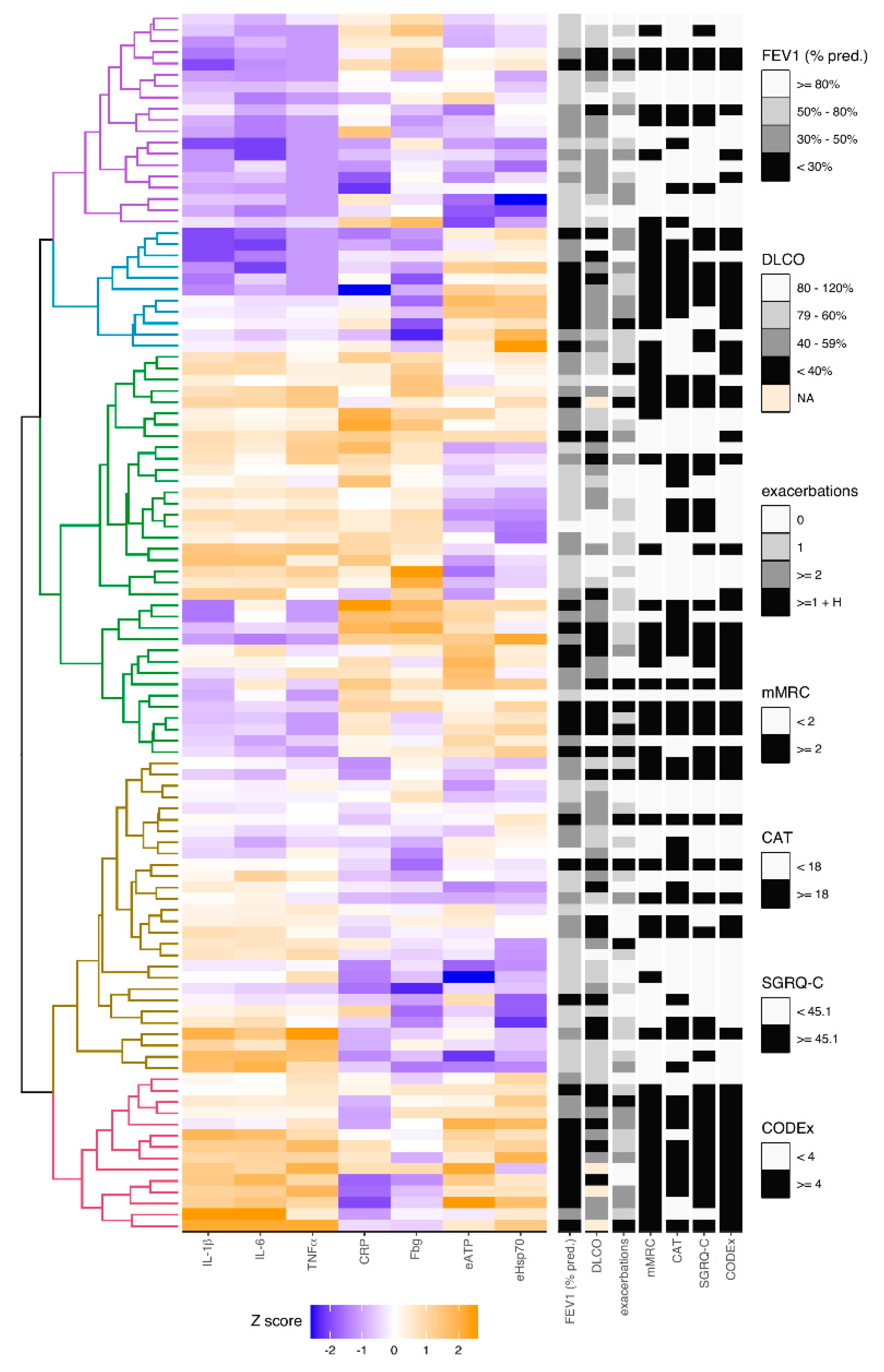
3.5. Analysis of Relations between Inflammation-Driven Parameters in COPD Patients and Identification of COPD Clusters Regarding Systemic Inflammation
3.6. Model Combined of IL-1β, eATP and eHsp70 as the Best Combination for Identifying COPD Patients
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Ramani, T.; Auletta, C.S.; Weinstock, D.; Mounho-Zamora, B.; Ryan, P.C.; Salcedo, T.W.; Bannish, G. Cytokines. Int. J. Toxicol. 2015, 34, 355–365. [Google Scholar] [CrossRef]
- Bade, G.; Khan, M.A.; Srivastava, A.; Khare, P.; Solaiappan, K.; Guleria, R.; Palaniyar, N.; Talwar, A. Serum cytokine profiling and enrichment analysis reveal the involvement of immunological and inflammatory pathways in stable patients with chronic obstructive pulmonary disease. Int. J. Chron. Obstruct. Pulmon. Dis. 2014, 9, 759–773. [Google Scholar]
- Boutou, A.K.; Pitsiou, G.G.; Stanopoulos, I.; Kontakiotis, T.; Kyriazis, G.; Argyropoulou, P. Levels of inflammatory mediators in chronic obstructive pulmonary disease patients with anemia of chronic disease: A case-control study. QJM 2012, 105, 657–663. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease (2020 Report); Global Initiative for Chronic Obstructive Lung Disease: Fontana, WI, USA, 2019; Available online: https://goldcopd.org/gold-reports/ (accessed on 30 October 2020).
- Bailey, K.L.; Goraya, J.; Rennard, S.L. Chronic obstructive pulmonary disease: Co-morbidities and systemic consequences. In The Role of Systemic Inflammation in COPD; Nici, L., ZuWallack, R., Eds.; Humana Press: Totowa, NJ, USA, 2012; pp. 15–30. [Google Scholar]
- Karadag, F.; Karul, A.B.; Cildag, O.; Yilmaz, M.; Ozcan, H. Biomarkers of Systemic Inflammation in Stable and Exacerbation Phases of COPD. Lung 2008, 186, 403–409. [Google Scholar] [CrossRef] [PubMed]
- Morello Gearhart, A.; Cavallazzi, R.; Peyrani, P.; Wiemken, T.L.; Furmanek, S.P.; Reyes-Vega, A.; Gauhar, U.; Rivas Perez, H.; Roman, J.; Ramirez, J.A.; et al. Lung Cytokines and Systemic Inflammation in Patients with COPD. J. Respir. Infect. 2017, 1, 4. [Google Scholar] [CrossRef]
- Barnes, P.J. Cellular and molecular mechanisms of asthma and COPD. Clin. Sci. 2017, 131, 1541–1558. [Google Scholar] [CrossRef] [PubMed]
- Gan, W.Q.; Man, S.F.P.; Senthilselvan, A.; Sin, D.D. Association between chronic obstructive pulmonary disease and systemic inflammation: A systematic review and a meta-analysis. Thorax 2004, 59, 574–580. [Google Scholar] [CrossRef]
- Sapey, E.; Ahmad, A.; Bayley, D.; Newbold, P.; Snell, N.; Rugman, P.; Stockley, R.A. Imbalances Between Interleukin-1 and Tumor Necrosis Factor Agonists and Antagonists in Stable COPD. J. Clin. Immunol. 2009, 29, 508–516. [Google Scholar] [CrossRef]
- Bradford, E.; Jacobson, S.; Varasteh, J.; Comellas, A.P.; Woodruff, P.; O’Neal, W.; DeMeo, D.L.; Li, X.; Kim, V.; Cho, M.; et al. The value of blood cytokines and chemokines in assessing COPD. Respir. Res. 2017, 18, 180. [Google Scholar] [CrossRef]
- Barnes, P.J. The cytokine network in asthma and chronic obstructive pulmonary disease. J. Clin. Investig. 2008, 118, 3546–3556. [Google Scholar] [CrossRef]
- Osei, E.T.; Brandsma, C.A.; Timens, W.; Heijink, I.H.; Hackett, T.L. Current perspectives on the role of interleukin-1 signalling in the pathogenesis of asthma and COPD. Eur. Respir. J. 2020, 55, 1900563. [Google Scholar] [CrossRef] [PubMed]
- Borthwick, L.A. The IL-1 cytokine family and its role in inflammation and fibrosis in the lung. Semin. Immunopathol. 2016, 38, 517–534. [Google Scholar] [CrossRef] [PubMed]
- Rumora, L.; Hlapčić, I.; Popović-Grle, S.; Rako, I.; Rogić, D.; Čepelak, I. Uric acid and uric acid to creatinine ratio in the assessment of chronic obstructive pulmonary disease: Potential biomarkers in multicomponent models comprising IL-1beta. PLoS ONE 2020, 15, e0234363. [Google Scholar] [CrossRef] [PubMed]
- El-Shimy, W.S.; El-Dib, A.S.; Nagy, H.M.; Sabry, W. A study of IL-6, IL-8, and TNF-α as inflammatory markers in COPD patients. Egypt. J. Bronchol. 2014, 8, 91. [Google Scholar]
- Wei, J.; Xiong, X.F.; Lin, Y.H.; Zheng, B.X.; Cheng, D.Y. Association between serum interleukin-6 concentrations and chronic obstructive pulmonary disease: A systematic review and meta-analysis. PeerJ. 2015, 2015, e1199. [Google Scholar] [CrossRef]
- Aghasafari, P.; George, U.; Pidaparti, R. A review of inflammatory mechanism in airway diseases. Inflamm. Res. 2019, 68, 59–74. [Google Scholar] [CrossRef]
- Cao, Y.; Gong, W.; Zhang, H.; Liu, B.; Li, B.; Wu, X.; Duan, X.; Dong, J. A Comparison of Serum and Sputum Inflammatory Mediator Profiles in Patients with Asthma and COPD. J. Int. Med. Res. 2012, 40, 2231–2242. [Google Scholar] [CrossRef]
- Karadag, F.; Kirdar, S.; Karul, A.B.; Ceylan, E. The value of C-reactive protein as a marker of systemic inflammation in stable chronic obstructive pulmonary disease. Eur. J. Intern. Med. 2008, 19, 104–108. [Google Scholar] [CrossRef]
- Agustí, A.; Edwards, L.D.; Rennard, S.I.; MacNee, W.; Tal-Singer, R.; Miller, B.E.; Vestbo, J.; Lomas, D.A.; Calverly, P.M.A.; Wouters, E.; et al. Persistent systemic inflammation is associated with poor clinical outcomes in copd: A novel phenotype. PLoS ONE 2012, 7, e37483. [Google Scholar] [CrossRef]
- Hlapčić, I.; Somborac-Bačura, A.; Popović-Grle, S.; Vukić Dugac, A.; Rogić, D.; Rako, I.; Žanić Grubišić, T.; Rumora, L. Platelet indices in stable chronic obstructive pulmonary disease—Association with inflammatory markers, comorbidities and therapy. Biochem. Med. 2020, 30, 60–73. [Google Scholar] [CrossRef]
- Hlapčić, I.; Hulina-Tomašković, A.; Somborac-Bačura, A.; Grdić Rajković, M.; Vukić Dugac, A.; Popović-Grle, S.; Rumora, L. Extracellular adenosine triphosphate is associated with airflow limitation severity and symptoms burden in patients with chronic obstructive pulmonary disease. Sci. Rep. 2019, 9, 15349. [Google Scholar] [CrossRef] [PubMed]
- Hlapčić, I.; Hulina-Tomašković, A.; Grdić Rajković, M.; Popović-Grle, S.; Vukić Dugac, A.; Rumora, L. Association of Plasma Heat Shock Protein 70 with Disease Severity, Smoking and Lung Function of Patients with Chronic Obstructive Pulmonary Disease. J. Clin. Med. 2020, 9, 3097. [Google Scholar] [CrossRef] [PubMed]
- Morales, D.R.; Flynn, R.; Zhang, J.; Trucco, E.; Quint, J.K.; Zutis, K. External validation of ADO, DOSE, COTE and CODEX at predicting death in primary care patients with COPD using standard and machine learning approaches. Respir. Med. 2018, 138, 150–155. [Google Scholar] [CrossRef] [PubMed]
- Simundic, A.M.; Bölenius, K.; Cadamuro, J.; Church, S.; Cornes, M.P.; van Dongen-Lases, E.C.; Eker, P.; Erdeljanovic, T.; Grankvist, K.; Tiago Guimaraes, J.; et al. Joint EFLM-COLABIOCLI Recommendation for venous blood sampling. Clin. Chem. Lab. Med. 2018, 56, 2015–2038. [Google Scholar] [CrossRef] [PubMed]
- GenABEL Project Developers. GenABEL: Genome-wide SNP association analysis. R package version 1.8-0. Available online: http://CRAN.R-project.org/package=GenABEL (accessed on 24 April 2020).
- Charrad, M.; Ghazzali, N.; Boiteau, V.; Niknafs, A. Nbclust: An R package for determining the relevant number of clusters in a data set. J. Stat. Softw. 2014, 61, 36. [Google Scholar] [CrossRef]
- 3.5.1. RDCT. A Language and Environment for Statistical Computing. Vol. 2, R Foundation for Statistical Computing. Available online: http://www.r-project.org (accessed on 27 April 2020).
- Landsberg, J. Pulmonary Function Testing. In Clinical Practice Manual for Pulmonary and Critical Care Medicine, 1st ed.; Elsevier: Philadelphia, PA, USA, 2018; pp. 23–27. [Google Scholar]
- Fragoso, E.; André, S.; Boleo-Tomé, J.P.; Areias, V.; Munhá, J.; Cardoso, J. Understanding COPD: A vision on phenotypes, comorbidities and treatment approach. Rev. Port. Pneumol. 2016, 22, 101–111. [Google Scholar] [CrossRef]
- Botelho, F.M.; Bauer, C.M.T.; Finch, D.; Nikota, J.K.; Zavitz, C.C.J.; Kelly, A.; Lambert, K.N.; Piper, S.; Foster, M.L.; Goldring, J.J.P.; et al. IL-1α/IL-1R1 Expression in Chronic Obstructive Pulmonary Disease and Mechanistic Relevance to Smoke-Induced Neutrophilia in Mice. PLoS ONE 2011, 6, e28457. [Google Scholar] [CrossRef]
- Zou, Y.; Chen, X.; Liu, J.; Zhou, D.B.; Kuang, X.; Xiao, J.; Yu, Q.; Lu, X.; Li, W.; Xie, B.; et al. Serum IL-1β and IL-17 levels in patients with COPD: Associations with clinical parameters. Int. J. Chron. Obstruct. Pulmon. Dis. 2017, 12, 1247–1254. [Google Scholar] [CrossRef]
- Donaldson, G.C.; Seemungal, T.A.R.; Patel, I.S.; Bhowmik, A.; Wilkinson, T.M.A.; Hurst, J.R.; Maccallum, P.K.; Wedzicha, J.A. Airway and Systemic Inflammation and Decline in Lung Function in Patients with COPD. Chesty 2005, 128, 1995–2004. [Google Scholar] [CrossRef]
- Zhang, J.; Dai, R.; Zhai, W.; Feng, N.; Lin, J. The efficacy of modified neutrophil alkaline phosphatase score, serum IL-6, IL-18 and CC16 levels on the prognosis of moderate and severe COPD patients. Biomed. Res. 2017, 28, 7651–7655. [Google Scholar]
- Singh, S.; George, K.; Kaleem, M.; King, A.; Kumar, S.; King, S. Association between serum cytokine levels and severity of chronic obstructive pulmonary disease in Northern India. Innov. Sp. Sci. Res. J. 2015, 18, 357–361. [Google Scholar]
- Pinto-Plata, V.; Toso, J.; Lee, K.; Park, D.; Bilello, J.; Mullerova, H.; De Souza, M.M.; Vessey, R.; Celli, B. Profiling serum biomarkers in patients with COPD: Associations with clinical parameters. Thorax 2007, 62, 595–601. [Google Scholar] [CrossRef] [PubMed]
- Queiroz, C.F.; Lemos, A.C.M.; de Lourdes Sanatana Bastos, M.; Neves, M.C.L.C.; Camelier, A.A.; Carvalho, N.B.; de Carvalho, E.M. Inflammatory and immunological profiles in patients with COPD: Relationship with FEV1 reversibility. J. Bras. Pneumol. 2016, 42, 241–247. [Google Scholar] [CrossRef] [PubMed]
- Kleniewska, A.; Walusiak-Skorupa, J.; Piotrowski, W.; Nowakowska-Świrta, E.; Wiszniewska, M. Comparison of biomarkers in serum and induced sputum of patients with occupational asthma and chronic obstructive pulmonary disease. J. Occup. Health. 2016, 58, 333–339. [Google Scholar] [CrossRef]
- Selvarajah, S.; Todd, I.; Tighe, P.J.; John, M.; Bolton, C.E.; Harrison, T.; Fairclough, L.C. Multiple Circulating Cytokines Are Coelevated in Chronic Obstructive Pulmonary Disease. Mediators Inflamm. 2016, 2016, 9. [Google Scholar] [CrossRef]
- Marević, S.; Petrik, J.; Popović-Grle, S.; Čepelak, I.; Grgić, I.; Gorenec, L.; Stjepanović, G.; Laskaj, R.; Židovec Lepej, S. Cytokines and statin therapy in chronic obstructive pulmonary disease patients. Scand. J. Clin. Lab. Investig. 2018, 78, 533–538. [Google Scholar]
- Vanfleteren, L.E.G.W.; Spruit, M.A.; Groenen, M.; Gaffron, S.; van Empel, V.P.M.; Bruijnzeel, P.L.B.; Rutten, E.P.A.; Op ‘t Roodt, J.; Wouters, E.F.M.; Franssen, F.M.E. Clusters of Comorbidities Based on Validated Objective Measurements and Systemic Inflammation in Patients with Chronic Obstructive Pulmonary Disease. Am. J. Respir. Crit. Care. Med. 2013, 187, 728–735. [Google Scholar] [CrossRef]
- Garcia-Aymerich, J.; Gomez, F.P.; Benet, M.; Farrero, E.; Basagana, X.; Gayete, A.; Paré, C.; Freixa, X.; Ferrer, J.; Ferrer, A.; et al. Identification and prospective validation of clinically relevant chronic obstructive pulmonary disease (COPD) subtypes. Thorax 2011, 66, 430–437. [Google Scholar] [CrossRef]
- Burgel, P.R.; Paillasseur, J.L.; Caillaud, D.; Tillie-Leblond, I.; Chanez, P.; Escamilla, R.; Court-Fortune, I.; Perez, T.; Carré, P.; Roche, N.; et al. Clinical COPD phenotypes: A novel approach using principal component and cluster analyses. Eur. Respir. J. 2010, 36, 531–539. [Google Scholar] [CrossRef]
- Rennard, S.I.; Locantore, N.; Delafont, B.; Tal-Singer, R.; Silverman, E.K.; Vestbo, J.; Miller, B.E.; Bakke, P.; Celli, B.; Calverley, P.M.A.; et al. Identification of Five Chronic Obstructive Pulmonary Disease Subgroups with Different Prognoses in the ECLIPSE Cohort Using Cluster Analysis. Ann. Am. Thorac. Soc. 2015, 12, 303–312. [Google Scholar] [CrossRef]
| Total Healthy Subjects n = 95 | Healthy Non-Smokers n = 48 | COPD Patients n = 109 | p1 | p2 | |
|---|---|---|---|---|---|
| age | 64 (46–83) | 65 (52–83) | 65 (45–87) | 0.069 | 0.600 |
| gender male female | 49 46 | 23 25 | 69 40 | 0.121 | 0.104 |
| FEV1 (L) | 2.60 (2.12–3.19) | 2.82 (2.28–3.19) | 1.08 (0.69–1.60) | <0.001 | <0.001 |
| FEV1 (% pred.) | 93.3 (86.4–104.2) | 101.1 (90.6–110.4) | 40.8 (27.9–61.7) | <0.001 | <0.001 |
| FVC (L) | 3.35 (2.77–4.16) | 3.58 (2.76–4.18) | 2.28 (1.74–2.77) | <0.001 | <0.001 |
| FEV1/FVC (%) | 80.6 (76.8–87.6) | 83.0 (78.1–91.8) | 51.3 (40.7–58.7) | <0.001 | <0.001 |
| IL-1α (pg/mL) | 0.30 (0.30–0.97) | 0.31 (0.31–0.71) | 0.43 (0.30–2.13) | 0.003 | 0.007 |
| IL-1β (pg/mL) | 0.10 (0.10–0.61) | 0.10 (0.10–0.17) | 6.90 (0.61–23.91) | <0.001 | <0.001 |
| IL-6 (pg/mL) | 4.85 (3.45–7.09) | 4.41 (3.29–6.17) | 32.17 (10.64–64.30) | <0.001 | <0.001 |
| IL-8 (pg/mL) | 6.36 (4.07–11.17) | 6.22 (4.34–11.33) | 8.73 (3.56–17.76) | 0.040 | 0.049 |
| TNFα (pg/mL) | 0.40 (0.35–1.36) | 0.35 (0.35–0.53) | 8.24 (0.35–19.23) | <0.001 | <0.001 |
| IL-1α (pg/mL) | IL-1β (pg/mL) | IL-6 (pg/mL) | IL-8 (pg/mL) | TNFα (pg/mL) | |
|---|---|---|---|---|---|
| controls n = 95 | 0.30 (0.30–0.97) | 0.10 (0.10–0.61) | 4.85 (3.45–7.09) | 6.36 (4.07–11.17) | 0.40 (0.35–1.36) |
| GOLD 2 n = 39 | 0.40 (0.30–2.37) 1 | 8.77 (0.70–20.40) 1 | 30.14 (10.54–58.01) 1 | 6.98 (3.27–15.25) | 11.04 (0.39–19.37) 1 |
| GOLD 3 n = 36 | 0.63 (0.30–2.04) 1 | 7.57 (0.75–22.63) 1 | 34.75 (8.25–56.75) 1 | 8.77 (3.50–23.59) 1 | 7.40 (0.77–14.08) 1 |
| GOLD 4 n = 34 | 0.48 (0.30–1.60) 1 | 5.54 (0.56–42.23) 1 | 27.23 (12.51–106.87) 1 | 9.74 (4.56–22.89) 1 | 6.63 (0.35–31.37) 1 |
| p1 | 0.031 | <0.001 | <0.001 | 0.041 | <0.001 |
| GOLD A n = 14 | 2.04 (0.30–3.09) 1 | 8.72 (3.55–20.63) 1 | 33.33 (11.95–56.65) 1 | 6.07 (3.55–14.00) | 12.31 (3.34–18.65) 1 |
| GOLD B n = 63 | 0.40 (0.30–1.84) 1 | 8.27 (0.56–25.78) 1 | 33.36 (10.85–71.16) 1 | 9.40 (3.64–19.89) | 8.60 (0.35–19.46) 1 |
| GOLD D n = 32 | 0.48 (0.30–2.56) 1 | 4.25 (0.53–21.72) 1 | 24.07 (8.25–52.93) 1 | 8.20 (3.37–18.60) | 4.29 (0.35–17.28) 1 |
| p2 | 0.018 | <0.001 | <0.001 | 0.398 | <0.001 |
| OR | p | 95% CI | Cases Correctly Classified (%) | |
|---|---|---|---|---|
| IL-1α | 1.00 | 0.536 | 0.99–1.01 | 53 |
| IL-1β | 5.53 | <0.001 | 2.05–14.90 | 84 |
| IL-6 | 1.14 | <0.001 | 1.08–1.19 | 80 |
| IL-8 | 1.03 | 0.010 | 1.01–1.05 | 56 |
| TNFα | 1.27 | <0.001 | 1.16–1.40 | 74 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hlapčić, I.; Belamarić, D.; Bosnar, M.; Kifer, D.; Vukić Dugac, A.; Rumora, L. Combination of Systemic Inflammatory Biomarkers in Assessment of Chronic Obstructive Pulmonary Disease: Diagnostic Performance and Identification of Networks and Clusters. Diagnostics 2020, 10, 1029. https://doi.org/10.3390/diagnostics10121029
Hlapčić I, Belamarić D, Bosnar M, Kifer D, Vukić Dugac A, Rumora L. Combination of Systemic Inflammatory Biomarkers in Assessment of Chronic Obstructive Pulmonary Disease: Diagnostic Performance and Identification of Networks and Clusters. Diagnostics. 2020; 10(12):1029. https://doi.org/10.3390/diagnostics10121029
Chicago/Turabian StyleHlapčić, Iva, Daniela Belamarić, Martina Bosnar, Domagoj Kifer, Andrea Vukić Dugac, and Lada Rumora. 2020. "Combination of Systemic Inflammatory Biomarkers in Assessment of Chronic Obstructive Pulmonary Disease: Diagnostic Performance and Identification of Networks and Clusters" Diagnostics 10, no. 12: 1029. https://doi.org/10.3390/diagnostics10121029
APA StyleHlapčić, I., Belamarić, D., Bosnar, M., Kifer, D., Vukić Dugac, A., & Rumora, L. (2020). Combination of Systemic Inflammatory Biomarkers in Assessment of Chronic Obstructive Pulmonary Disease: Diagnostic Performance and Identification of Networks and Clusters. Diagnostics, 10(12), 1029. https://doi.org/10.3390/diagnostics10121029





