The AST/ALT (De Ritis) Ratio Predicts Survival in Patients with Oral and Oropharyngeal Cancer
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Considerations
2.2. Radiation Technique
2.3. Staging und Follow-up
2.4. Statistical Analysis
3. Results
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Ferlay, J.; Shin, H.R.; Bray, F.; Forman, D.; Mathers, C.; Parkin, D.M. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int. J. Cancer 2010, 127, 2893–2917. [Google Scholar] [CrossRef] [PubMed]
- Warnakulasuriya, S. Global epidemiology of oral and oropharyngeal cancer. Oral Oncol. 2009, 45, 309–316. [Google Scholar] [CrossRef] [PubMed]
- Chaturvedi, A.K.; Anderson, W.F.; Lortet-Tieulent, J.; Curado, M.P.; Ferlay, J.; Franceschi, S.; Rosenberg, P.S.; Bray, F.; Gillison, M.L. Worldwide trends in incidence rates for oral cavity and oropharyngeal cancers. J. Clin. Oncol. 2013, 31, 4550–4559. [Google Scholar] [CrossRef] [PubMed]
- Jin, S.; Yang, X.; Li, J.; Yang, W.; Ma, H.; Zhang, Z. p53-targeted lincRNA-p21 acts as a tumor suppressor by inhibiting JAK2/STAT3 signaling pathways in head and neck squamous cell carcinoma. Mol. Cancer 2019, 18, 38. [Google Scholar] [CrossRef] [PubMed]
- Binabaj, M.M.; Soleimani, A.; Rahmani, F.; Avan, A.; Khazaei, M.; Fiuji, H.; Soleimanpour, S.; Ryzhikov, M.; Ferns, G.; Bahrami, A.; et al. Prognostic value of high mobility group protein A2 (HMGA2) over-expression in cancer progression. Gene 2019, 706, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Templeton, A.J.; McNamara, M.G.; Seruga, B.; Vera-Badillo, F.E.; Aneja, P.; Ocaña, A.; Leibowitz-Amit, R.; Sonpavde, G.; Knox, J.J.; Tran, B.; et al. Prognostic role of neutrophil-to-lymphocyte ratio in solid tumors: A systematic review and meta-analysis. J. Natl. Cancer Inst. 2014, 106, dju124. [Google Scholar] [CrossRef]
- Meyer, F.; Samson, E.; Douville, P.; Duchesne, T.; Liu, G.; Bairati, I. Serum prognostic markers in head and neck cancer. Clin. Cancer Res. 2010, 16, 1008–1015. [Google Scholar] [CrossRef]
- Proctor, M.J.; Talwar, D.; Balmar, S.M.; O’Reilly, D.S.J.; Foulis, A.K.; Horgan, P.G.; Morrison, D.S.; McMillan, D.C. The relationship between the presence and site of cancer, an inflammation-based prognostic score and biochemical parameters. Initial results of the Glasgow Inflammation Outcome Study. Br. J. Cancer 2010, 103, 870–876. [Google Scholar] [CrossRef]
- Koppenol, W.H.; Bounds, P.L.; Dang, C.V. Otto Warburg’s contributions to current concepts of cancer metabolism. Nat. Rev. Cancer 2011, 11, 325–337. [Google Scholar] [CrossRef]
- Botros, M.; Sikaris, K.A. The de ritis ratio: The test of time. Clin. Biochem. Rev. 2013, 34, 117–130. [Google Scholar]
- De Ritis, F.; Coltorti, M.; Giusti, G. An enzymic test for the diagnosis of viral hepatitis; the transaminase serum activities. Clin. Chim. Acta 1957, 2, 70–74. [Google Scholar] [CrossRef]
- Nishikawa, M.; Miyake, H.; Fujisawa, M. De Ritis (aspartate transaminase/alanine transaminase) ratio as a significant predictor of recurrence-free survival in patients with upper urinary tract urothelial carcinoma following nephroureterectomy. Urol. Oncol. 2016, 34, e415–e419. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Fang, K.; Zhang, J.; Jiang, Y.; Wang, G.; Zhang, H.; Chen, T.; Shi, X.; Li, Y.; Duan, F.; et al. The significance of De Ritis (aspartate transaminase/alanine transaminase) ratio in predicting pathological outcomes and prognosis in localized prostate cancer patients. Int. Urol. Nephrol. 2017, 49, 1391–1398. [Google Scholar] [CrossRef] [PubMed]
- Ha, Y.S.; Kim, S.W.; Chun, S.Y.; Chung, J.-W.; Choi, S.H.; Lee, J.N.; Kim, B.S.; Kim, H.T.; Yoo, E.S.; Kwon, T.G.; et al. Association between De Ritis ratio (aspartate aminotransferase/alanine aminotransferase) and oncological outcomes in bladder cancer patients after radical cystectomy. BMC Urol. 2019, 19, 10. [Google Scholar] [CrossRef] [PubMed]
- Ishihara, H.; Kondo, T.; Yoshida, K.; Omae, K.; Takagi, T.; Iizuka, J.; Tanabe, K. Evaluation of Preoperative Aspartate Transaminase/Alanine Transaminase Ratio as an Independent Predictive Biomarker in Patients with Metastatic Renal Cell Carcinoma Undergoing Cytoreductive Nephrectomy: A Propensity Score Matching Study. Clin. Genitourin. Cancer 2017, 15, 598–604. [Google Scholar] [CrossRef]
- Miyake, H.; Matsushita, Y.; Watanabe, H.; Tamura, K.; Suzuki, T.; Motoyama, D.; Ito, T.; Sugiyama, T.; Otsuka, A. Significance of De Ritis (Aspartate Transaminase/Alanine Transaminase) Ratio as a Significant Prognostic But Not Predictive Biomarker in Japanese Patients with Metastatic Castration-resistant Prostate Cancer Treated with Cabazitaxel. Anticancer Res. 2018, 38, 4179–4185. [Google Scholar] [CrossRef]
- Takenaka, Y.; Takemoto, N.; Yasui, T.; Yamamoto, Y.; Uno, A.; Miyabe, H.; Ashida, N.; Shimizu, K.; Nakahara, S.; Hanamoto, A.; et al. Transaminase Activity Predicts Survival in Patients with Head and Neck Cancer. PLoS ONE 2016, 11, e0164057. [Google Scholar] [CrossRef]
- Thurner, E.M.; Krenn-Pilko, S.; Langsenlehner, U.; Stojakovic, T.; Pichler, M.; Gerger, A.; Kapp, K.S.; Langsenlehner, T. The association of an elevated plasma fibrinogen level with cancer-specific and overall survival in prostate cancer patients. World J. Urol. 2015, 33, 1467–1473. [Google Scholar] [CrossRef]
- Holzinger, D.; Danilovic, I.; Seemann, R.; Kornek, G.; Engelmann, J.; Pillerstorff, R.; Holawe, S.; Psyrri, A.; Erovic, B.M.; Farwell, G.; et al. Prognostic Impact of Pretreatment Plasma Fibrinogen in Patients with Locally Advanced Oral and Oropharyngeal Cancer. PLoS ONE 2016, 11, e0158697. [Google Scholar] [CrossRef]
- Bezan, A.; Mrsic, E.; Krieger, D.; Stojakovic, T.; Pummer, K.; Zigeuner, R.; Hutterer, G.C.; Pichler, M. The preoperative AST/ALT (De Ritis) ratio represents a poor prognostic factor in a cohort of patients with nonmetastatic renal cell carcinoma. J. Urol. 2015, 194, 30–35. [Google Scholar] [CrossRef]
- Tan, X.; Xiao, K.; Liu, W.; Chang, S.; Zhang, T.; Tang, H. Prognostic factors of distal cholangiocarcinoma after curative surgery: A series of 84 cases. Hepatogastroenterology 2013, 60, 1892–1895. [Google Scholar] [PubMed]
- Lindmark, G.; Gerdin, B.; Pahlman, L.; Bergström, R.; Glimelius, B. Prognostic predictors in colorectal cancer. Dis. Colon Rectum 1994, 37, 1219–1227. [Google Scholar] [CrossRef] [PubMed]
- Stocken, D.D.; Hassan, A.B.; Altman, D.G.; Billingham, L.J.; Bramhall, S.R.; Johnson, P.J.; Freemantle, N. Modelling prognostic factors in advanced pancreatic cancer. Br. J. Cancer 2008, 99, 883–893. [Google Scholar] [CrossRef] [PubMed]
- Chougule, A.; Hussain, S.; Agarwal, D.P. Prognostic and diagnostic value of serum pseudocholinesterase, serum aspartate transaminase, and serum alinine transaminase in malignancies treated by radiotherapy. J. Cancer Res. Ther. 2008, 4, 21–25. [Google Scholar] [CrossRef]
- Conde, V.R.; Oliveira, P.F.; Nunes, A.R.; Rocha, C.S.; Ramalhosa, E.; Pereira, J.A.; Alves, M.G.; Silva, B.M. The progression from a lower to a higher invasive stage of bladder cancer is associated with severe alterations in glucose and pyruvate metabolism. Exp. Cell Res. 2015, 335, 91–98. [Google Scholar] [CrossRef]
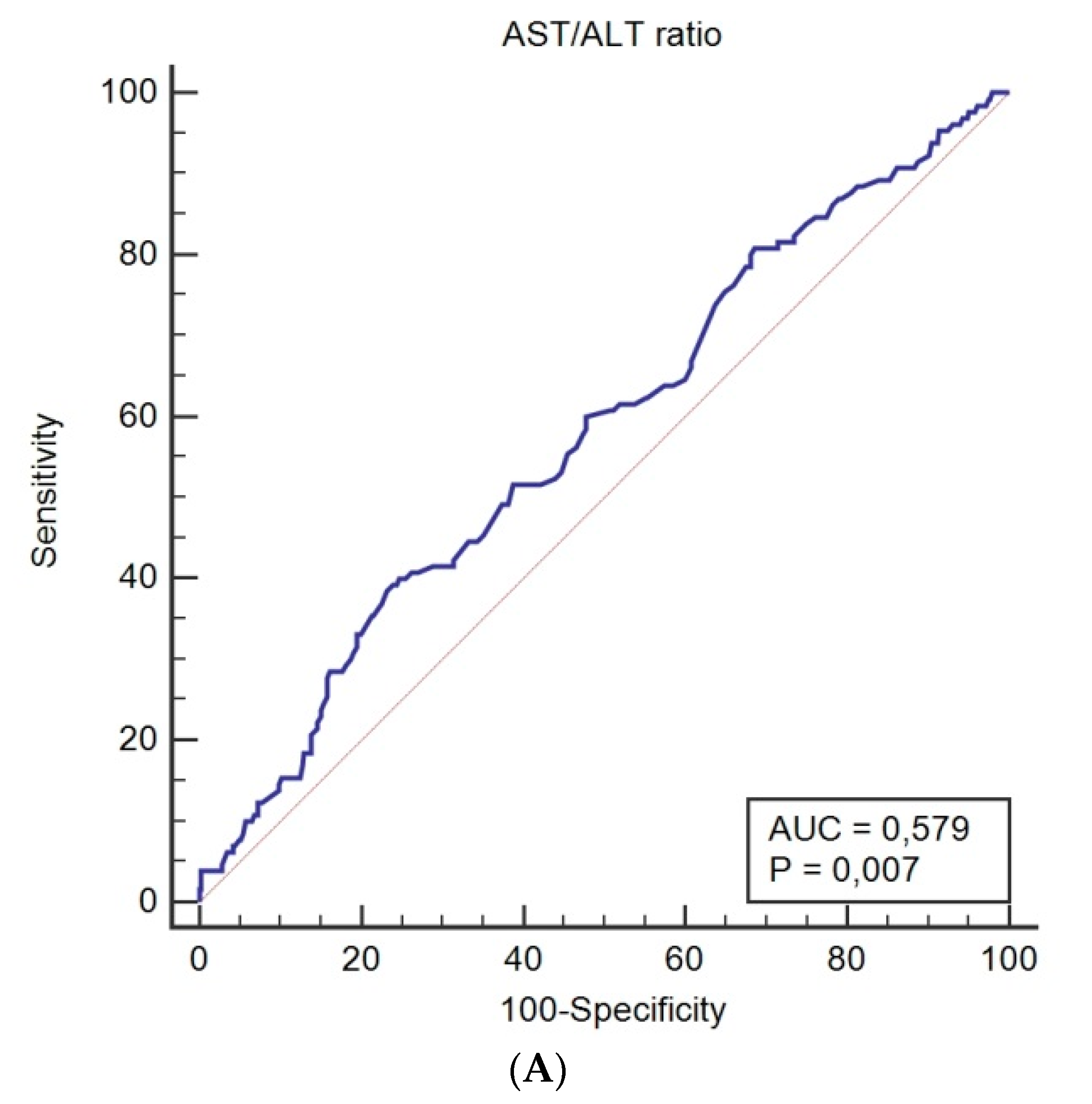


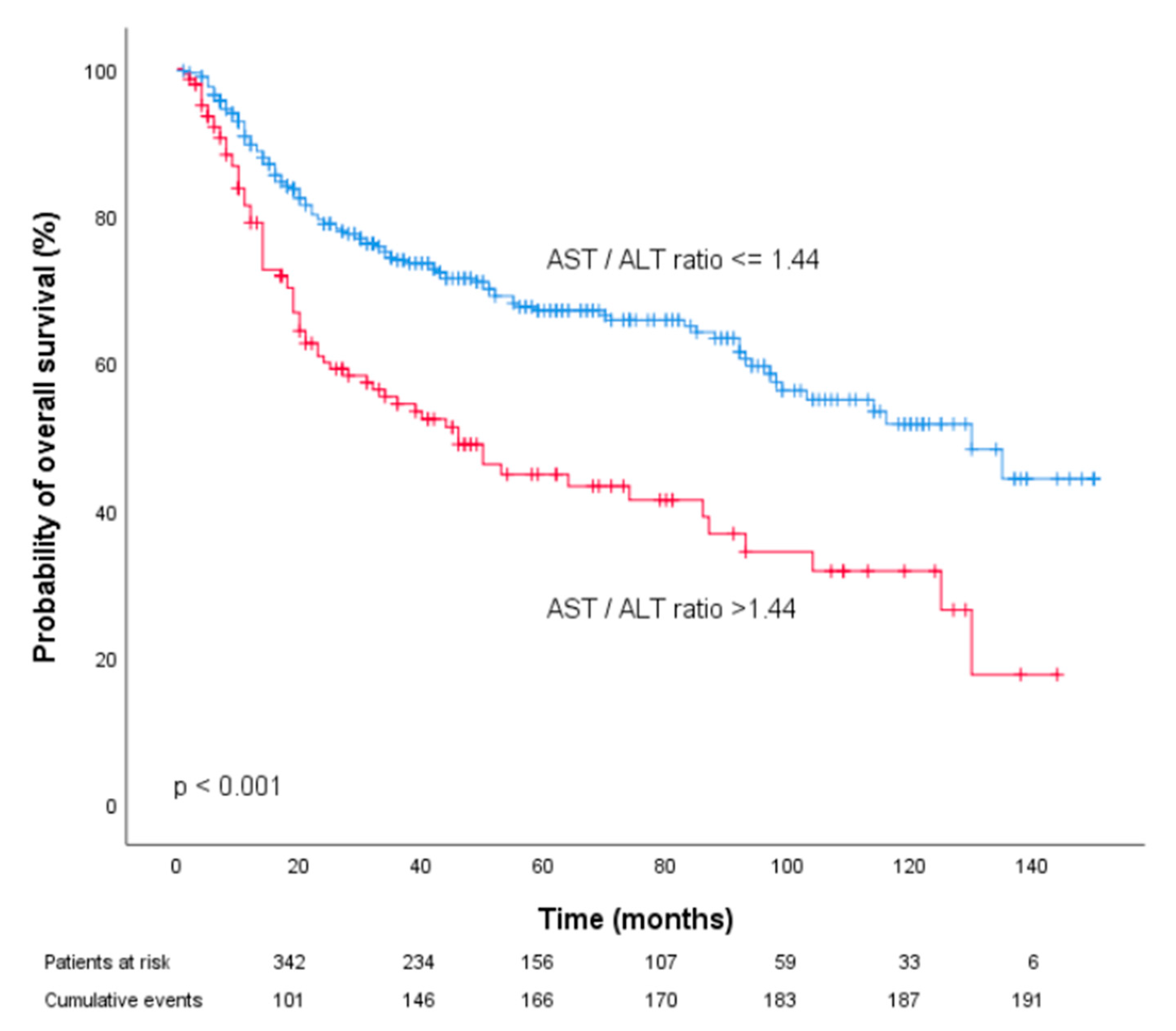
| Patient Characteristics, n (%) | |
|---|---|
| Sex | |
| Male | 388 (75.3%) |
| Female | 127 (24.7%) |
| Age at diagnosis | |
| <60 | 279 (54.2) |
| >60 | 236 (45.8%) |
| Body mass index | |
| Median (mean ± SD) | 24.1 (24.6 ± 4.42) |
| Smoking status | |
| Former or never * | 188 (36.5%) |
| Current ** | 320 (62.1%) |
| Missing data | 7 (1.4%) |
| Alcohol consumption | |
| Former or never * | 309 (60.0%) |
| Current ** | 191 (37.1%) |
| Missing data | 15 (2.9%) |
| Primary site | |
| Oral cavity | 206 (40%) |
| Oropharynx | 309 (60%) |
| Tumor grade | |
| G 1/2 | 252 (48.9%) |
| G 3/4 | 256 (49.7%) |
| Missing data | 7 (1.4%) |
| Tumor stage | |
| T 1/2 | 215 (41.7%) |
| T 3/4 | 290 (56.3%) |
| Missing data | 10 (1.9%) |
| HPV status | |
| Negative | 30 (5.8%) |
| Positive | 35 (6.8%) |
| Missing data | 450 (87.4%) |
| Nodal involvement | |
| N0 | 100 (19.4%) |
| N+ | 409 (79.4%) |
| Missing data | 6 (1.2%) |
| UICC stage | |
| I | 9 (1.7%) |
| II | 30 (5.8%) |
| III | 99 (19.1%) |
| IV | 372 (71.8%) |
| Missing data | 8 (1.5%) |
| Primary treatment | |
| Surgery | 269 (52.2%) |
| Radio(chemo-) therapy | 246 (47.8%) |
| Induction Chemotherapy | |
| Yes | 76 (14.8%) |
| No | 439 (85.2%) |
| Concomitant Chemotherapy | |
| Yes | 197 (38.3%) |
| No | 317 (61.6%) |
| Pre-treatment AST/ALT ratio | |
| Median (mean ± SD) | 1.14 (1.26 ± 0.66) |
| Cancer Specific Survival | ||||
|---|---|---|---|---|
| Univariate Analysis | Multivariate Analysis * | |||
| HR (95% CI) | p-Value | HR (95% CI) | p-Value | |
| Sex | ||||
| Male | 1 | |||
| Female | 1.22 (0.83–1.79) | 0.307 | ||
| Age at diagnosis | ||||
| <60 | 1 | |||
| >60 | 1.15 (0.81–1.63) | 0.446 | ||
| Body mass index (continuous) | 0.93 (0.89–0.98) | 0.004 | 0.98 (0.93–1.03) | 0.358 |
| Smoking status | ||||
| Former/never | 1 | 1 | ||
| Current | 1.48 (1.00–2.17) | 0.048 | 1.17 (0.75–1.83) | 0.493 |
| Alcohol consumption | ||||
| Former/never | 1 | 1 | ||
| Current | 1.76 (1.24–2.51) | 0.002 | 1.47 (0.99–2.18) | 0.058 |
| Primary site | ||||
| Oropharynx | 1 | 1 | ||
| Oral cavity | 1.69 (1.19–2.40) | 0.002 | 1.93 (1.32–2.82) | 0.001 |
| Tumor grade | ||||
| G 1/2 | 1 | |||
| G 3/4 | 0.99 (0.70–1.42) | 0.984 | ||
| Tumor stage | ||||
| T 1/2 | 1 | 1 | ||
| T 3/4 | 2.20 (1.50–3.23) | <0.001 | 1.24 (0.77–2.01) | 0.379 |
| Nodal involvement | ||||
| N0 | 1 | |||
| N+ | 1.16 (0.73–1.86) | 0.532 | ||
| UICC stage | ||||
| I | 1 | |||
| II | 0.74 (0.14–3.81) | 0.716 | ||
| III | 0.82 (0.19–3.54) | 0.794 | ||
| IV | 1.28 (0.31–5.18) | 0.733 | ||
| Primary treatment | ||||
| Radio(chemo-) therapy | 1 | 1 | ||
| Surgery | 0.36 (0.25–0.52) | <0.001 | 0.34 (0.21–0.55) | <0.001 |
| Induction chemotherapy | ||||
| No | 1 | 1 | ||
| Yes | 1.72 (1.13–2.63) | 0.012 | 1.05 (0.73–1.51) | 0.798 |
| Concomitant chemotherapy | ||||
| No | 1 | |||
| Yes | 1.01 (0.70–1.44) | 0.974 | ||
| Pre-treatment | ||||
| AST/ALR ratio (continuous) | 1.71 (1.38–2.12) | <0.001 | 1.45 (1.12–1.88) | 0.005 |
| Overall Survival | ||||
|---|---|---|---|---|
| Univariate Analysis | Multivariate Analysis * | |||
| HR (95% CI) | p-Value | HR (95% CI) | p-Value | |
| Sex | ||||
| Male | 1 | |||
| Female | 0.97 (0.70–1.35) | 0.866 | ||
| Age at diagnosis | ||||
| <60 | 1 | |||
| >60 | 1.17 (0.88–1.56) | 0.284 | ||
| Body mass index (continuous) | 0.93 (0.90–0.97) | <0.001 | 0.96 (0.92–1.00) | 0.048 |
| Smoking status | ||||
| Former/never | 1 | 1 | ||
| Current | 1.55 (1.13–2.13) | 0.006 | 1.19 (0.82–1.72) | 0.359 |
| Alcohol consumption | ||||
| Former or never | 1 | 1 | ||
| Current | 1.83 (1.37–2.45) | <0.001 | 1.52 (1.10–2.12) | 0.011 |
| Primary site | ||||
| Oropharynx | 1 | |||
| Oral cavity | 1.16 (0.87–1.55) | 0.308 | ||
| Tumor grade | ||||
| G 1/2 | 1 | |||
| G 3/4 | 0.98 (0.73–1.30) | 0.862 | ||
| Tumor stage | ||||
| T 1/2 | 1 | 1 | ||
| T 3/4 | 1.91 (1.41–2.59) | <0.001 | 1.04 (0.71–1.53) | 0.845 |
| Nodal involvement | ||||
| N0 | 1 | |||
| N+ | 1.13 (0.77–1.65) | 0.53 | ||
| UICC stage | ||||
| I | 1 | |||
| II | 0.65 (0.17–2.51) | 0.529 | ||
| III | 0.91 (0.28–2.96) | 0.87 | ||
| IV | 1.20 (0.38–3.76) | 0.759 | ||
| Primary treatment | ||||
| Radio(chemo-) therapy | 1 | 1 | ||
| Surgery | 0.42 (0.31–0.56) | <0.001 | 0.44 (0.30–0.64) | <0.001 |
| Induction chemotherapy | ||||
| No | 1 | |||
| Yes | 1.40 (0.97–2.01) | 0.073 | ||
| Concomitant chemotherapy | ||||
| No | 1 | |||
| Yes | 1.05 (0.78–1.41) | 0.746 | ||
| Pre-treatment | ||||
| AST/ALR ratio (continuous) | 1.69 (1.41–2.02) | <0.001 | 1.42 (1.14–1.77) | 0.002 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Knittelfelder, O.; Delago, D.; Jakse, G.; Reinisch, S.; Partl, R.; Stranzl-Lawatsch, H.; Renner, W.; Langsenlehner, T. The AST/ALT (De Ritis) Ratio Predicts Survival in Patients with Oral and Oropharyngeal Cancer. Diagnostics 2020, 10, 973. https://doi.org/10.3390/diagnostics10110973
Knittelfelder O, Delago D, Jakse G, Reinisch S, Partl R, Stranzl-Lawatsch H, Renner W, Langsenlehner T. The AST/ALT (De Ritis) Ratio Predicts Survival in Patients with Oral and Oropharyngeal Cancer. Diagnostics. 2020; 10(11):973. https://doi.org/10.3390/diagnostics10110973
Chicago/Turabian StyleKnittelfelder, Olivia, Daniela Delago, Gabi Jakse, Sabine Reinisch, Richard Partl, Heidi Stranzl-Lawatsch, Wilfried Renner, and Tanja Langsenlehner. 2020. "The AST/ALT (De Ritis) Ratio Predicts Survival in Patients with Oral and Oropharyngeal Cancer" Diagnostics 10, no. 11: 973. https://doi.org/10.3390/diagnostics10110973
APA StyleKnittelfelder, O., Delago, D., Jakse, G., Reinisch, S., Partl, R., Stranzl-Lawatsch, H., Renner, W., & Langsenlehner, T. (2020). The AST/ALT (De Ritis) Ratio Predicts Survival in Patients with Oral and Oropharyngeal Cancer. Diagnostics, 10(11), 973. https://doi.org/10.3390/diagnostics10110973







