Univariate Analysis of Short-Chain Fatty Acids Related to Sudden Infant Death Syndrome
Abstract
1. Introduction
2. Materials and Methods
- (1)
- Case-control study;
- (2)
- Short-chain fatty acids profile for cases and controls;
- (3)
- SIDS related study.
2.1. Data Description
2.2. Data Availability
2.3. Data Preprocessing
2.4. Generalized Linear Model
2.5. Validation
3. Experiments and Results
4. Discussion and Conclusions
- This case-control study was performed using a dataset from the University of Michigan, in the Boston children’s Hospital. From the literature, we are aware that NBS differ methodologically and in the disorders screened worldwide, meaning that this study can not be generalized onto the worldwide population. Several factors must be considered when the results are generalized, among them the profiles of SCFAs that were obtained to perform the data-set, which were postmortem. Thus, it is unknown if the found values fluctuate as the postmortem interval increases, and therefore whether they differ or not from a living subject. It is also recognized that race could also influence these values, since it has been described in various investigations that the prevalence is higher in certain race groups as opposed to the white race. In addition to racial characteristics, environmental influence, population lifestyles, access to health services (prenatal care, childbirth care, well-child care), socioeconomic status, among many other factors that vary according to the population, the territory must also be considered, which can directly and indirectly affect the appearance of SIDS;
- As described in Section 2, this study is comprised of 18 subjects, which is prone to overfitting even when a blind-test approach is performed to validate experimentation. As this is a small number of cases, valuable features for case identification could be excluded. One of them is that most of the study subjects were close to one year of age, so there could be a risk of overfitting and not identifying younger patients, since a higher range of risk has been identified in the literature, which is between the ages of 2 and 8 months, data that varies according to the authors, this age could mark significant differences in SCFAs levels according to weeks of life;
- As presented in Section 3, the SCFAs profile gives us insights to possible SIDS complications, however, we are aware that SIDS is a complex, multifactorial disorder, which can be influenced by other risk factors, it being a disease described as a syndrome and remembering that the concept refers to a set of signs and symptoms that characterize a disease, so the absence or presence of a particular sign or symptom is not decisive for suffering it. However, since several symptoms or signs are present in a patient, they have become relevant for their study. Until now, the most significant factors that describe this pathology have not been identified. Thus, long lists grouped into genetic or inheritance factors, maternal factors, environmental factors, and newborn factors can be found, just to mention some. It has been shown that each of them could intervene in the presentation of SIDS;
- Therefore, other clinical data from patients is not available in this study and can influence the results. Among which stands out the way of obtaining the product of conception, that is, by delivery or cesarean, which when compared will have different types of bacterial colonization, and remember that the production of SCFAs depends largely on the fermentation of food by part of the bacteria in the colon. This is also influenced by the type of feeding, whether exclusively breastfeeding, supplementary, or combinations of both. In the same sequence of ideas, the age of ablation and weaning have an important role in variations in the gut microbiota among newborns.
Author Contributions
Funding
Conflicts of Interest
References
- Bajanowski, T.; Brinkmann, B.; Vennemann, M. The San Diego definition of SIDS: Practical application and comparison with the GeSID classification. Int. J. Leg. Med. 2006, 120, 331–336. [Google Scholar] [CrossRef]
- Shipstone, R.; Young, J.T.J.; Byard, R. An evaluation of pathologists’ application of the diagnostic criteria from the San Diego definition of SIDS and unclassified sudden infant death. Int. J. Leg. Med. 2019. [Google Scholar] [CrossRef]
- Oyarzún, M.; Brockmann Veloso, P. Pediatric Respiratory Diseases. Sudden Infant Death Syndrome, 1st ed.; Pediatric Respiratory Diseases; Springer: Cham, Switzerland, 2020; pp. 495–500. [Google Scholar]
- Krous, H. Sudden Infant Death Syndrome and Unclassified Sudden Infant Deaths: A Definitional and Diagnostic Approach. Pediatrics 2004, 114, 234–238. [Google Scholar] [CrossRef]
- CDC. Data and Statistics for SIDS and SUID|CDC, 2019. Available online: https://www.cdc.gov/sids/data.htm (accessed on 25 March 2020).
- Informativo, P. Uno de cada dos mil bebés fallece por la llamada muerte de cuna. Technical Report, 2016. Available online: https://planoinformativo.com/449892/uno-de-cada-dos-mil-bebes-fallece-por-la-llamada-imuerte-de-cunai-slp (accessed on 1 December 2019).
- Flores-Huerta, S. Síndrome de muerte súbita del lactante. Prevención en la práctica hospitalaria. Rev. Med. Inst. Mex. Seguro Soc. 2006, 44, 511–518. [Google Scholar]
- Bryant, V.; Sebire, N. Natural Diseases Causing Sudden Death in Infancy and Early Childhood, Sudden Infant and Early Childhood Death: The Past, the Present and the Future, 1st ed.; University of Adelaide Press: Adelaide, Australia, 2018; pp. 539–588. [Google Scholar]
- Goldstein, R.; Blair, P.; Sens, M.; Shapiro-Mendoza, C.; Krous, H.; Rognum, T.; Moon, R. Inconsistent classification of unexplained sudden deaths in infants and children hinders surveillance, prevention and research: Recommendations from The 3rd International Congress on Sudden Infant and Child Death. Forensic Sci. Med. Pathol. 2019, 15, 622–628. [Google Scholar] [CrossRef]
- Fu, L.; Moon, R. Apparent Life-threatening Events (ALTEs) and the Role of Home Monitors. Pediatr. Rev. 2007, 28, 203–208. [Google Scholar] [CrossRef] [PubMed]
- Carlin, R.; Moon, R. Risk Factors, Protective Factors, and Current Recommendations to Reduce Sudden Infant Death Syndrome: A Review. JAMA Pediatr. 2017, 171, 175–180. [Google Scholar] [CrossRef] [PubMed]
- Zubov, L.; Bolganov, Y.; Valkov, A. Sindrom Vnezapnoy Detskoy Smerti. Med. Neotdozhiykh Sostoyaniy 2007, 2, 114–124. [Google Scholar]
- Liu, X.; Takeuchi, K.; Ogunfunmi, T.; Mathapathi, S. Video-based IoT baby monitor for SIDS prevention. In Proceedings of the 2017 IEEE Global Humanitarian Technology Conference (GHTC), San Jose, CA, USA, 19–22 October 2017; pp. 1–7. [Google Scholar]
- Jayatilleka, I.; Halgamuge, M.N. Chapter 1—Internet of Things in healthcare: Smart devices, sensors, and systems related to diseases and health conditions. In Real-Time Data Analytics for Large Scale Sensor Data; Advances in Ubiquitous Sensing Applications for Healthcare; Das, H., Dey, N., Balas, V.E., Eds.; Academic Press: New York, NY, USA, 2020; Volume 6, pp. 1–35. [Google Scholar] [CrossRef]
- Kroll, M.; Kurinczuk, J.Q.M.; Hollowell, J. Ethnic disparity in risk of SIDS and other unexplained infant death is not due to deprivation; examining ethnic patterns may help to clarify aetiology. J. Epidemiol. Community Health 2018, 72, 911–918. [Google Scholar]
- Mitterauer, B. The gliocentric hypothesis of the pathophysiology of the sudden infant death syndrome (SIDS). Med. Hypotheses 2011, 76, 482–485. [Google Scholar] [CrossRef]
- Moon, R. SIDS and Other Sleep-Related Infant Deaths: Expansion of Recommendations for a Safe Infant Sleeping Environment. Pediatrics 2011, 128, 1030–1039. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, M. SIDS—A developmental neurological perspective. Early Human Development. Early Hum. Dev. 1993, 34, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Harpey, J.; Charpentier, C.; Paturneau-Jouas, M. Sudden Infant Death Syndrome and Inherited Disorders of Fatty Acid p-Oxidation. Neonatology 1990, 58, 70–80. [Google Scholar] [CrossRef] [PubMed]
- Álvarez Fumero, R. ¿Son los errores congénitos del metabolismo causa prevenible de muerte súbita? Rev. Cuba. Pediatra 2004, 76, 0–0. [Google Scholar]
- Chavez-Ocaña, S.; Bravata-Alcantara, J.; Sierra-Martinez, M. Errores innatos del metabolismo, una mirada a un tópico poco valorado. Revista del Hospital Juárez de México 2018, 85, 159–167. [Google Scholar]
- Kumta, N. Inborn errors of metabolism (IEM)—An Indian perspective. Indian J. Pediatr. 2005, 72, 325–332. [Google Scholar] [CrossRef]
- Gill, P.; van Zelm, M.; Muir, J.; Gibson, P. Review article: Short chain fatty acids as potential therapeutic agents in human gastrointestinal and inflammatory disorders. Aliment. Pharmacol. Ther. 2018, 48, 15–34. [Google Scholar] [CrossRef]
- Jorda Lope, A. Libro Blanco de la Muerte Subita Infantil, 3th ed.; Grupo de Trabajo de Muerte Súbita Infantil-AEP: Madrid, España, 2013; pp. 149–158. ISBN 978-84-15351-90-0. [Google Scholar]
- Villoria, J.G.; Pajares, S.; López, R.M.; Marin, J.L.; Ribes, A. Neonatal screening for inherited metabolic diseases in 2016. In Proceedings of the Seminars in Pediatric Neurology; Elsevier: Amsterdam, The Netherlands, 2016; Volume 23, pp. 257–272. [Google Scholar]
- Ogier de Baulny, H. Management and emergency treatments of neonates with a suspicion of inborn errors of metabolism. Semin. Neonatol. 2002, 7, 17–26. [Google Scholar] [CrossRef]
- Ibdah, J. Fatty Acid Oxidation Defects as a Cause of SIDS, 2001. Available online: https://www.sleepreviewmag.com/sleep-health/prevailing-attitude/sleep-safety/fatty-acid-oxidation-defects-as-a-cause-of-sids/ (accessed on 26 March 2020).
- Kononenko, I. Machine learning for medical diagnosis: History, state of the art and perspective. Artif. Intell. Med. 2001, 23, 89–109. [Google Scholar] [CrossRef]
- Rodríguez-Ruiz, J.G.; Galván-Tejada, C.E.; Zanella-Calzada, L.A.; Celaya-Padilla, J.M.; Galván-Tejada, J.I.; Gamboa-Rosales, H.; Luna-García, H.; Magallanes-Quintanar, R.; Soto-Murillo, M.A. Comparison of Night, Day and 24 h Motor Activity Data for the Classification of Depressive Episodes. Diagnostics 2020, 10, 162. [Google Scholar] [CrossRef]
- Alcalá-Rmz, V.; Zanella-Calzada, L.A.; Galván-Tejada, C.E.; García-Hernández, A.; Cruz, M.; Valladares-Salgado, A.; Galván-Tejada, J.I.; Gamboa-Rosales, H. Identification of diabetic patients through clinical and para-clinical features in Mexico: An approach using deep neural networks. Int. J. Environ. Res. Public Health 2019, 16, 381. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Ceja, E.; Osmani, V.; Mayora, O. Automatic stress detection in working environments from smartphones’ accelerometer data: A first step. IEEE J. Biomed. Health Inform. 2015, 20, 1053–1060. [Google Scholar] [CrossRef]
- Oriol, J.D.V.; Vallejo, E.E.; Estrada, K.; Peña, J.G.T. The Alzheimer’s Disease Neuroimaging Initiative Benchmarking machine learning models for late-onset alzheimer’s disease prediction from genomic data. BMC Bioinform. 2019, 20, 1–17. [Google Scholar]
- Kokol, P.; Završnik, J.; Blažun Vošner, H. Artificial intelligence and pediatrics: A synthetic mini review. Pediatr. Dimens. 2017, 2, 1–5. [Google Scholar] [CrossRef]
- Mueller, M.; Almeida, J.S.R.; Wagner, C. Can Machine Learning Methods Predict Extubation Outcome in Premature Infants as well as Clinicians? J. Neonatal Biol. 2013, 2, 1–6. [Google Scholar] [CrossRef]
- Wadhwani, S.I.; Hsu, E.K.; Shaffer, M.L.; Anand, R.; Ng, V.L.; Bucuvalas, J.C. Predicting ideal outcome after pediatric liver transplantation: An exploratory study using machine learning analyses to leverage Studies of Pediatric Liver Transplantation Data. Pediatr. Transplant. 2019, 23, e13554. [Google Scholar] [CrossRef]
- NIH Common Fund’s National Metabolomics Data Repository (NMDR) Website, t.M.W. SCFA Analysis in SIDS, Project ID ST000512. 2017. Available online: https://www.metabolomicsworkbench.org/data/DRCCMetadata.php?Mode=ProjectProjectID=PR000512 (accessed on 12 December 2019).
- Galal, M.; Symonds, I.; Murray, H.; Petraglia, F.; Smith, R. Postterm pregnancy. Facts Views Vis. ObGyn 2012, 4, 175–187. [Google Scholar] [PubMed]
- Joshi, A.V. Machine Learning and Artificial Intelligence, 1st ed.; Springer International Publishing: Berlin/Heidelberg, Germany, 2020. [Google Scholar]
- Altman, D.G.; Bland, J.M. Diagnostic tests. 1: Sensitivity and specificity. BMJ Br. Med. J. 1994, 308, 1552. [Google Scholar] [CrossRef]
- Hajian-Tilaki, K. Receiver operating characteristic (ROC) curve analysis for medical diagnostic test evaluation. Casp. J. Intern. Med. 2013, 4, 627. [Google Scholar]
- Nardo, L.G.; Gelbaya, T.A.; Wilkinson, H.; Roberts, S.A.; Yates, A.; Pemberton, P.; Laing, I. Circulating basal anti-Müllerian hormone levels as predictor of ovarian response in women undergoing ovarian stimulation for in vitro fertilization. Fertil. Steril. 2009, 92, 1586–1593. [Google Scholar] [CrossRef]
- Yip, S.S.; Kim, J.; Coroller, T.P.; Parmar, C.; Velazquez, E.R.; Huynh, E.; Mak, R.H.; Aerts, H.J. Associations between somatic mutations and metabolic imaging phenotypes in non—Small cell lung cancer. J. Nucl. Med. 2017, 58, 569–576. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, A.R.; Ferreri, C.; Ruggiero, S.; Deplano, S.; Sunda, V.; Galloro, G.; Formisano, C.; Faraone Mennella, M.R. Automodification of PARP and fatty acid-based membrane lipidome as a promising integrated biomarker panel in molecular medicine. Biomark. Med. 2016, 10, 229–242. [Google Scholar] [CrossRef] [PubMed]
- Caminha, T.C.; Ferreira, H.S.; Costa, N.S.; Nakano, R.P.; Carvalho, R.E.S.; Xavier, A.F., Jr.; Assunção, M.L. Waist-to-height ratio is the best anthropometric predictor of hypertension: A population-based study with women from a state of northeast of Brazil. Medicine 2017, 96, e5874. [Google Scholar] [CrossRef]
- Mandrekar, J.N. Receiver operating characteristic curve in diagnostic test assessment. J. Thorac. Oncol. 2010, 5, 1315–1316. [Google Scholar] [CrossRef] [PubMed]
- Schieda, N.; Dilauro, M.; Moosavi, B.; Hodgdon, T.; Gregory, O.C.; McInnes, M.D.F.; Flood, T.A. MRI evaluation of small (<4cm) solid renal masses: Multivariate modeling improves diagnostic accuracy for angiomyolipoma without visible fat compared to univariate analysis. Eur. Radiol. 2015, 26, 2242–2251. [Google Scholar] [CrossRef]
- Wu, J.; Gong, G.; Cui, Y.; Li, R. Intratumor partitioning and texture analysis of dynamic contrast-enhanced (DCE)-MRI identifies relevant tumor subregions to predict pathological response of breast cancer to neoadjuvant chemotherapy. J. Magn. Reson. Imaging 2016, 44, 1107–1115. [Google Scholar] [CrossRef] [PubMed]
- Asllani, I.; Habeck, C.; Scarmeas, N.; Borogovac, A.; Brown, T.R.; Stern, Y. Multivariate and univariate analysis of continuous arterial spin labeling perfusion MRI in Alzheimer’s disease. J. Cereb. Blood Flow Metab. 2008, 28, 725–736. [Google Scholar] [CrossRef]
- El-Hattab, A.W.; Sutton, V.R. Approach to inborn errors of metabolism in pediatrics. Pediatr. Clin. 2018, 65. [Google Scholar] [CrossRef]
- Optiz, E.; Gilbert-Barness, D.E.; Spicer, T.S.S. Handbook of Pediatric Autopsy Pathology, 2nd ed.; Springer: New York, NY, USA, 2013; pp. 653–673. [Google Scholar]
- Byard, R.W.; Shipstone, R.A.; Young, J. Continuing major inconsistencies in the classification of unexpected infant deaths. J. Forensic Leg. Med. 2019, 64, 20–22. [Google Scholar] [CrossRef]
- Loeber, J.G. Neonatal screening in Europe; the situation in 2004. J. Inherit. Metab. Dis. 2007, 30, 430–438. [Google Scholar] [CrossRef]
- Wilcken, B.; Wiley, V.; Hammond, J.; Carpenter, K. Screening newborns for inborn errors of metabolism by tandem mass spectrometry. N. Engl. J. Med. 2003, 348, 2304–2312. [Google Scholar] [CrossRef]
- Takiishi, T.; Fenero, C.I.M.; Câmara, N.O.S. Intestinal barrier and gut microbiota: Shaping our immune responses throughout life. Tissue Barriers 2017, 5, e1373208. [Google Scholar] [CrossRef]
- Dai, X.; Yuan, T.; Zhang, X.; Zhou, Q.; Bi, H.; Yu, R.; Wei, W.; Wang, X. Short-chain fatty acid (SCFA) and medium-chain fatty acid (MCFA) concentrations in human milk consumed by infants born at different gestational ages and the variations in concentration during lactation stages. Food Funct. 2020, 11, 1869–1880. [Google Scholar] [CrossRef]
| Feature | |
|---|---|
| 1 | Postmortem interval PMI hours |
| 2 | Gestational age weeks |
| 3 | Postnatal age weeks |
| 4 | Isovaleric acid |
| 5 | Octanoic acid |
| 6 | Propionic acid |
| 7 | Isobutyric acid |
| 8 | Butyric acid |
| 9 | Hexanoic acid |
| 10 | Valeric acid |
| 11 | Acetic acid |
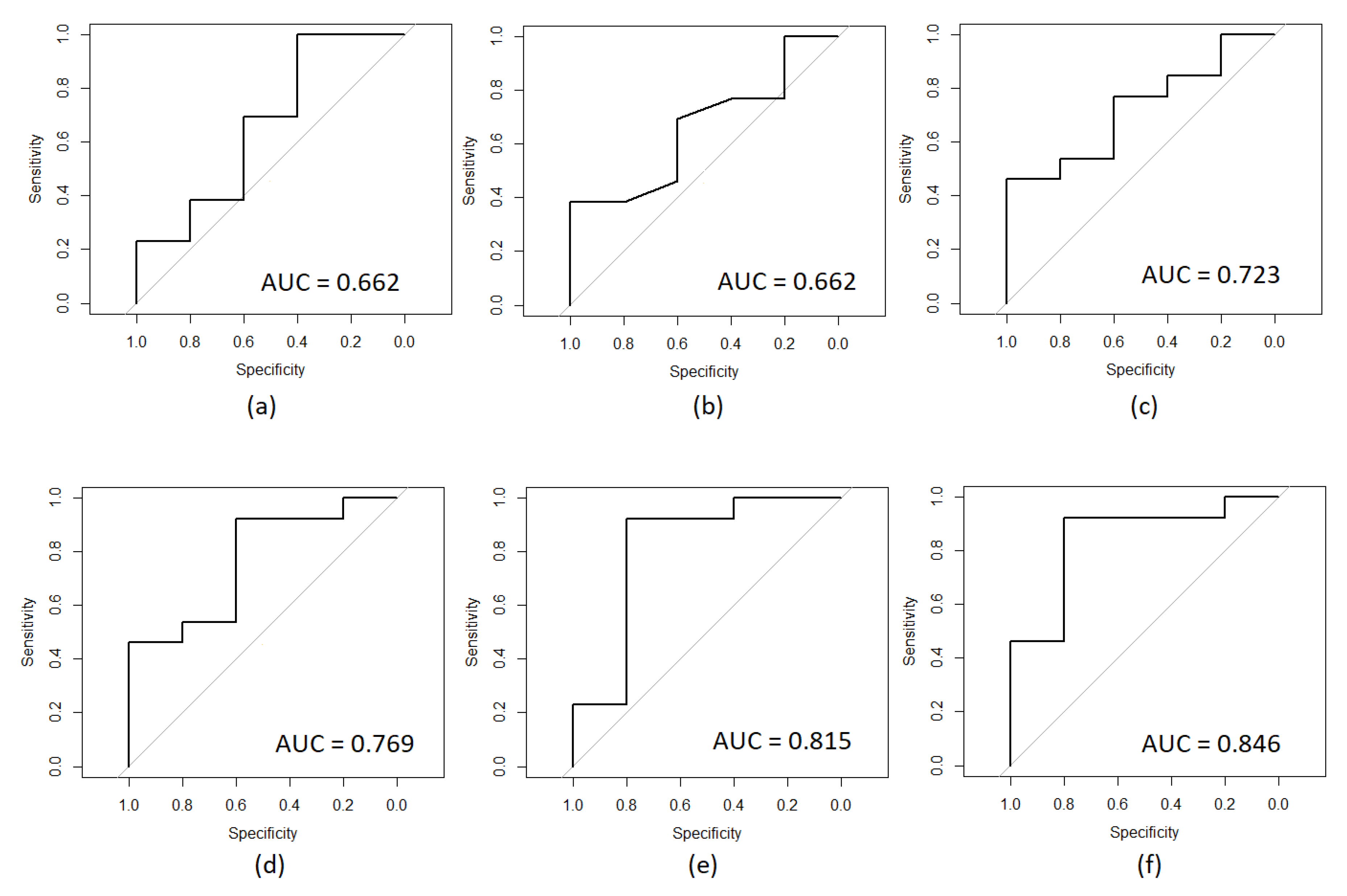
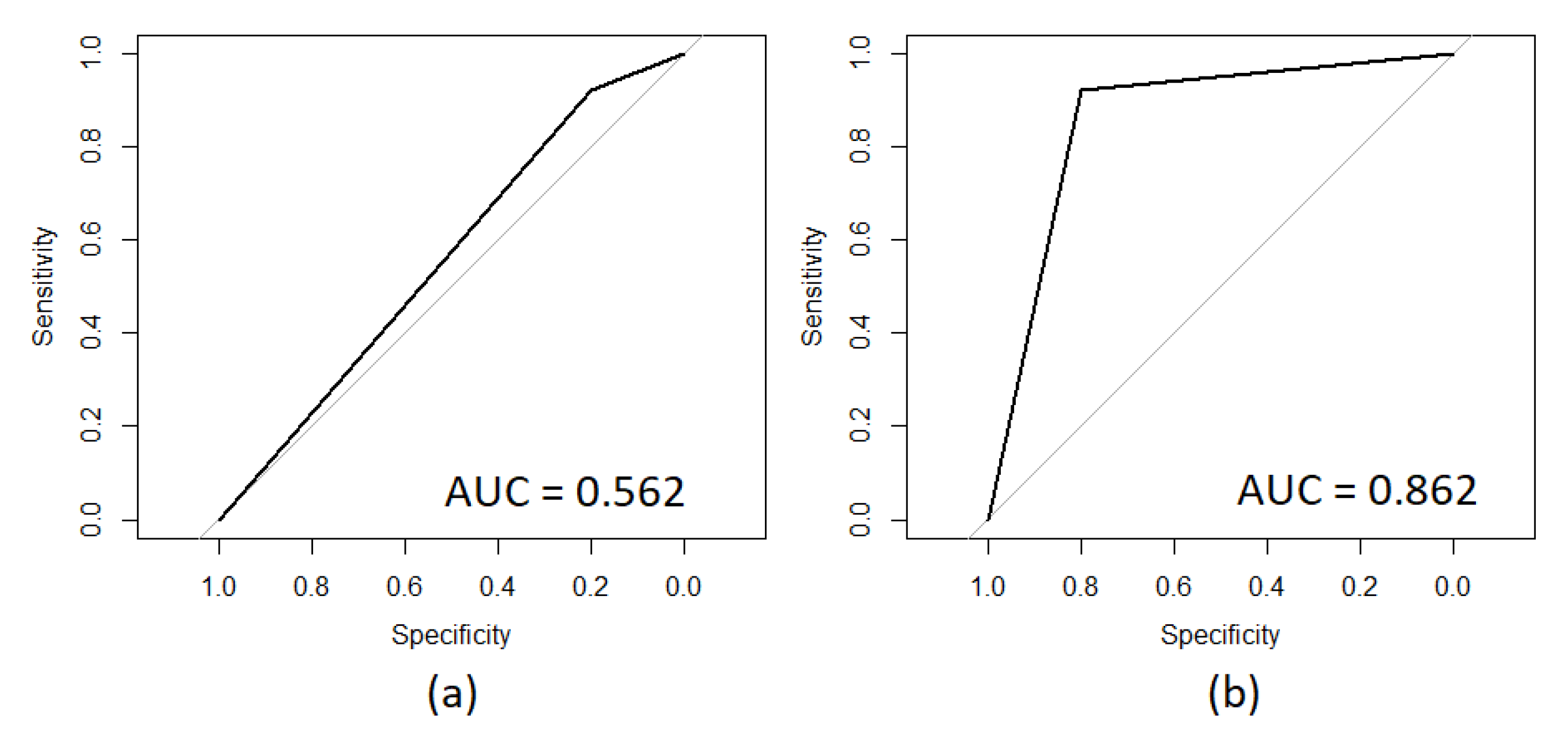
| Feature | AUC Value |
|---|---|
| Isovaleric acid | 0.508 |
| Octanoic acid | 0.538 |
| Gestational age weeks | 0.592 |
| Postmortem interval PMI hours | 0.600 |
| Propionic acid | 0.646 |
| Isobutyric acid | 0.662 |
| Postnatal age weeks | 0.662 |
| Butyric acid | 0.723 |
| Hexanoic acid | 0.769 |
| Valeric acid | 0.815 |
| Acetic acid | 0.846 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Galván-Tejada, C.E.; Villagrana-Bañuelos, K.E.; Zanella-Calzada, L.A.; Moreno-Báez, A.; Luna-García, H.; Celaya-Padilla, J.M.; Galván-Tejada, J.I.; Gamboa-Rosales, H. Univariate Analysis of Short-Chain Fatty Acids Related to Sudden Infant Death Syndrome. Diagnostics 2020, 10, 896. https://doi.org/10.3390/diagnostics10110896
Galván-Tejada CE, Villagrana-Bañuelos KE, Zanella-Calzada LA, Moreno-Báez A, Luna-García H, Celaya-Padilla JM, Galván-Tejada JI, Gamboa-Rosales H. Univariate Analysis of Short-Chain Fatty Acids Related to Sudden Infant Death Syndrome. Diagnostics. 2020; 10(11):896. https://doi.org/10.3390/diagnostics10110896
Chicago/Turabian StyleGalván-Tejada, Carlos E., Karen E. Villagrana-Bañuelos, Laura A. Zanella-Calzada, Arturo Moreno-Báez, Huizilopoztli Luna-García, Jose M. Celaya-Padilla, Jorge I. Galván-Tejada, and Hamurabi Gamboa-Rosales. 2020. "Univariate Analysis of Short-Chain Fatty Acids Related to Sudden Infant Death Syndrome" Diagnostics 10, no. 11: 896. https://doi.org/10.3390/diagnostics10110896
APA StyleGalván-Tejada, C. E., Villagrana-Bañuelos, K. E., Zanella-Calzada, L. A., Moreno-Báez, A., Luna-García, H., Celaya-Padilla, J. M., Galván-Tejada, J. I., & Gamboa-Rosales, H. (2020). Univariate Analysis of Short-Chain Fatty Acids Related to Sudden Infant Death Syndrome. Diagnostics, 10(11), 896. https://doi.org/10.3390/diagnostics10110896








