Delta-Like Canonical Notch Ligand 1 in Patients Following Liver Transplantation—A Secondary Analysis of a Prospective Cohort Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
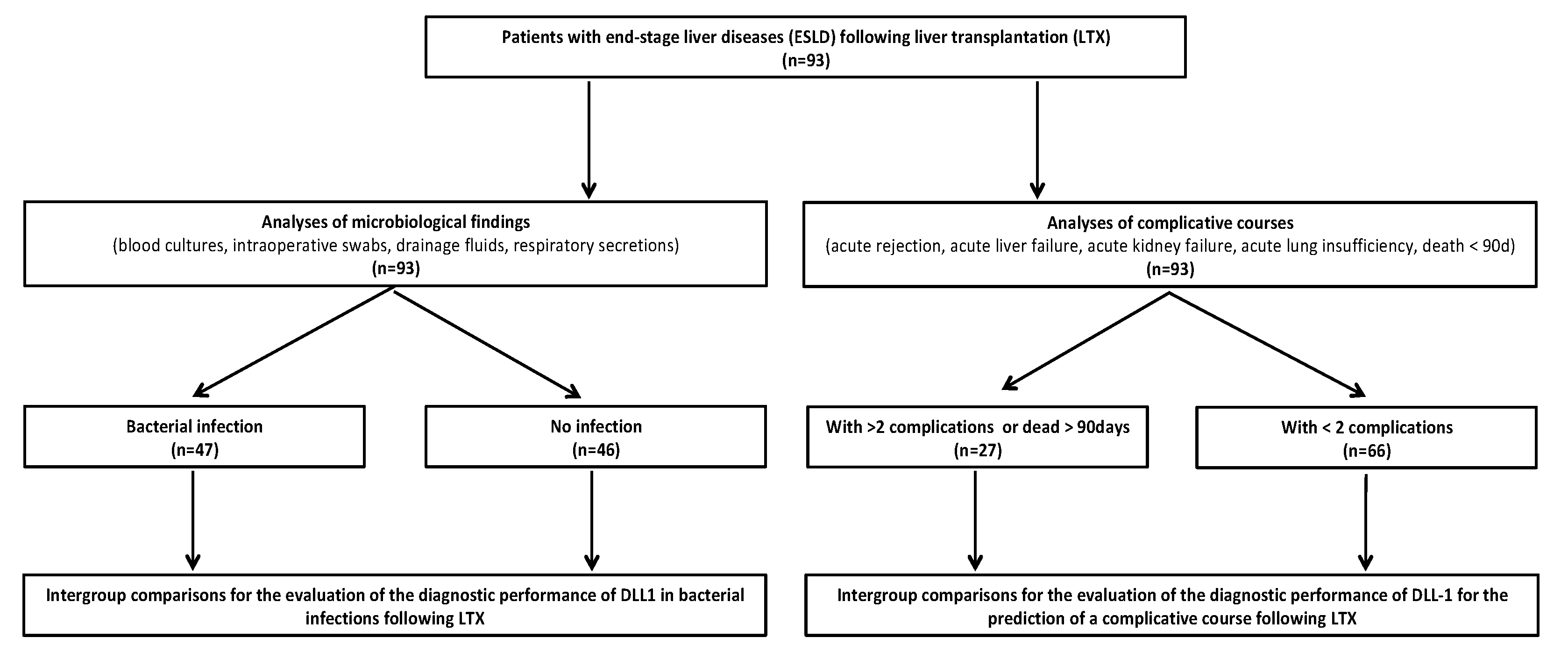
2.2. Subgroup Definitions
2.3. Measurement of DLL1
2.4. Statistical Analyses
3. Results
3.1. Patients’ Characteristics
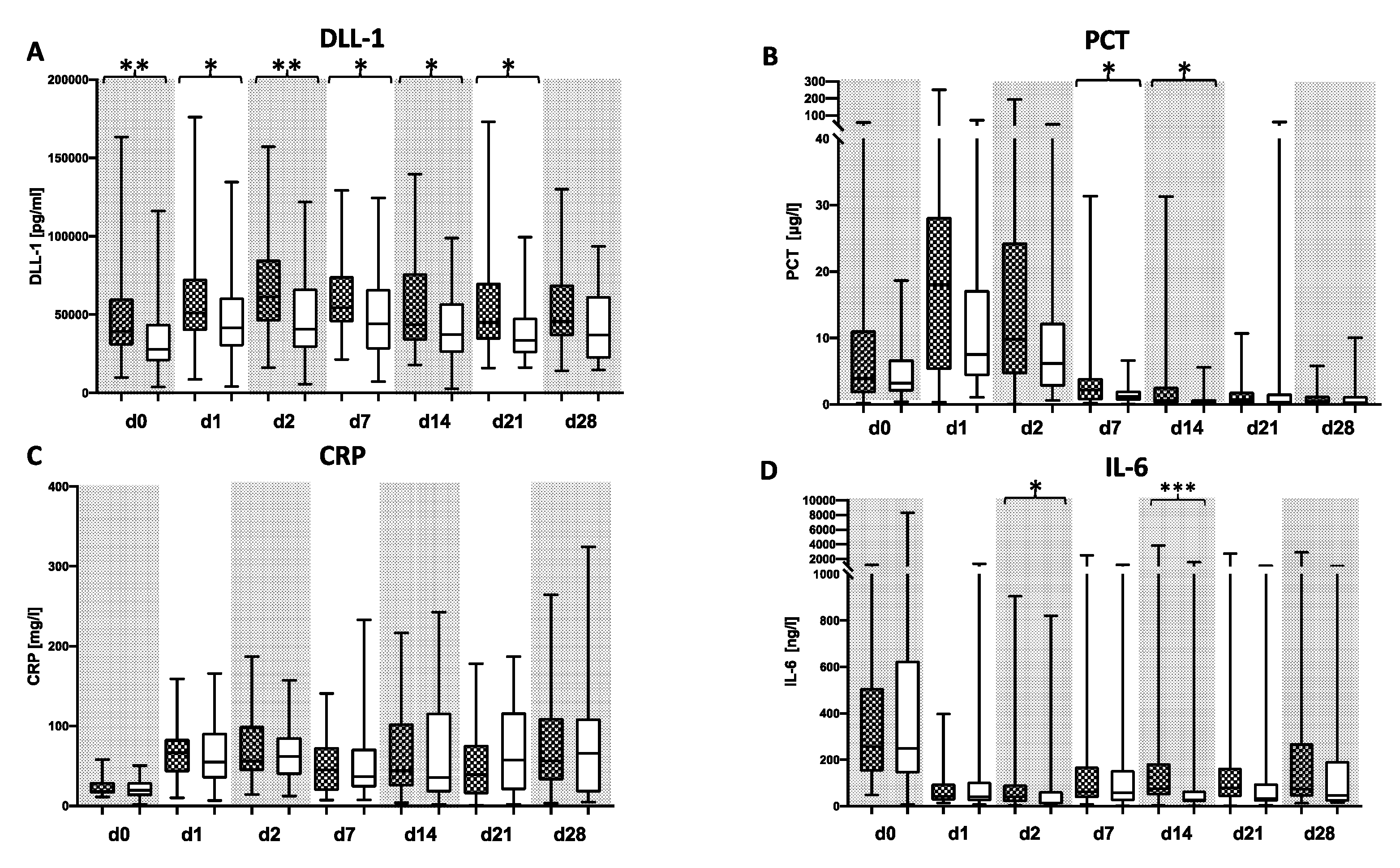
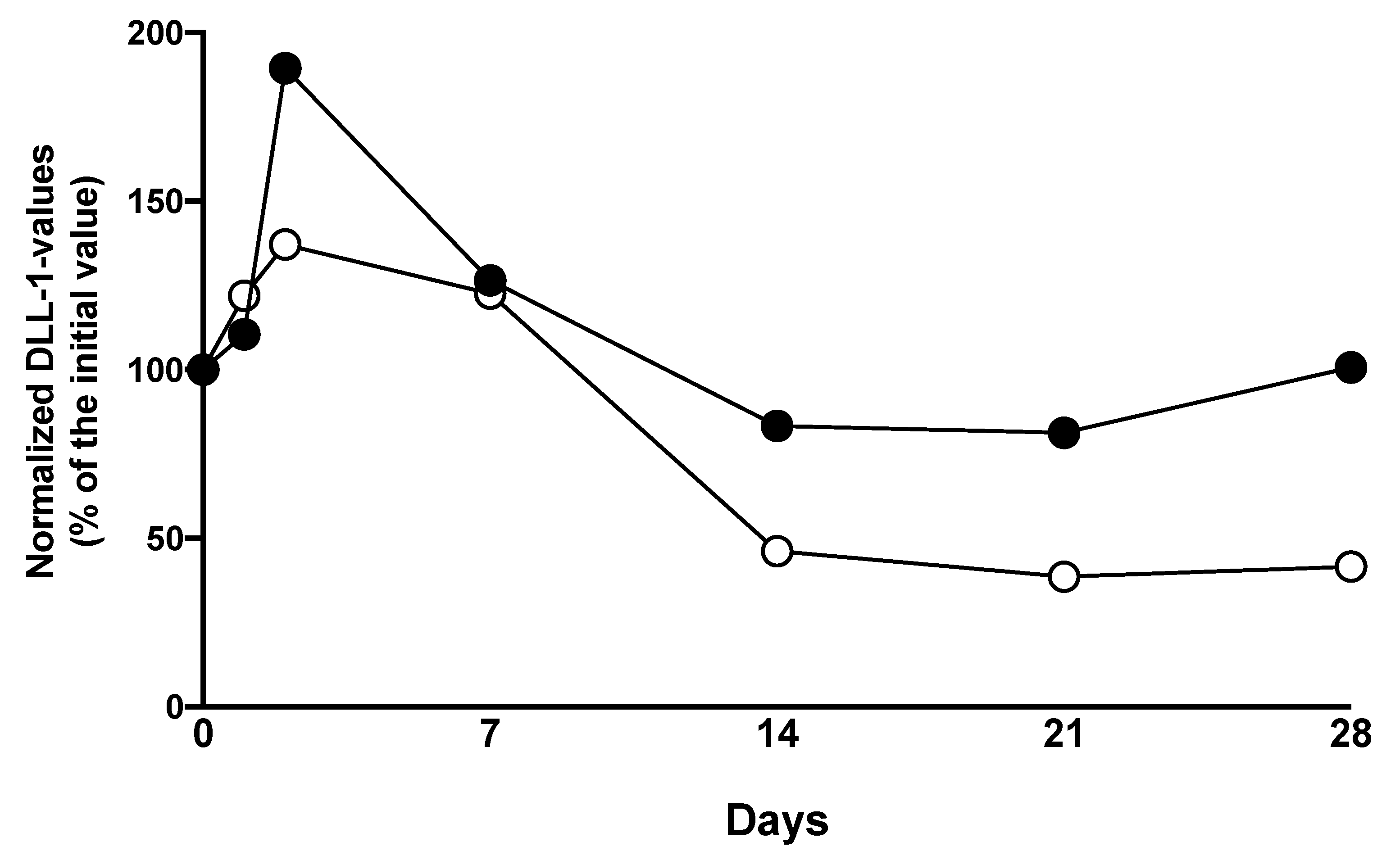
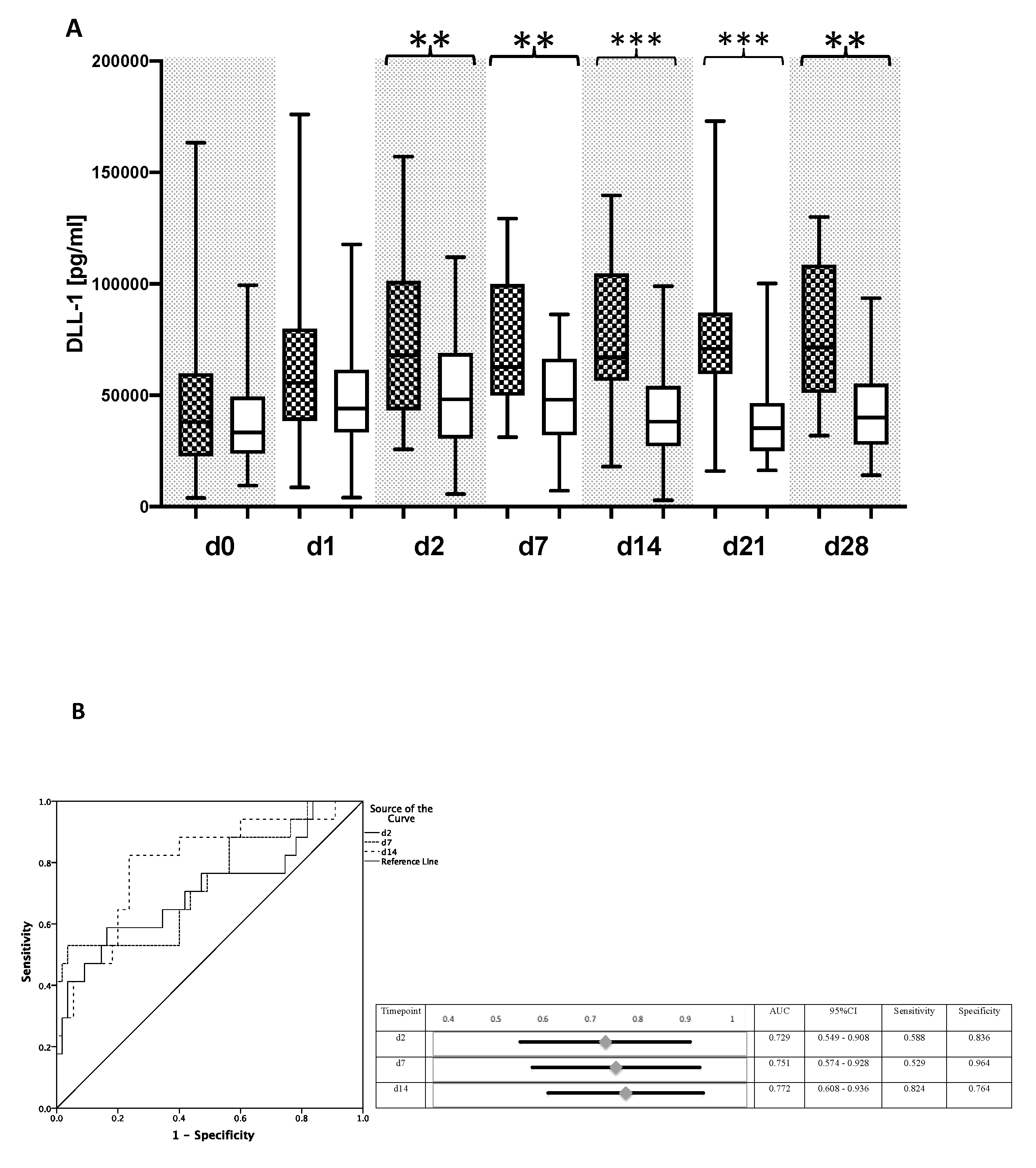
3.2. DLL1 and PCT for the Detection of Bacterial Infection after LTX
3.3. DLL1 for the Stratification of Patients with High Postoperative Risk
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Starzl, T.E.; Marchioro, T.L.; Von Kaulla, K.N.; Hermann, G.; Brittain, R.S.; Waddell, W.R. Homotransplantation of the Liver in Humans. Surg. Gynecol. Obstet. 1963, 117, 659–676. [Google Scholar] [PubMed]
- Trunečka, P. Immunosuppression after liver transplant, now and in future. Vnitr. Lek. 2013, 59, 671–677. [Google Scholar] [PubMed]
- Onghena, L.; Develtere, W.; Poppe, C.; Geerts, A.; Troisi, R.; Vanlander, A.; Berrevoet, F.; Rogiers, X.; Van Vlierberghe, H.; Verhelst, X. Quality of life after liver transplantation: State of the art. World J. Hepatol. 2016, 8, 749–756. [Google Scholar] [CrossRef] [PubMed]
- Van Delden, C.; Stampf, S.; Hirsch, H.H.; Manuel, O.; Meylan, P.; Cusini, A.; Hirzel, C.; Khanna, N.; Weisser, M.; Garzoni, C.; et al. Burden and Timeline of Infectious Diseases in the First Year After Solid Organ. Transplantation in the Swiss Transplant. Cohort Study. Clin. Infect. Dis. 2020, 71, e159–e169. [Google Scholar] [CrossRef] [PubMed]
- Ayvazoglu Soy, E.H.; Akdur, A.; Yildirim, S.; Arslan, H.; Haberal, M. Early Postoperative Infections after Liver Transplant. Exp. Clin. Transplant. 2018, 16 (Suppl. S1), 145–148. [Google Scholar] [PubMed]
- Biron, B.M.; Ayala, A.; Lomas-Neira, J.L. Biomarkers for Sepsis: What is and What Might Be? Biomark. Insights 2015, 10 (Suppl. S4), 7–17. [Google Scholar] [CrossRef] [PubMed]
- Gür, A.; Oğuztürk, H.; Köse, A.; Turtay, M.G.; Ersan, V.; Bayindir, Y.; Ince, V.; Gurbuz, S.; Yucel, N. Prognostic Value of Procalcitonin, CRP, Serum Amyloid A, Lactate and IL-6 Markers in Liver Transplant Patients Admitted to ED with Suspected Infection. In Vivo 2017, 31, 1179–1185. [Google Scholar] [PubMed]
- Zant, R.; Melter, M.; Knoppke, B.; Ameres, M.; Kunkel, J. Kinetics of interleukin-6, procalcitonin, and C-reactive protein after pediatric liver transplantation. Transplant. Proc. 2014, 46, 3507–3510. [Google Scholar] [CrossRef] [PubMed]
- Agudo, R.L.; Grande, M.A.; Roux, P.D.; Martín, E.E.; Pérez, A.P.; Martín, G.L. Procalcitonin after liver transplantation. Kinetics and correlation with postoperative complications: 12AP5-10. Eur. J. Anaesthesiol. 2014, 31, 209. [Google Scholar] [CrossRef][Green Version]
- Hildebrand, D.; Uhle, F.; Sahin, D.; Krauser, U.; Weigand, M.A.; Heeg, K. The Interplay of Notch Signaling and STAT3 in TLR-Activated Human Primary Monocytes. Front. Cell. Infect. Microbiol. 2018, 8, 241. [Google Scholar] [CrossRef]
- Hildebrand, D.; Decker, S.O.; Koch, C.; Schmitt, F.C.F.; Ruhrmann, S.; Schneck, E.; Sander, M.; Weigand, M.A.; Brenner, T.; Heeg, K.; et al. Host-Derived Delta-Like Canonical Notch Ligand 1 as a Novel Diagnostic Biomarker for Bacterial Sepsis-Results From a Combinational Secondary Analysis. Front. Cell. Infect. Microbiol. 2019, 9, 267. [Google Scholar] [CrossRef]
- Decker, S.O.; Krüger, A.; Wilk, H.; Grumaz, S.; Vainshtein, Y.; Schmitt, F.C.F.; Uhle, F.; Bruckner, T.; Zimmermann, S.; Mehrabi, A.; et al. New approaches for the detection of invasive fungal diseases in patients following liver transplantation-results of an observational clinical pilot study. Langenbecks Arch. Surg. 2019, 404, 309–325. [Google Scholar] [CrossRef] [PubMed]
- Heidelberg University Hospital. Heidelberger Manual der Lebertransplantation, 2nd ed.; Heidelberg University Hospital: Heidelberg, Germany, 2006. [Google Scholar]
- Humar, A.; Michaels, M.; AST ID Working Group on Infectious Disease Monitoring. American Society of Transplantation recommendations for screening, monitoring and reporting of infectious complications in immunosuppression trials in recipients of organ transplantation. Am. J. Transplant. 2006, 6, 262–274. [Google Scholar] [CrossRef]
- Demetris, A.J.; Bellamy, C.; Hübscher, S.G.; O’Leary, J.; Randhawa, P.; Feng, S.; Neil, D.A.H.; Colvin, R.B.; McCaughan, G.; Fung, J.; et al. 2016 Comprehensive Update of the Banff Working Group on Liver Allograft Pathology: Introduction of Antibody-Mediated Rejection. Am. J. Transplant. 2016, 16, 2816–2835. [Google Scholar] [CrossRef]
- Khwaja, A. KDIGO clinical practice guidelines for acute kidney injury. Nephron Clin. Pract. 2012, 120, c179–c184. [Google Scholar] [CrossRef]
- Force, A.D.T.; Ranieri, V.M.; Rubenfeld, G.D.; Thompson, B.T.; Ferguson, N.D.; Caldwell, E.; Fan, E.; Camporota, L.; Slutsky, A.S. Acute respiratory distress syndrome: The Berlin Definition. JAMA 2012, 307, 2526–2533. [Google Scholar]
- Fishman, J.A. Infection in solid-organ transplant recipients. N. Engl. J. Med. 2007, 357, 2601–2614. [Google Scholar] [CrossRef]
- Kim, S.I. Bacterial infection after liver transplantation. World J. Gastroenterol. 2014, 20, 6211–6220. [Google Scholar] [CrossRef] [PubMed]
- Bagante, F.; Beal, E.; Tumin, D.; Azoulay, D.; Mumtaz, K.; Black, S.M.; Washburn, K.; Pawlik, T.M. Early mortality after liver transplantation: Defining the course and the cause. Surgery 2018, 164, 694–704. [Google Scholar] [CrossRef]
- Dellinger, R.P.; Levy, M.M.; Rhodes, A.; Annane, D.; Gerlach, H.; Opal, S.M.; Sevransky, J.E.; Sprung, C.L.; Douglas, I.S.; Jaeschke, R.; et al. Surviving sepsis campaign: International guidelines for management of severe sepsis and septic shock: 2012. Crit. Care Med. 2013, 41, 580–637. [Google Scholar] [CrossRef]
- Vincent, J.L.; Brealey, D.; Libert, N.; Abidi, N.E.; O’Dwyer, M.; Zacharowski, K.; Mikaszewska-Sokolewicz, M.; Schrenzel, J.; Simon, F.; Wilks, M.; et al. Rapid Diagnosis of Infection in the Critically Ill, a Multicenter Study of Molecular Detection in Bloodstream Infections, Pneumonia, and Sterile Site Infections. Crit. Care Med. 2015, 43, 2283–2291. [Google Scholar] [CrossRef] [PubMed]
- Rath, P.M.; Saner, F.; Paul, A.; Lehmann, N.; Steinmann, J.; Buer, J.; Steinmann, J. Multiplex PCR for rapid and improved diagnosis of bloodstream infections in liver transplant recipients. J. Clin. Microbiol. 2012, 50, 2069–2071. [Google Scholar] [CrossRef] [PubMed]
- Kita, Y.; Iwaki, Y.; Noguchi, K.; Tzakis, A.; Todo, S.; Fung, J.; Starzl, T. Daily serum inflammatory cytokine (tumor necrosis factor-alpha, interleukin-6) monitoring in liver transplantation focusing on allograft rejection: A five-case report. Transplant. Proc. 1996, 28, 1237–1240. [Google Scholar]
- Krishnasamy, K.; Limbourg, A.; Kapanadze, T.; Gamrekelashvili, J.; Beger, C.; Häger, C.; Lozanovski, V.J.; Falk, C.S.; Napp, L.C.; Bauersachs, J.; et al. Blood vessel control of macrophage maturation promotes arteriogenesis in ischemia. Nat. Commun. 2017, 8, 952. [Google Scholar] [CrossRef] [PubMed]
- Six, E.; Ndiaye, D.; Laâbi, Y.; Brou, C.; Gupta-Rossi, N.; Israël, A.; Logeat, F. The Notch ligand Delta1 is sequentially cleaved by an ADAM protease and gamma-secretase. Proc. Natl. Acad. Sci. USA 2003, 100, 7638–7643. [Google Scholar] [CrossRef]
- Dyczynska, E.; Sun, D.; Yi, H.; Sehara-Fujisawa, A.; Blobel, C.P.; Zolkiewska, A. Proteolytic processing of delta-like 1 by ADAM proteases. J. Biol. Chem. 2007, 282, 436–444. [Google Scholar] [CrossRef]
- Norum, H.M.; Michelsen, A.E.; Lekva, T.; Arora, S.; Otterdal, K.; Olsen, M.B.; Kong, X.Y.; Gude, E.; Andreassen, A.K.; Solbu, D.; et al. Circulating delta-like Notch ligand 1 is correlated with cardiac allograft vasculopathy and suppressed in heart transplant recipients on everolimus-based immunosuppression. Am. J. Transplant. 2019, 19, 1050–1060. [Google Scholar] [CrossRef]
- Kirk, A.D. Induction immunosuppression. Transplantation 2006, 82, 593–602. [Google Scholar] [CrossRef]
- Bush, W.W. Overview of transplantation immunology and the pharmacotherapy of adult solid organ transplant recipients: Focus on immunosuppression. AACN Clin. Issues 1999, 10, 253–269, quiz 304–306. [Google Scholar] [CrossRef]
- Moini, M.; Schilsky, M.L.; Tichy, E.M. Review on immunosuppression in liver transplantation. World J. Hepatol. 2015, 7, 1355–1368. [Google Scholar] [CrossRef]
- Staatz, C.E.; Tett, S.E. Pharmacology and toxicology of mycophenolate in organ transplant recipients: An update. Arch. Toxicol. 2014, 88, 1351–1389. [Google Scholar] [CrossRef]
- Neria, F.; Castilla, M.A.; Sanchez, R.F.; Pacheco, F.R.G.; Deudero, J.J.; Calabia, O.; Tejedor, A.; Manzarbeitia, F.; Ortiz, A.; Caramelo, C. Inhibition of JAK2 protects renal endothelial and epithelial cells from oxidative stress and cyclosporin A toxicity. Kidney Int. 2009, 75, 227–234. [Google Scholar] [CrossRef] [PubMed]
- Norum, H.M.; Gullestad, L.; Abraityte, A.; Broch, K.; Aakhus, S.; Aukrust, P.; Ueland, T. Increased Serum Levels of the Notch Ligand DLL1 are Associated with Diastolic Dysfunction, Reduced Exercise Capacity, and Adverse Outcome in Chronic Heart Failure. J. Card. Fail. 2016, 22, 218–223. [Google Scholar] [CrossRef]
- Norum, H.M.; Broch, K.; Michelsen, A.E.; Lunde, I.G.; Lekva, T.; Abraityte, A.; Dahl, C.P.; Fiane, A.E.; Andreassen, A.K.; Christensen, G.; et al. The Notch Ligands DLL1 and Periostin Are Associated with Symptom Severity and Diastolic Function in Dilated Cardiomyopathy. J. Cardiovasc. Transl. Res. 2017, 10, 401–410. [Google Scholar] [CrossRef] [PubMed]
- Berg, J.; Halvorsen, A.R.; Bengtson, M.-B.; Taskén, K.A.; Maelandsmo, G.M.; Yndestad, A.; Halvorsen, B.; Brustugun, O.T.; Aukrust, P.; Ueland, T.; et al. Levels and prognostic impact of circulating markers of inflammation, endothelial activation and extracellular matrix remodelling in patients with lung cancer and chronic obstructive pulmonary disease. BMC Cancer 2018, 18, 739. [Google Scholar] [CrossRef] [PubMed]
- Iida, T.; Kaido, T.; Yagi, S.; Yoshizawa, A.; Hata, K.; Mizumoto, M.; Mori, A.; Ogura, Y.; Oike, F.; Uemoto, S. Posttransplant bacteremia in adult living donor liver transplant recipients. Liver Transplant. 2010, 16, 1379–1385. [Google Scholar] [CrossRef] [PubMed]
- Gallardo, M.L.; Herrera-Gutiérrez, M.E.; Seller-Pérez, G.; Balsera, E.C.; Fernández-Ortega, J.F.; García, G.Q. Risk factors for renal dysfunction in the postoperative course of liver transplant. Liver Transplant. 2004, 10, 1379–1385. [Google Scholar] [CrossRef]
- Morell, C.M.; Strazzabosco, M. Notch signaling and new therapeutic options in liver disease. J. Hepatol. 2014, 60, 885–890. [Google Scholar] [CrossRef]

| Parameter | Unit | All Patients (n = 93) | With Bacterial Infection (n = 47) | Without Bacterial Infection (n = 46) | p-Value for Patients with Bacterial Infection vs. without Bacterial Infection |
|---|---|---|---|---|---|
| Male gender | 58 (62.3%) | 27 (57.5%) | 31 (67.4%) | 0.219 | |
| Age | (years) | 52 (42–58) | 52 (45–58) | 52.5 (40–59) | 0.721 |
| BMI | (kg/m2) | 25.53 (22.99–29.86) | 26.45 (23.88–30.03) | 24.94 (22.69–29.07) | 0.440 |
| MELD score | 18.0 (11.0–28.0) | 20.0 (13.0–30.0) | 14.5 (10.0–22.8) | 0.053 | |
| Causes of liver cirrhosis | |||||
| Alcohol | 27 (29.0%) | 15 (31.91%) | 12 (26.1%) | 0.348 | |
| Hepatitis B | 6 (6.5%) | 2 (4.3%) | 4 (8.7%) | 0.328 | |
| Hepatitis C | 10 (10.8%) | 5 (10.6%) | 5 (10.9%) | 0.616 | |
| HCC | 25 (26.9%) | 10 (21.3%) | 15 (32.6%) | 0.159 | |
| PSC | 16 (17.2%) | 9 (19.1%) | 7 (15.2%) | 0.411 | |
| PBC | 5 (5.4%) | 4 (8.5%) | 1 (2.2%) | 0.187 | |
| NASH | 7 (7.5%) | 4 (8.5%) | 3 (6.5%) | 0.512 | |
| Others | 20 (21.5%) | 8 (17.0%) | 12 (26.1%) | 0.209 | |
| Pre-existing MDR colonization | 25 (26.9%) | 18 (38.3%) | 7 (15.2K%) | 0.013 * | |
| Need for catecholamines before LTX | 3 (3.2%) | 2 (4.3%) | 1 (2.2%) | 0.492 | |
| NYHA 0-I | 90 (96.8%) | 45 (95.7%) | 45 (97.8%) | 0.508 | |
| Diabetes mellitus | 18 (19.4%) | 12 (25.5%) | 6 (13.0%) | 0.103 | |
| Arterial hypertension | 28 (30.1%) | 13 (27.6%) | 15 (32.6%) | 0.384 | |
| Coronary heart disease | 10 (11.4%) | 7 (14.9%) | 3 (6.5%) | 0.388 | |
| Chronic obstructive lung disease | 7 (7.5%) | 3 (6.4%) | 4 (8.7%) | 0.450 | |
| Smoker | 21 (22.5%) | 13 (27.7%) | 8 (17.4%) | 0.189 | |
| Pre-existing renal insufficiency | 20 (21.5%) | 13 (27.7%) | 7 (15.2%) | 0.400 | |
| Pre-existing ARF | 10 (10.8%) | 8 (17.0%) | 2 (4.3%) | 0.119 | |
| Pre-existing thrombosis | 16 (18.2%) | 10 (21.3%) | 6 (13.0%) | 0.269 | |
| Neurological disorder | 40 (45.5%) | 21 (44.7%) | 19 (41.3%) | 0.228 | |
| High-urgency listing | 32 (34.4%) | 16 (34.0%) | 16 (34.8%) | 0.557 | |
| Re-LTX | 16 (17.2%) | 10 (21.3%) | 6 (13.0%) | 0.219 | |
| Immunosuppressive medication | |||||
| Corticosteroids | 93 (100%) | 11 (100%) | 8 (100%) | ||
| Mycophenolat | 92 (98.6%) | 47 (100%) | 45 (97.8%) | 0.495 | |
| mofetil | |||||
| Ciclosporin | 39 (41.9%) | 20 (42.6%) | 19 (41.3%) | 0.535 | |
| Tacrolimus | 54 (58.1%) | 27 (57.4%) | 27 (58.7%) | 0.535 | |
| APACHE II score + | 27 (17–32) | 26 (18–34) | 28 (15–31) | 0.252 | |
| SOFA score + | 13 (7–15) | 13 (9–7) | 12 (5–15) | 0.160 | |
| SAPS + | 52 (30–69) | 56 (3–71) | 49 (26–67) | 0.254 | |
| Duration of mechanical ventilation | (days) | 1 (1–4) | 2 (1–5) | 1 (0–3) | 0.035 * |
| Tracheostomy | 11 (11.8%) | 8 (17.0%) | 3 (6.5%) | 0.106 | |
| Hospital stay | (days) | 1 (1–7) | 1 (1–13) | 1 (1–1) | 0.065 |
| before LTX | |||||
| ICU stay | (days) | 13 (8–24) | 15 (10–36) | 11 (7–7) | 0.005 ** |
| Hospital stay | (days) | 34 (25–52) | 43 (29–62) | 31 (21–46) | 0.012 * |
| 90-day survival | 73 (78.5%) | 36 (76.6%) | 37 (80.4%) | 0.422 | |
| 28-day survival | 86 (92.8%) | 45 (95.7%) | 41 (89.1%) | 0.209 | |
| TLF after LTX | 14 (16.1%) | 6 (12.8%) | 8 (17.4%) | 0.370 | |
| ARF after LTX | 31 (33.3%) | 16 (34.0%) | 15 (32.6%) | 0.529 | |
| ALI after LTX | 6 (6.5%) | 4 (8.5%) | 2 (4.3%) | 0.337 | |
| Dialysis | |||||
| Directly after LTX | 6 (6.5%) | 5 (10.6%) | 1 (2.2%) | 0.107 | |
| In time course | 27 (20%) | 20 (42.6%) | 7 (15.2%) | 0.003 ** | |
| Duration of surgery | (min) | 347 (289–405) | 360 (313–418) | 330 (281–401) | 0.108 |
| Intraoperative blood loss | (L) | 3.0 (1.5–4.4) | 3.0 (1.5–5.0) | 3.0 (2.3–7.5) | 0.292 |
| Rejection | 20 (21.5%) | 12 (25.6%) | 8 (17.4%) | 0.241 | |
| Perforation of the intestine or stomach | 4 (4.3%) | 3 (6.4%) | 1 (2.2%) | 0.317 | |
| Stenosis of the bile duct | 9 (9.7%) | 5 (12.8%) | 4 (6.5%) | 0.254 | |
| Leakage of the bile duct | 10 (10.8%) | 5 (10.6%) | 5 (10.9%) | 0.500 | |
| Need for surgical intervention | 44 (47.3%) | 23 (48.9%) | 21 (45.7%) | 0.456 | |
| Vascular complications | 13 (14.0%) | 5 (10.6%) | 8 (17.4%) | 0.262 | |
| Need for endoscopic diagnostics | 13 (14.0%) | 10 (21.3%) | 3 (6.5%) | 0.038 * | |
| Parameter | Unit | With >2 Complications or Dead within 90 Days (n = 27) | With <2 Complications (n = 66) | p-Value for Patients with >2 Complications or Dead within 90 Days vs. <2 Complications |
|---|---|---|---|---|
| Male gender | 18 (66.7%) | 40 (60.6%) | 0.381 | |
| Age | (years) | 52 (39–58) | 52 (45–58) | 0.909 |
| BMI | (kg/m2) | 25.53 (23.08–30.72) | 25.86 (22.99–29.49) | 0.024 * |
| MELD score | 20 (14–35) | 17 (10–23) | 0.406 | |
| Causes of liver cirrhosis | ||||
| Alcohol | 7 (25.61%) | 20 (30.3%) | 0.438 | |
| Hepatitis B | 0 (0.0%) | 6 (9.1%) | 0.119 | |
| Hepatitis C | 2 (7.4%) | 8 (12.1%) | 0.399 | |
| HCC | 3 (11%) | 22 (33.3%) | 0.022 * | |
| PSC | 6 (22.2%) | 10 (15.2%) | 0.296 | |
| PBC | 1 (3.7%) | 4 (6.1%) | 0.546 | |
| NASH | 4 (14.8%) | 3 (4.5%) | 0.105 | |
| Others | 7 (25.9%) | 13 (19.7%) | 0.343 | |
| Pre-existing MDR colonization | 9 (33.3%) | 16 (24.2%) | 0.272 | |
| Need for catecholamines before LTX | 0 (0.0%) | 3 (4.5%) | 0.353 | |
| NYHA 0-I | 27 (100%) | 63 (95.5%) | 0.353 | |
| Diabetes mellitus | 5 (18.5%) | 13 (19.7%) | 0.573 | |
| Arterial hypertension | 10 (37.0%) | 18 (27.3%) | 0.254 | |
| Coronary heart disease | 3 (11.1%) | 7 (10.6%) | 0.111 | |
| Chronic obstructive lung disease | 1 (3.7%) | 6 (9.1%) | 0.344 | |
| Smoker | 6 (22.2%) | 15 (22.7%) | 0.551 | |
| Pre-existing renal insufficiency | 3 (11.1%) | 17 (15.8%) | 0.111 | |
| Pre-existing ARF | 2 (7.4%) | 8 (12.1%) | 0.384 | |
| Pre-existing thrombosis | 2 (7.4%) | 14 (21.2%) | 0.151 | |
| Neurological disorder | 10 (37.0%) | 30 (45.5%) | 0.311 | |
| High-urgency listing | 9 (33.3%) | 23 (34.8%) | 0.544 | |
| Re-LTX | 4 (14.8%) | 12 (18.2%) | 0.477 | |
| Immunosuppressive medication | ||||
| Corticosteroids | 27 (100%) | 66 (100%) | ||
| Mycophenolat | 27 (100%) | 65 (90.1%) | 0.710 | |
| mofetil | ||||
| Ciclosporin | 12 (44.4%) | 27 (40.9%) | 0.465 | |
| Tacrolimus | 15 (55.5%) | 39 (59.1%) | 0.465 | |
| APACHE II score + | 29 (21–34) | 25 (16–31) | 0.593 | |
| SOFA score + | 13 (11–18) | 12 (6 -15) | 0.436 | |
| SAPS + | 61 (43–77) | 43 (29–67) | 0.359 | |
| Duration of mechanical ventilation | (days) | 5 (2–14) | 1 (1–2) | 0.742 |
| Tracheostomy | 6 (18.5%) | 5 (7.6%) | 0.056 | |
| Hospital stay | (days) | 1 (1–9) | 1 (1–6) | 0.503 |
| before LTX | ||||
| ICU stay | (days) | 27 (9–47) | 13 (8–17) | 0.986 |
| Hospital stay | (days) | 44 (26–72) | 32 (25–50) | 0.452 |
| 90-day survival | 36 (76.6%) | 37 (80.4%) | 0.313 | |
| 28-day survival | 25 (92.6%) | 61 (92.4%) | 0.328 | |
| TLF after LTX | 3 (11.1%) | 11 (16.7%) | 0.477 | |
| ARF after LTX | 9 (33.3%) | 22 (33.3%) | 0.600 | |
| ALI after LTX | 3 (11.1%) | 3 (4.5%) | 0.150 | |
| Dialysis | ||||
| Directly after LTX | 0 (0.0%) | 6 (9.1%) | 0.119 | |
| In time course | 8 (29.7%) | 19 (28.8%) | 0.562 | |
| Duration of surgery | (min) | 375 (293–420) | 346 (293–401) | 0.479 |
| Intraoperative blood loss | (L) | 2.0 (1.0–3.8) | 3.0 (1.5–4.6) | 0.026 * |
| Rejection | 11 (40.7%) | 9 (13.6%) | 0.006 ** | |
| Perforation of the intestine or stomach | 0 (0.0%) | 4 (6.1%) | 0.247 | |
| Stenosis of the bile duct | 3 (11.1%) | 6 (9.1%) | 0.516 | |
| Leakage of the bile duct | 2 (7.4%) | 8 (12.1%) | 0.475 | |
| Need for surgical intervention | 14 (51.9%) | 30 (45.5%) | 0.370 | |
| Vascular complications | 2 (7.4%) | 11 (16.7%) | 0.204 | |
| Need for endoscopic diagnostics | 6 (22.2%) | 7 (10.6%) | 0.129 | |
| Variable | Univariate Analysis with 95% CI | p-Value | Multivariate Analysis with 95% CI | p-Value |
|---|---|---|---|---|
| DLL-1 d2 | 3.03 (1.26–7.28) | 0.010 ** | 1.76 (0.57–5.42) | 0.323 |
| Pretransplant diabetes mellitus | 2.74 (0.87–8.55) | 0.103 | 3.20 (0.88–11.57) | 0.075 |
| Dialysis following LTX | 4.02 (1.49–10.48) | 0.003 ** | 2.40 (0.62–9.19) | 0.200 |
| MELD | 1.03 (0.99–1.08) | 0.053 | 1.02 (0.97–1.07) | 0.306 |
| Duration of surgery | 1.03 (0.99–1.08) | 0.108 | 1.00 (0.99–1.00) | 0.573 |
| Pretransplant MDR | 3.36 (1.24–9.14) | 0.013 * | 2.26 (0.76–6.74) | 0.141 |
| Variable | Univariate Analysis with 95% CI | p-Value | Multivariate Analysis with 95% CI | p-Value |
|---|---|---|---|---|
| DLL d2 | 4.40 (1.55–12.41) | 0.05 * | 2.11 (0.36–12.38) | 0.407 |
| Dialysis following LTX | 13.57 (4.60–40.03) | 0.001 *** | 6.87 (1.28–34.20) | 0.018 * |
| HCC | 0.25 (0.06–0.92) | 0.022 * | 0.00 (0.00–0.00) | 0.998 |
| Rejection | 4.76 (1.67–13.47) | 0.006 ** | 0.00 (0.00–0.00) | 0.998 |
| BMI | 4.72 (1.65–13.47) | 0.024 * | 0.90 (0.78–1.04) | 0.158 |
| Intraoperative blood loss | 1.04 (0.94–1.16) | 0.026 * | 0.99 (0.86–1.12) | 0.880 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Decker, S.O.; Hildebrand, D.; Bruckner, T.; Lichtenstern, C.; Heeg, K.; Weigand, M.A.; Brenner, T.; Uhle, F. Delta-Like Canonical Notch Ligand 1 in Patients Following Liver Transplantation—A Secondary Analysis of a Prospective Cohort Study. Diagnostics 2020, 10, 894. https://doi.org/10.3390/diagnostics10110894
Decker SO, Hildebrand D, Bruckner T, Lichtenstern C, Heeg K, Weigand MA, Brenner T, Uhle F. Delta-Like Canonical Notch Ligand 1 in Patients Following Liver Transplantation—A Secondary Analysis of a Prospective Cohort Study. Diagnostics. 2020; 10(11):894. https://doi.org/10.3390/diagnostics10110894
Chicago/Turabian StyleDecker, Sebastian O., Dagmar Hildebrand, Thomas Bruckner, Christoph Lichtenstern, Klaus Heeg, Markus A. Weigand, Thorsten Brenner, and Florian Uhle. 2020. "Delta-Like Canonical Notch Ligand 1 in Patients Following Liver Transplantation—A Secondary Analysis of a Prospective Cohort Study" Diagnostics 10, no. 11: 894. https://doi.org/10.3390/diagnostics10110894
APA StyleDecker, S. O., Hildebrand, D., Bruckner, T., Lichtenstern, C., Heeg, K., Weigand, M. A., Brenner, T., & Uhle, F. (2020). Delta-Like Canonical Notch Ligand 1 in Patients Following Liver Transplantation—A Secondary Analysis of a Prospective Cohort Study. Diagnostics, 10(11), 894. https://doi.org/10.3390/diagnostics10110894







