Kappa Index versus CSF Oligoclonal Bands in Predicting Multiple Sclerosis and Infectious/Inflammatory CNS Disorders
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Laboratory Procedures
2.3. Statistical Methods
3. Results
3.1. Patients
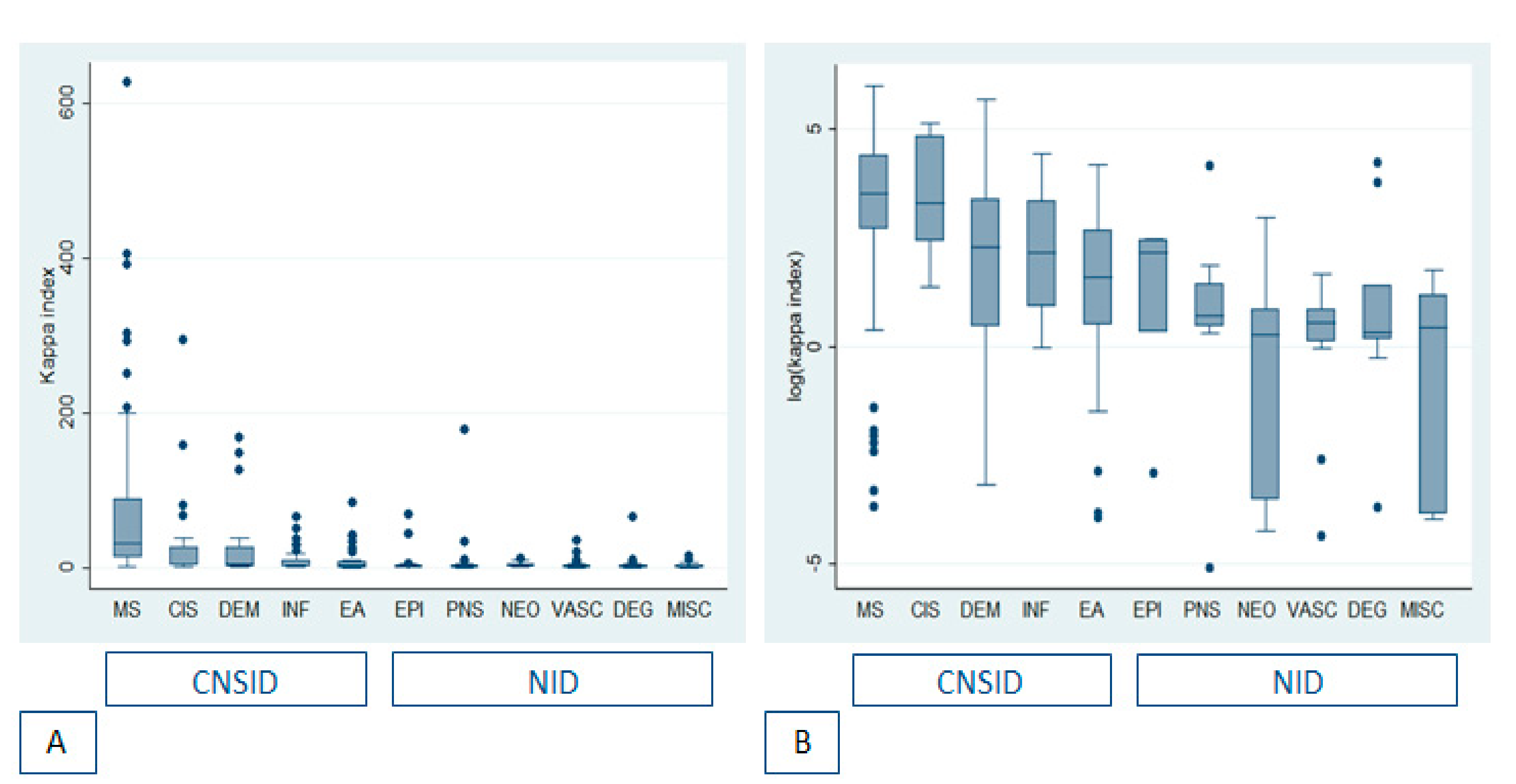
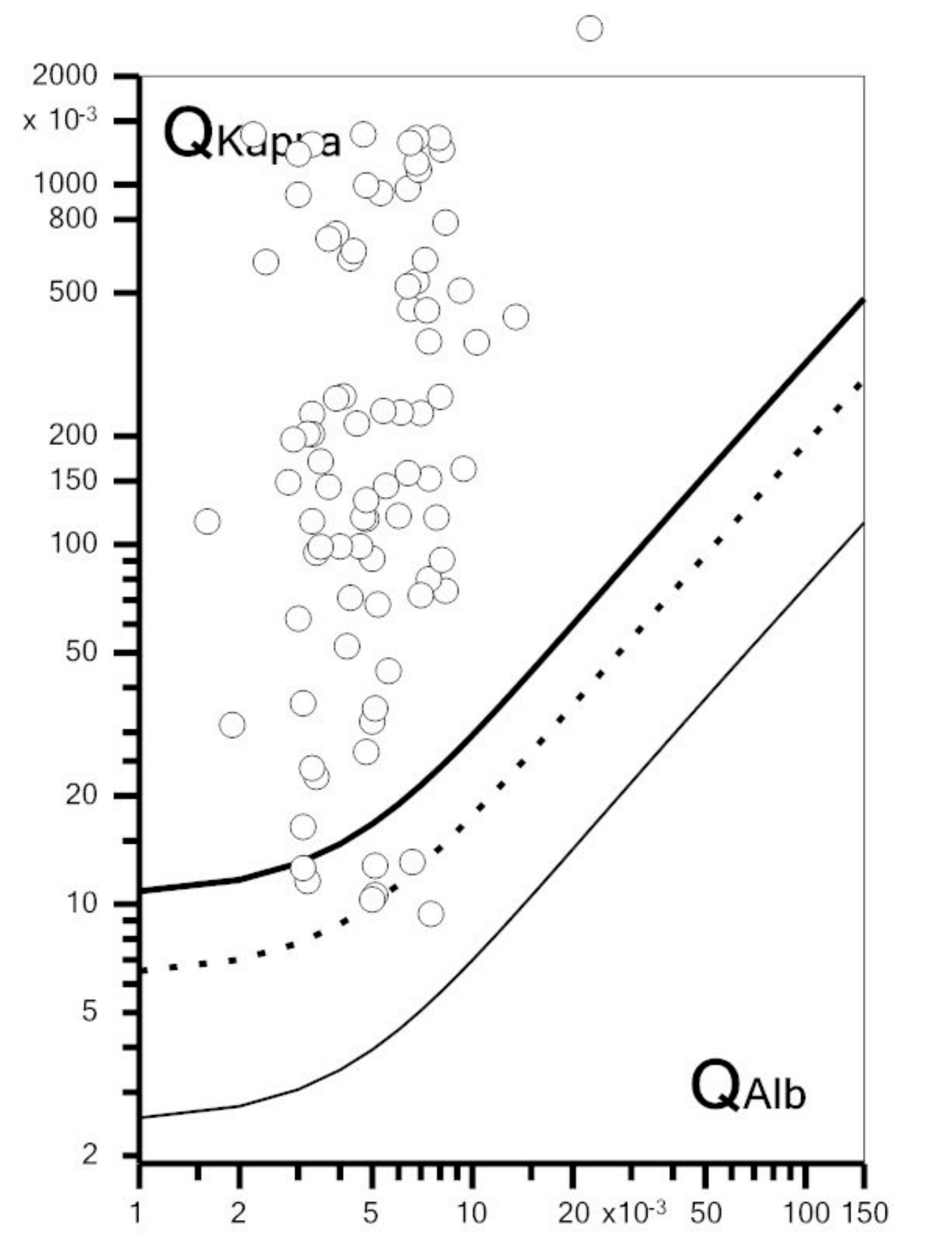
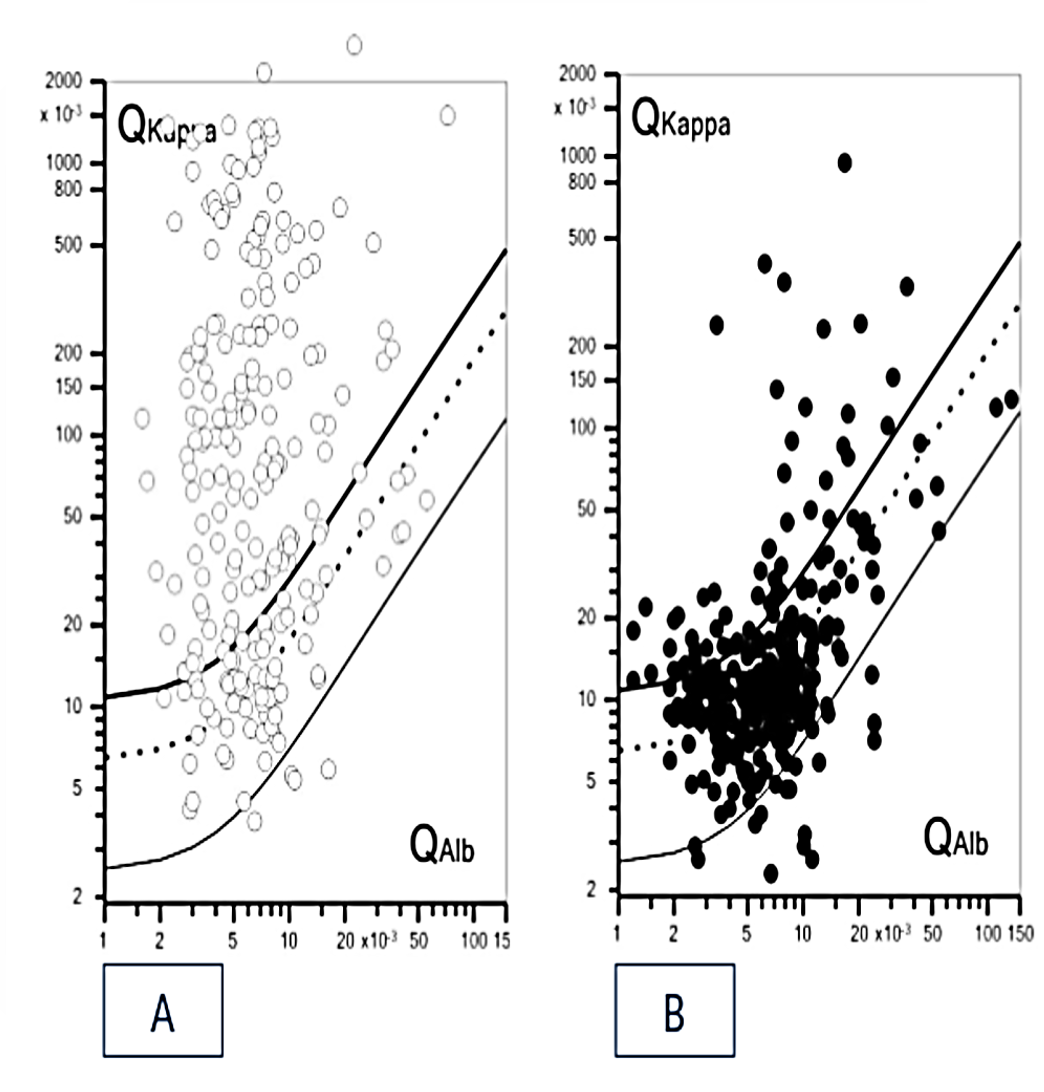
3.2. Concordance between Kappa Index and OCB
3.3. Sensitivity and Specificity of OCB and Kappa Index for a MS Diagnosis
3.4. Sensitivity and Specificity of OCB and Kappa Index for a CNSID Diagnosis
3.5. OCB-Negative Patients and Patients with a Single CSF IgG Band
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gastaldi, M.; Zardini, E.; Franciotta, D. An update on the use of cerebrospinal fluid analysis as a diagnostic tool in multiple sclerosis. Expert Rev. Mol. Diagn. 2017, 17, 31–46. [Google Scholar] [CrossRef] [PubMed]
- Thompson, A.J.; Banwell, B.L.; Barkhof, F.; Carroll, W.M.; Coetzee, T.; Comi, G.; Correale, J.; Fazekas, F.; Filippi, M.; Freedman, M.S.; et al. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol. 2018, 17, 162–173. [Google Scholar] [CrossRef]
- Lee, D.-H.; Peschke, M.; Utz, K.S.; Linker, R.A. Diagnostic value of the 2017 McDonald criteria in patients with a first demyelinating event suggestive of relapsing–remitting multiple sclerosis. Eur. J. Neurol. 2019, 26, 540–545. [Google Scholar] [CrossRef] [PubMed]
- Nakano, T.; Matsui, M.; Inoue, I.; Awata, T.; Katayama, S.; Murakoshi, T. Free immunoglobulin light chain: Its biology and implications in diseases. Clin. Chim. Acta 2011, 412, 843–849. [Google Scholar] [CrossRef] [PubMed]
- Reiber, H.; Zeman, D.; Kušnierová, P.; Mundwiler, E.; Bernasconi, L. Diagnostic relevance of free light chains in cerebrospinal fluid—The hyperbolic reference range for reliable data interpretation in quotient diagrams. Clin. Chim. Acta 2019, 497, 153–162. [Google Scholar] [CrossRef] [PubMed]
- Rajkumar, S.V.; Dimopoulos, M.A.; Palumbo, A.; Blade, J.; Merlini, G.; Mateos, M.V.; Kumar, S.; Hillengass, J.; Kastritis, E.; Richardson, P.; et al. International Myeloma Working Group updated criteria for the diagnosis of multiple myeloma. Lancet Oncol. 2014, 15, e538–e548. [Google Scholar] [CrossRef]
- Ferraro, D.; Trovati, A.; Bedin, R.; Natali, P.; Franciotta, D.; Santangelo, M.; Camera, V.; Vitetta, F.; Varani, M.; Trenti, T.; et al. Cerebrospinal fluid free light kappa and lambda chains in oligoclonal band-negative patients with suspected Multiple Sclerosis. Eur. J. Neurol. 2019, 27, 461–467. [Google Scholar] [CrossRef] [PubMed]
- Presslauer, S.; Milosavljevic, D.; Brücke, T.; Bayer, P.; Hübl, W. Elevated levels of kappa free light chains in CSF support the diagnosis of multiple sclerosis. J. Neurol. 2008, 255, 1508–1514. [Google Scholar] [CrossRef]
- Duranti, F.; Pieri, M.; Centonze, D.; Buttari, F.; Bernardini, S.; Dessi, M. Determination of kFLC and K Index in cerebrospinal fluid: A valid alternative to assessintrathecal immunoglobulin synthesis. J. Neuroimmunol. 2013, 263, 116–120. [Google Scholar] [CrossRef]
- Gaetani, L.; Di Carlo, M.; Brachelente, G.; Valletta, F.; Eusebi, P.; Mancini, A.; Gentili, L.; Borrelli, A.; Calabresi, P.; Sarchielli, P.; et al. Cerebrospinal fluid free light chains compared to oligoclonal bands as biomarkers in multiple sclerosis. J. Neuroimmunol. 2020, 339, 577108. [Google Scholar] [CrossRef]
- Duell, F.; Evertsson, B.; Al Nimer, F.; Sandin, Å.; Olsson, D.; Olsson, T.; Khademi, M.; Hietala, M.A.; Piehl, F.; Hansson, M. Diagnostic accuracy of intrathecal kappa free light chains compared with OCBs in MS. Neurol. Neuroimmunol. Neuroinflamm. 2020, 7, e775. [Google Scholar] [CrossRef] [PubMed]
- Senel, M.; Tumani, H.; Lauda, F.; Presslauer, S.; Mojib-Yezdani, R.; Otto, M.; Brettschneider, J. Cerebrospinal fluid immunoglobulin kappa light chain in clinically isolated syndrome and multiple sclerosis. PLoS ONE 2014, 9, e88680. [Google Scholar] [CrossRef]
- Schwenkenbecher, P.; Konen, F.F.; Wurster, U.; Jendretzky, K.F.; Gingele, S.; Sühs, K.; Pul, R.; Witte, T.; Stangel, M.; Skripuletz, T. The Persisting Significance of Oligoclonal Bands in the Dawning Era of Kappa Free Light Chains for the Diagnosis of Multiple Sclerosis. Int. J. Mol. Sci. 2018, 19, 3796. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valencia-Vera, E.; Martinez-Escribano Garcia-Ripoll, A.; Enguix, A.; Abalos-Garcia, C.; Segovia-Cuevas, M.J. Application of κ free light chains in cerebrospinal fluid as a biomarker in multiple sclerosis diagnosis: Development of a diagnosis algorithm. Clin. Chem. Lab. Med. 2018, 56, 609–613. [Google Scholar] [CrossRef] [PubMed]
- Menéndez-Valladares, P.; García-Sánchez, M.I.; Adorna Martínez, M.; De Veas Silva, L.G.; Guitarte, C.B.; Ayuso, G.I. Validation and meta-analysis of kappa index biomarker in multiple sclerosis diagnosis. Autoimmun. Rev. 2018, 18, 43–49. [Google Scholar] [CrossRef]
- Leurs, C.; Twaalfhoven, H.; Lissenberg-Witte, B.; Van Pesch, V.; Dujmovic, I.; Drulovic, J.; Castellazzi, M.; Bellini, T.; Pugliatti, M.; Kuhle, J.; et al. Kappa free light chains is a valid tool in the diagnostics of MS: A large multicenter study. Mult. Scler. J. 2019, 135245851984584. [Google Scholar] [CrossRef] [Green Version]
- Cavalla, P.; Caropreso, P.; Limoncelli, S.; Bosa, C.; Pasanisi, M.B.; Schillaci, V.; Alteno, A.; Costantini, G.; Giordana, M.T.; Mengozzi, G.; et al. Kappa free light chains index in the differential diagnosis of Multiple Sclerosis from Neuromyelitis optica spectrum disorders and other immune-mediated central nervous system disorders. J. Neuroimmunol. 2020, 339, 577122. [Google Scholar] [CrossRef]
- Süße, M.; Reiber, H.; Grothe, M.; Petersmann, A.; Nauck, M.; Dressel, A.; Hannich, M.J. Free light chain kappa and the polyspecific immune response in MS and CIS–Application of the hyperbolic reference range for most reliable data interpretation. J. Neuroimmunol. 2020, 346, 577287. [Google Scholar] [CrossRef]
- Puthenparampil, M.; Altinier, S.; Stropparo, E.; Zywicki, S.; Poggiali, D.; Cazzola, C.; Toffanin, E.; Ruggero, S.; Grassivaro, F.; Zaninotto, M.; et al. Intrathecal K free light chain synthesis in multiple sclerosis at clinical onset associates with local IgG production and improves the diagnostic value of cerebrospinal fluid examination. Mult. Scler. Relat. Disord. 2018, 25, 241–245. [Google Scholar] [CrossRef]
- Goffette, S.; Schluep, M.; Henry, H.; Duprez, T.; Sindic, C.J.M. Detection of oligoclonal free kappa chains in the absence of oligoclonal IgG in the CSF of patients with suspected multiple sclerosis. J. Neurol. Neurosurg. Psychiatry 2004, 75, 308–310. Available online: http://www.ncbi.nlm.nih.gov/pubmed/14742614 (accessed on 4 July 2017). [CrossRef] [Green Version]
- Ferraro, D.; Franciotta, D.; Bedin, R.; Solaro, C.; Cocco, E.; Santangelo, M.; Immovilli, P.; Gajofatto, A.; Calabrese, M.; Di Filippo, M.; et al. A multicenter study on the diagnostic significance of a single cerebrospinal fluid IgG band. J. Neurol. 2017, 264, 973–978. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Franciotta, D.; Bergamaschi, R.; Amato, M.P.; Zardini, E.; Persico, A.; Portaccio, E.; Lolli, F. Clinical correlations of CSF single IgG bands. J. Neurol. 2005, 252, 1274–1275. [Google Scholar] [CrossRef] [PubMed]
- Andersson, M.; Alvarez-Cermeño, J.; Bernardi, G.; Cogato, I.; Fredman, P.; Frederiksen, J.; Fredrikson, S.; Gallo, P.; Grimaldi, L.M.; Grønning, M.; et al. Cerebrospinal fluid in the diagnosis of multiple sclerosis: A consensus report. J. Neurol. Neurosurg. Psychiatry 1994, 57, 897–902. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gobbin, F.; Zanoni, M.; Marangi, A.; Orlandi, R.; Crestani, L.; Benedetti, M.D.; Gajofatto, A. 2017 McDonald criteria for multiple sclerosis: Earlier diagnosis with reduced specificity? Mult. Scler. Relat. Disord. 2019, 29, 23–25. [Google Scholar] [CrossRef]
- Villar, L.; García-Barragán, N.; Espiño, M.; Roldán, E.; Sádaba, M.C.; Gómez-Rial, J.; González-Porqué, P.; Álvarez-Cermeño, J.C. Influence of oligoclonal IgM specificity in multiple sclerosis disease course. Mult. Scler. 2008, 14, 183–187. [Google Scholar] [CrossRef]
- Ferraro, D.; Simone, A.M.; Bedin, R.; Galli, V.; Vitetta, F.; Federzoni, L.; D’Amico, R.; Merelli, E.; Nichelli, P.F.; Sola, P. Cerebrospinal fluid oligoclonal IgM bands predict early conversion to clinically definite multiple sclerosis in patients with Clinically Isolated Syndrome. J. Neuroimmunol. 2013, 257, 76–81. [Google Scholar] [CrossRef]
- Sharief, M.K.; Keir, G.; Thompson, E.J. Intrathecal synthesis of IgM in neurological diseases: A comparison between detection of oligoclonal bands and quantitative estimation. J. Neurol. Sci. 1990, 96, 131–142. [Google Scholar] [CrossRef]
- Villar, L.M.; Masjuan, J.; González-Porqué, P.; Plaza, J.; Sádaba, M.C.; Roldán, E.; Bootello, A.; Alvarez–Cermeño, J.C. Intrathecal IgM synthesis in neurologic diseases: Relationship with disability in MS. Neurology 2002, 58, 824–826. Available online: http://www.ncbi.nlm.nih.gov/pubmed/11889253 (accessed on 1 March 2012). [CrossRef]
- Hegen, H.; Milosavljevic, D.; Schnabl, C.; Manowiecka, A.; Walde, J.; Deisenhammer, F.; Presslauer, S. Cerebrospinal fluid free light chains as diagnostic biomarker in neuroborreliosis. Clin. Chem. Lab. Med. 2018, 56, 1383–1391. [Google Scholar] [CrossRef]
- Tjernberg, I.; Johansson, M.; Henningsson, A.J. Diagnostic performance of cerebrospinal fluid free light chains in Lyme neuroborreliosis–a pilot study. Clin. Chem. Lab. Med. 2019, 57, 2008–2018. [Google Scholar] [CrossRef]
- Regeniter, A.; Kuhle, J.; Mehling, M.; Möller, H.; Wurster, U.; Freidank, H.; Siede, W.H. A modern approach to CSF analysis: Pathophysiology, clinical application, proof of concept and laboratory reporting. Clin. Neurol. Neurosurg. 2009, 111, 313–318. [Google Scholar] [CrossRef] [PubMed]
| MS (n = 84) | Non-MS (n = 456) | p Value | |
|---|---|---|---|
| Sex (F/M) | 54/30 | 222/236 | 0.029 |
| Age (years) (mean ± SD) | 38 ± 14 | 57± 20 | <0.001 |
| Serum KFLC (mg/L) (mean ± SD) | 12.4 ± 5.6 | 19.2 ± 15.5 | <0.001 |
| CSF KFLC (mg/L) (mean ± SD) | 4.1 ± 4.5 | 0.8 ± 2.0 | <0.001 |
| Kappa Index (mean ± SD) | 78.6 ± 105.8 | 7.4 ± 22.0 | <0.001 |
| KFLC IF (%) (mean ± SD) | 78.4 ± 28.6 | 14.4 ±28.3 | <0.001 |
| Patients with KFLC IF > 0, nr (%) | 77 (91.7) | 132 (29) | <0.001 |
| IgG Index (mean ± SD) | 0.8 ± 0.3 | 0.6 ± 1.1 | <0.001 |
| Intrathecal IgG synthesis according to Reiber (mean ± SD) | 0.3 ± 1.4 | −1.8 ± 3.5 | <0.001 |
| CSF/serum albumin (mean ± SD) | 5.5 × 10−3 ± 2.8 × 10−3 | 9.2 × 10−3 ± 11.1 × 10−3 | <0.001 |
| IEF pattern | <0.001 | ||
| Polyclonal, nr (%) | 2 (2.4) | 185 (40.5) | |
| Mirror pattern, nr (%) | 4 (4.8) | 192 (42.1) | |
| Single CSF IgG band, nr (%) | 6 (7.1) | 9 (2.0) | |
| Mirror pattern + single CSF IgG band, nr (%) | 1 (1.2) | 15 (3.3) | |
| CSF-restricted IgG OCB, nr (%) | 54 (64.3) | 30 (6.6) | |
| Mirror pattern + OCB, nr (%) | 17 (20.2) | 21 (4.6) | |
| Monoclonal gammopathy, nr (%) | 0 (0.0) | 4 (0.9) |
| Kappa Index Positive (≥5.8), OCB-Negative Patients (nr = 41) | OCB-Positive, Kappa Index-Negative (<5.8) Patients (nr = 15) | |
|---|---|---|
| MS, nr (%) | 6 (14.6) | 3 (20%) |
| CIS, nr (%) | 1 (2.4) | 0 |
| Other acquired CNS demyelinating disease, nr (%) | 2 (4.9) | 1 (6.6) |
| CNS autoimmune or paraneoplastic disease, nr (%) | 5 (12.2) | 3 (20%) |
| CNS infectious disease, nr (%) | 8 (19.5) | 3 (20%) |
| CNS neoplasm, nr (%) | 3 (7.3) | 1 (6.7) |
| Neurological degenerative disorder, nr (%) | 2 (4.9) | 0 |
| Vascular disorder, nr (%) | 4 (9.8) | 0 |
| PNS disorder, nr (%) | 6 (14.6) | 2 (13.3) |
| Epilepsy, nr (%) | 0 | 1 (6.7) |
| Miscellaneous, nr (%) | 4 (9.8) | 1 (6.7) |
| Sensitivity (95%CI) | Specificity (95%CI) | PPV (95%CI) | NPV (95%CI) | AUC (95%CI) | |
|---|---|---|---|---|---|
| OCB | 85 (75–92) | 89 (86–92) | 58 (49–67) | 97 (95–98) | 87 (83–91) |
| OCB + single CSF IgG band | 93 (85–97) | 84 (80–87) | 51 (43–59) | 98 (97–99) | 88 (85–92) |
| Kappa Index ≥ 5.8 | 89 (81–95) | 84 (80–87) | 51 (42–59) | 98 (96–99) | 87 (83–90) |
| Kappa Index ≥ 6.2 | 89 (81–95) | 84 (81–88) | 51 (43–60) | 98 (96–99) | 87 (83–91) |
| KFLC IF (%) > 0 | 92 (84–97) | 71 (67–75) | 37 (30–44) | 98 (96–99) | 81 (78–85) |
| KFLC IF (%) > 37 | 90 (82–96) | 83 (80–96) | 50 (42–58) | 98 (96–99) | 87 (83–90) |
| Sensitivity (95%CI) | Specificity (95%CI) | PPV (95%CI) | NPV (95%CI) | AUC (95%CI) | |
|---|---|---|---|---|---|
| OCB | 50 (44–57) | 97 (94–99) | 92 (85–96) | 73 (69–78) | 74 (70–77) |
| OCB + single CSF IgG band | 57 (50–63) | 92 (88–94) | 82 (75–88) | 75 (70–79) | 74 (70–78) |
| Kappa Index ≥ 5.8 | 56 (49–62) | 92 (89–95) | 84 (77–89) | 75 (70–79) | 74 (70–78) |
| Kappa Index ≥ 3.9 | 67 (60–73) | 81 (77–86) | 72 (65–78) | 78 (73–82) | 74 (70–78) |
| KFLC IF (%) > 0 | 69 (63–75) | 83 (78–87) | 74 (67–80) | 79 (74–83) | 76 (72–80) |
| KFLC IF (%) > 11 | 65 (58–72) | 87 (83–91) | 78 (74–82) | 78 (74–82) | 76 (73–80) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferraro, D.; Bedin, R.; Natali, P.; Franciotta, D.; Smolik, K.; Santangelo, M.; Immovilli, P.; Camera, V.; Vitetta, F.; Gastaldi, M.; et al. Kappa Index versus CSF Oligoclonal Bands in Predicting Multiple Sclerosis and Infectious/Inflammatory CNS Disorders. Diagnostics 2020, 10, 856. https://doi.org/10.3390/diagnostics10100856
Ferraro D, Bedin R, Natali P, Franciotta D, Smolik K, Santangelo M, Immovilli P, Camera V, Vitetta F, Gastaldi M, et al. Kappa Index versus CSF Oligoclonal Bands in Predicting Multiple Sclerosis and Infectious/Inflammatory CNS Disorders. Diagnostics. 2020; 10(10):856. https://doi.org/10.3390/diagnostics10100856
Chicago/Turabian StyleFerraro, Diana, Roberta Bedin, Patrizia Natali, Diego Franciotta, Krzysztof Smolik, Mario Santangelo, Paolo Immovilli, Valentina Camera, Francesca Vitetta, Matteo Gastaldi, and et al. 2020. "Kappa Index versus CSF Oligoclonal Bands in Predicting Multiple Sclerosis and Infectious/Inflammatory CNS Disorders" Diagnostics 10, no. 10: 856. https://doi.org/10.3390/diagnostics10100856
APA StyleFerraro, D., Bedin, R., Natali, P., Franciotta, D., Smolik, K., Santangelo, M., Immovilli, P., Camera, V., Vitetta, F., Gastaldi, M., Trenti, T., Meletti, S., & Sola, P. (2020). Kappa Index versus CSF Oligoclonal Bands in Predicting Multiple Sclerosis and Infectious/Inflammatory CNS Disorders. Diagnostics, 10(10), 856. https://doi.org/10.3390/diagnostics10100856





