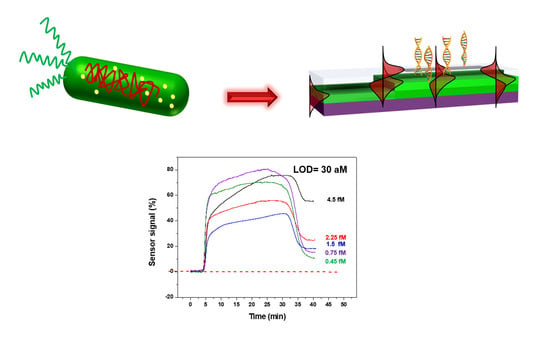
Ultrasensitive Label-Free Detection of Unamplified Multidrug-Resistance Bacteria Genes with a Bimodal Waveguide Interferometric Biosensor
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemical Reagents
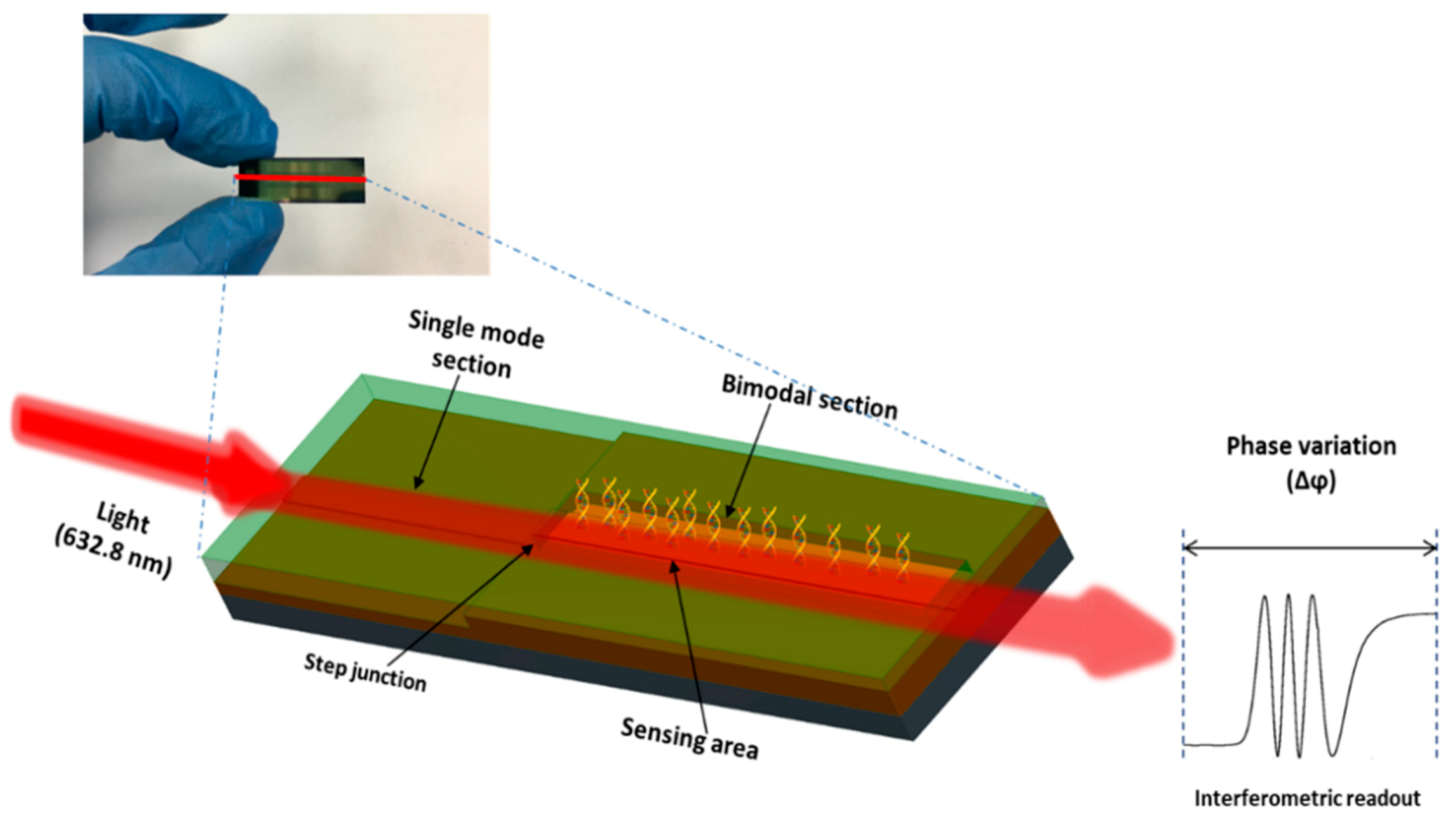
2.2. BiMW Device and Experimental Set-Up
2.3. Microfluidic System
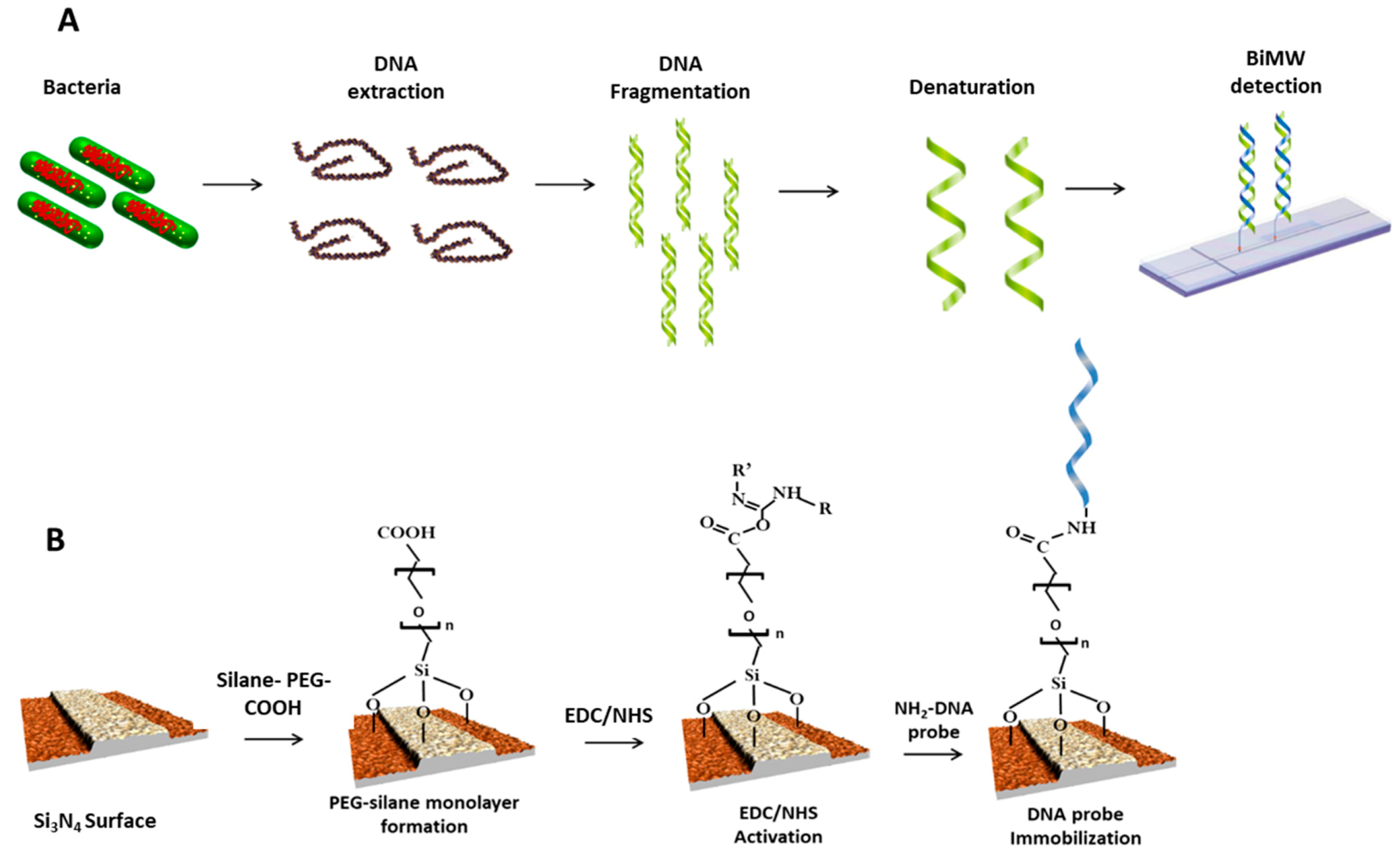
2.4. BiMW Surface Cleaning, Activation, and Biofunctionalization
2.5. Probes Design
2.6. Bacteria Culture, DNA Extraction, and PCR Validation
2.7. BiMW Assay Development
3. Results and Discussion
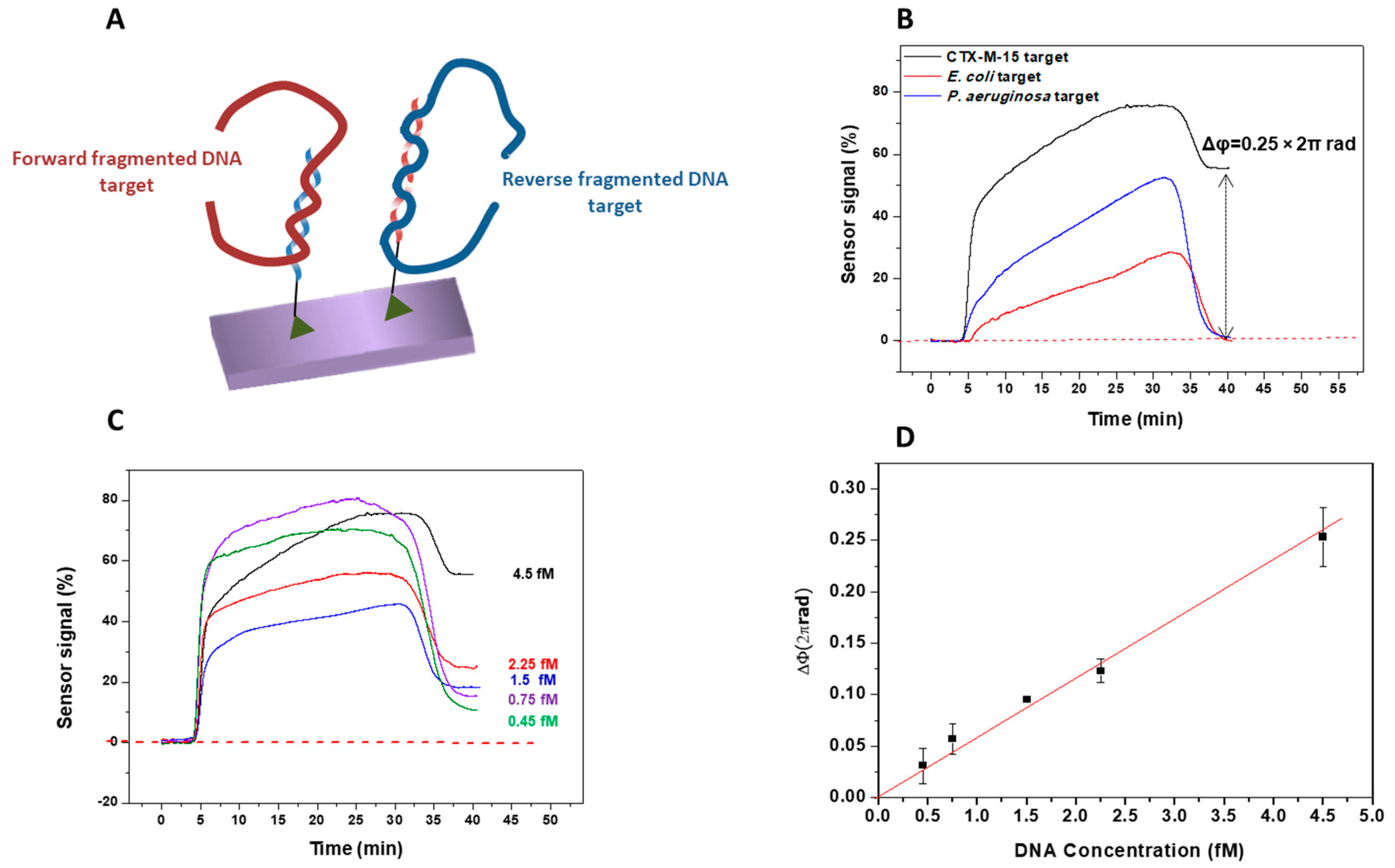
3.1. Bioassay Strategy and Evaluation with Synthetic DNA Target Sequences
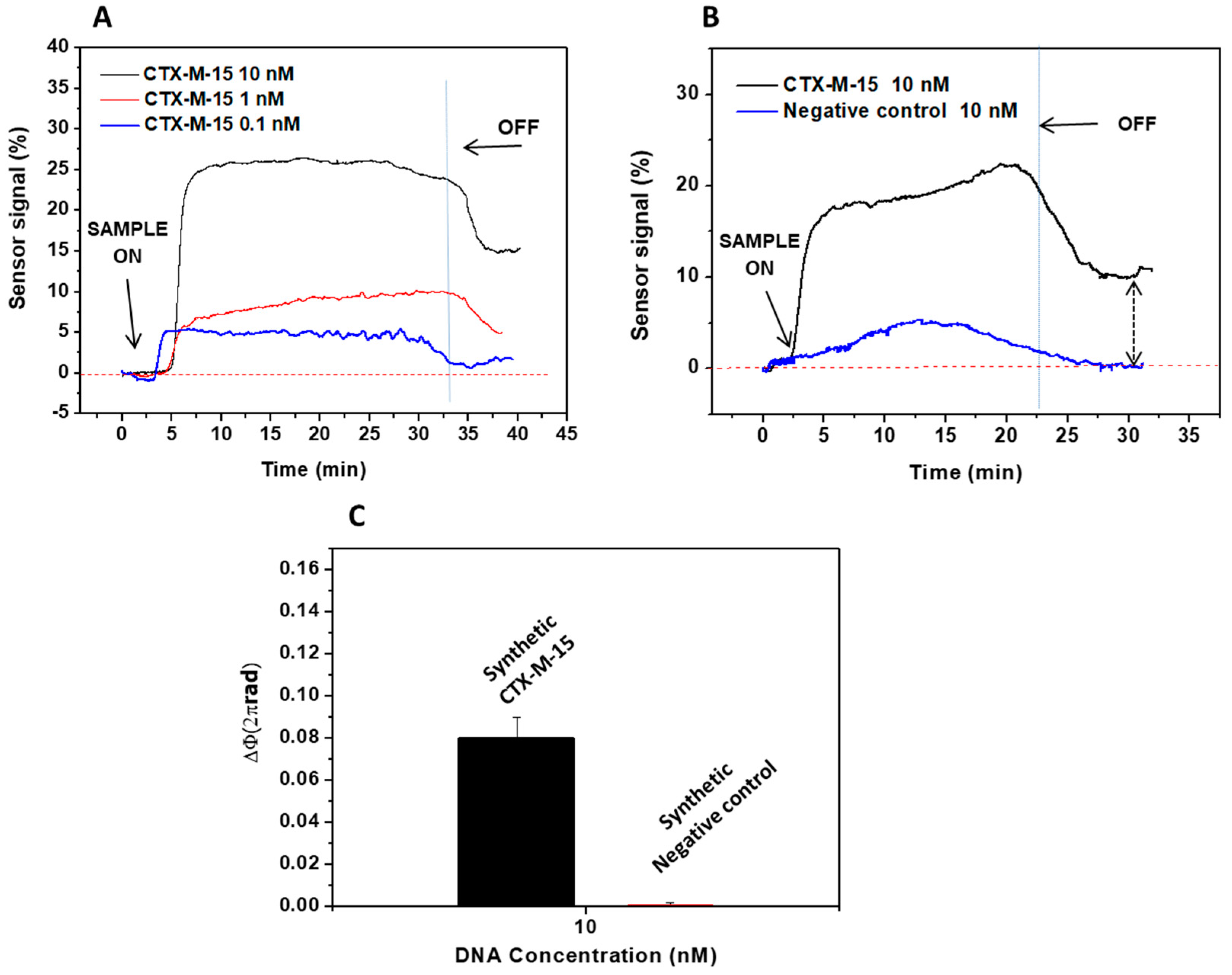
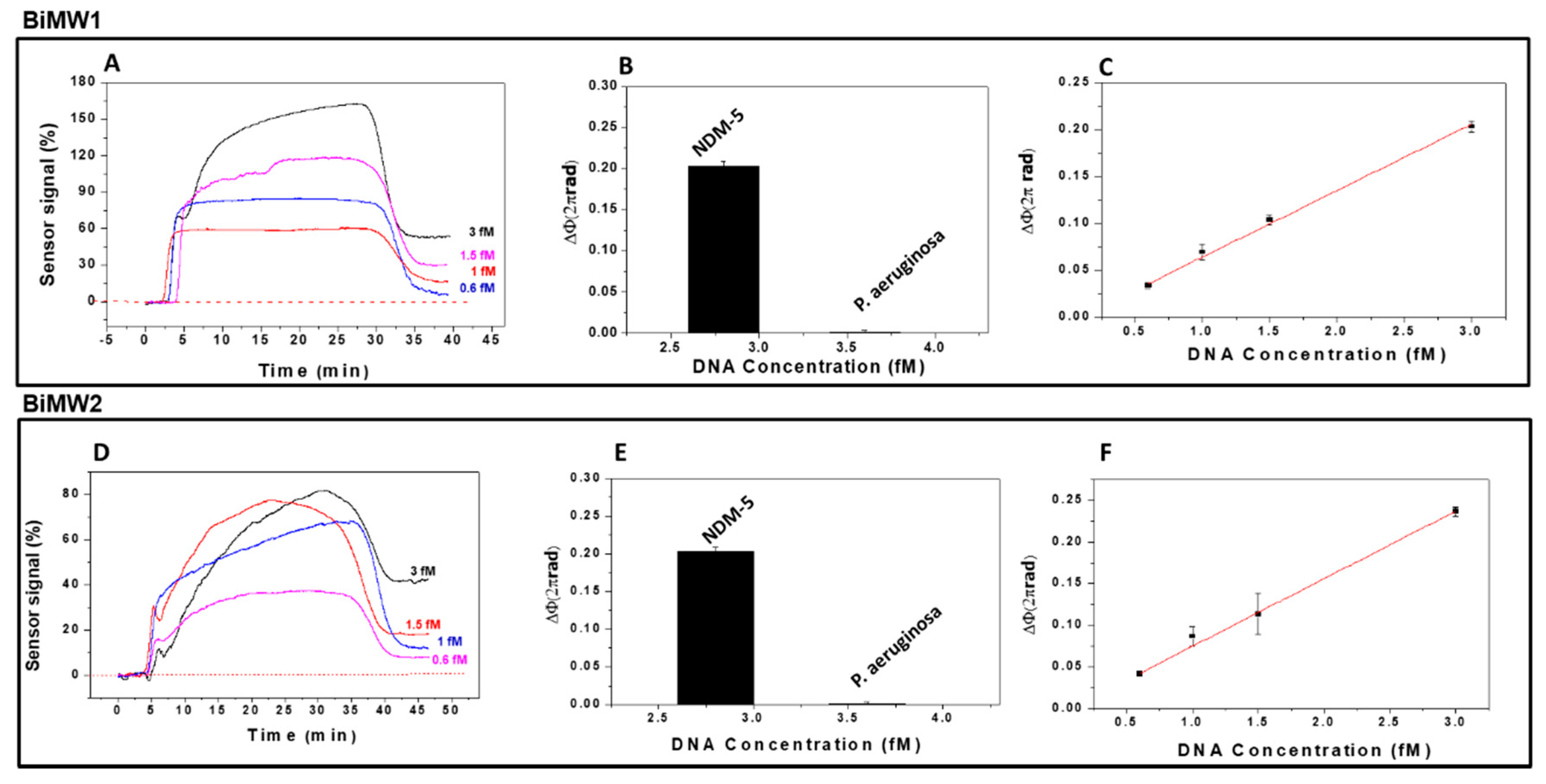
3.2. Evaluation of the Genes Encoding Betalactamase CTX-M-15 and Carbapenemase NDM-5
3.2.1. Betalactamase CTX-M-15
3.2.2. Carbapenemase NDM-5
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- McFee, R.B. Nosocomial or Hospital-acquired Infections: An Overview. Dis. Month 2009, 55, 422–438. [Google Scholar] [CrossRef] [PubMed]
- Brusaferro, S.; Arnoldo, L.; Cattani, G.; Fabbro, E.; Cookson, B.; Gallagher, R.; Hartemann, P.; Holt, J.; Kalenic, S.; Popp, W.; et al. Harmonizing and supporting infection control training in Europe. J. Hosp. Infect. 2015, 89, 351–356. [Google Scholar] [CrossRef]
- Center for Disease Control and Prevention. Antinbiotic Resistance threats in the United States. In US Deparment of Health and Human Services; Center for Disease Control and Prevention: Atlanta, GA, USA, 2013. [Google Scholar]
- Center for Disease Control and Prevention. Antinbiotic Resistance threats in the United States. In US Deparment of Health and Human Services; Center for Disease Control and Prevention: Atlanta, GA, USA, 2019. [Google Scholar]
- Zimlichman, E.; Henderson, D.; Tamir, O.; Franz, C.; Song, P.; Yamin, C.K.; Keohane, C.; Denham, C.R.; Bates, D.W. Health Care–Associated Infections. JAMA Intern. Med. 2013, 173, 2039–2046. [Google Scholar] [CrossRef]
- Huber, F.; Lang, H.P.; Gerber, C. New leverage against superbugs. Nat. Nanotechnol. 2008, 3, 645–646. [Google Scholar] [CrossRef]
- Freire-Moran, L.; Aronsson, B.; Manz, C.; Gyssens, I.C.; So, A.D.; Monnet, D.L.; Cars, O.; ECDC-EMA Working Group. Critical shortage of new antibiotics in development against multidrug-resistant bacteria—Time to react is now. Drug Resist. Updates 2011, 14, 118–124. [Google Scholar] [CrossRef]
- Dark, P.; Dean, P.; Warhurst, G. Bench-to-bedside review: The promise of rapid infection diagnosis during sepsis using polymerase chain reaction-based pathogen detection. Crit. Care 2009, 13, 217. [Google Scholar] [CrossRef]
- Loman, N.J.; Constantinidou, C.; Chan, J.Z.M.; Halachev, M.; Sergeant, M.; Penn, C.W.; Robinson, E.R.; Pallen, M.J. High-throughput bacterial genome sequencing: An embarrassment of choice, a world of opportunity. Nat. Rev. Genet. 2012, 10, 599–606. [Google Scholar] [CrossRef]
- Ottesen, E.A.; Hong, J.W.; Quake, S.R.; Leadbetter, J.R.; Gomendio, M.; Malo, A.; Soler, A.J.; Fernández-Santos, M.R.; Esteso, M.C.; García, A.J.; et al. Microfluidic Digital PCR Enables Multigene Analysis of Individual Environmental Bacteria. Science 2006, 314, 1464–1467. [Google Scholar] [CrossRef] [PubMed]
- Pechorsky, A.; Nitzan, Y.; Lazarovitch, T. Identification of pathogenic bacteria in blood cultures: Comparison between conventional and PCR methods. J. Microbiol. Methods 2009, 78, 325–330. [Google Scholar] [CrossRef] [PubMed]
- Zanoli, L.M.; D’Agata, R.; Spoto, G. Functionalized gold nanoparticles for ultrasensitive DNA detection. Anal. Bioanal. Chem. 2011, 402, 1759–1771. [Google Scholar] [CrossRef]
- Shi, L.; Perkins, R.G.; Fang, H.; Tong, W. Reproducible and reliable microarray results through quality control: Good laboratory proficiency and appropriate data analysis practices are essential. Curr. Opin. Biotechnol. 2008, 19, 10–18. [Google Scholar] [CrossRef] [PubMed]
- D’Agata, R.; Breveglieri, G.; Zanoli, L.M.; Borgatti, M.; Spoto, G.; Gambari, R. Direct Detection of Point Mutations in Nonamplified Human Genomic DNA. Anal. Chem. 2011, 83, 8711–8717. [Google Scholar] [CrossRef] [PubMed]
- D’Agata, R.; Corradini, R.; Ferretti, C.; Zanoli, L.M.; Gatti, M.; Marchelli, R.; Spoto, G. Ultrasensitive detection of non-amplified genomic DNA by nanoparticle-enhanced surface plasmon resonance imaging. Biosens. Bioelectron. 2010, 25, 2095–2100. [Google Scholar] [CrossRef] [PubMed]
- Scarano, S.; Ermini, M.L.; Spiriti, M.M.; Mascini, M.; Bogani, P.; Minunni, M.E. Simultaneous Detection of Transgenic DNA by Surface Plasmon Resonance Imaging with Potential Application to Gene Doping Detection. Anal. Chem. 2011, 83, 6245–6253. [Google Scholar] [CrossRef] [PubMed]
- Kaewphinit, T.; Santiwatanakul, S.; Promptmas, C.; Chansiri, K. Detection of Non-Amplified Mycobacterium tuberculosis Genomic DNA Using Piezoelectric DNA-Based Biosensors. Sensors 2010, 10, 1846–1858. [Google Scholar] [CrossRef] [PubMed]
- Minunni, M.E.; Tombelli, S.; Fonti, J.; Spiriti, M.M.; Mascini, M.; Bogani, P.; Buiatti, M. Detection of Fragmented Genomic DNA by PCR-Free Piezoelectric Sensing Using a Denaturation Approach. J. Am. Chem. Soc. 2005, 127, 7966–7967. [Google Scholar] [CrossRef]
- Almadidy, A.; Watterson, J.; Piunno, P.A.; Raha, S.; Foulds, I.V.; Horgen, P.A.; Castle, A.; Krull, U.J. Direct selective detection of genomic DNA from coliform using a fiber optic biosensor. Anal. Chim. Acta 2002, 461, 37–47. [Google Scholar] [CrossRef]
- Sethi, S.; Nanda, R.; Chakraborty, T. Clinical Application of Volatile Organic Compound Analysis for Detecting Infectious Diseases. Clin. Microbiol. Rev. 2013, 26, 462–475. [Google Scholar] [CrossRef]
- Turner, A.P.; Magan, N. Electronic noses and disease diagnostics. Nat. Rev. Microbiol. 2004, 2, 161–166. [Google Scholar] [CrossRef]
- Banday, K.M.; Pasikanti, K.K.; Chan, E.C.Y.; Singla, R.; Rao, K.V.S.; Chauhan, V.S.; Nanda, R.K. Use of Urine Volatile Organic Compounds to Discriminate Tuberculosis Patients from Healthy Subjects. Anal. Chem. 2011, 83, 5526–5534. [Google Scholar] [CrossRef]
- Savelev, S.; Perry, J.D.; Bourke, S.; Jary, H.; Taylor, R.; Fisher, A.J.; Corris, P.; Petrie, M.; De Soyza, A. Volatile biomarkers of Pseudomonas aeruginosa in cystic fibrosis and noncystic fibrosis bronchiectasis. Lett. Appl. Microbiol. 2011, 52, 610–613. [Google Scholar] [CrossRef]
- Zhu, J.; Bean, H.D.; Kuo, Y.-M.; Hill, J.E. Fast Detection of Volatile Organic Compounds from Bacterial Cultures by Secondary Electrospray Ionization-Mass Spectrometry. J. Clin. Microbiol. 2011, 49, 769. [Google Scholar] [CrossRef]
- Duval, D.; González-Guerrero, A.B.; Dante, S.; Osmond, J.; Monge, R.; Fernández, L.J.; Zinoviev, K.E.; Domínguez, C.; Lechuga, L.M. Nanophotonic lab-on-a-chip platforms including novel bimodal interferometers, microfluidics and grating couplers. Lab Chip 2012, 12, 1987–1994. [Google Scholar] [CrossRef] [PubMed]
- Zinoviev, K.E.; Gonzalez-Guerrero, A.B.; Dominguez, C.; Lechuga, L.M. Integrated Bimodal Waveguide Interferometric Biosensor for Label-Free Analysis. J. Light. Technol. 2011, 29, 1926–1930. [Google Scholar] [CrossRef]
- Dante, S.; Duval, D.; Fariña, D.; González-Guerrero, A.B.; Lechuga, L.M. Linear readout of integrated interferometric biosensors using a periodic wavelength modulation. Laser Photonics Rev. 2015, 9, 248–255. [Google Scholar] [CrossRef]
- Huertas, C.S.; Farina, D.; Lechuga, L.M. Direct and Label-Free Quantification of Micro-RNA-181a at Attomolar Level in Complex Media Using a Nanophotonic Biosensor. ACS Sens. 2016, 1, 748–756. [Google Scholar] [CrossRef]
- Huertas, C.S.; Domínguez-Zotes, S.; Lechuga, L.M. Analysis of alternative splicing events for cancer diagnosis using a multiplexing nanophotonic biosensor. Sci. Rep. 2017, 7, srep41368. [Google Scholar] [CrossRef]
- Maldonado, J.; González-Guerrero, A.B.; Domínguez, C.; Lechuga, L.M. Label-free bimodal waveguide immunosensor for rapid diagnosis of bacterial infections in cirrhotic patients. Biosens. Bioelectron. 2016, 85, 310–316. [Google Scholar] [CrossRef]
- Pitart, C.; Solé, M.; Roca, I.; Román, A.; Moreno, A.; Vila, J.; Marco, F. Molecular Characterization of blaNDM-5 Carried on an IncFII Plasmid in an Escherichia coli Isolate from a Nontraveler Patient in Spain. Antimicrob. Agents Chemother. 2015, 59, 659–662. [Google Scholar] [CrossRef]
- Oteo, J.; Diestra, K.; Juan, C.; Bautista, V.; Novais, Â.; Pérez-Vázquez, M.; Moyà, B.; Miró, E.; Coque, T.M.; Oliver, A.; et al. Extended-spectrum β-lactamase-producing Escherichia coli in Spain belong to a large variety of multilocus sequence typing types, including ST10 complex/A, ST23 complex/A and ST131/B2. Int. J. Antimicrob. Agents 2009, 34, 173–176. [Google Scholar] [CrossRef]
- Coelho, A.; Mora, A.; Mamani, R.; López, C.; González-López, J.J.; Larrosa, N.; Quintero-Zarate, J.N.; Dahbi, G.; Herrera, A.; Blanco, J.; et al. Spread of Escherichia coli O25b:H4-B2-ST131 producing CTX-M-15 and SHV-12 with high virulence gene content in Barcelona (Spain). J. Antimicrob. Chemother. 2010, 66, 517–526. [Google Scholar] [CrossRef]
- González-López, J.J.; Piedra-Carrasco, N.; Salvador, F.; Rodriguez, V.; Sánchez-Montalvá, A.; Planes, A.M.; Molina, I.; Larrosa, N. ESBL-ProducingSalmonella entericaSerovar Typhi in Traveler Returning from Guatemala to Spain. Emerg. Infect. Dis. 2014, 20, 1918–1920. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhang, W.; Pan, W.; Yin, J.; Pan, Z.; Gao, S.; Jiao, X. Prevalence of qnr, aac(6′)-Ib-cr, qepA, and oqxAB in Escherichia coli isolates from humans, animals, and the environment. Antimicrob. Agents Chemother. 2012, 56, 3423–3427. [Google Scholar] [CrossRef] [PubMed]
- Lavilla, S.; Gonzalez-Lopez, J.J.; Sabate, M.; Garcia-Fernandez, A.; Larrosa, M.N.; Bartolome, R.M.; Carattoli, A.; Prats, G. Prevalence of qnr genes among extended-spectrum beta-lactamase-producing enterobacterial isolates in Barcelona, Spain. J. Antimicrob. Chemother. 2008, 61, 291–295. [Google Scholar] [CrossRef] [PubMed]
- Maldonado, J.; Estévez, M.-C.; Fernández-Gavela, A.; González-López, J.J.; González-Guerrero, A.B.; Lechuga, L.M. Label-free detection of nosocomial bacteria using a nanophotonic interferometric biosensor. Analyst 2020, 145, 497–506. [Google Scholar] [CrossRef]
| Probe | Target Sequences |
|---|---|
| CTX-M-15 synthetic target | 5′ TTT GCG CAT ACA GCG GCA CAC TTC CTA ACA ACA GCG TGA CGG TTG CCG TCG CCA TCA GCG TGA ACT GGC GCA GTG ATT TTT TAA CCA T 3′ |
| Negative synthetic control | 5′ ACG CUG UCG GUG AGU 3′ |
| CTX-M-15 F probe | 5′ NH2-(CH2)6-TTG TTA GGA AGT GTG CCG 3′ |
| CTX-M-15 R probe | 5′ NH2-(CH2)6-TAA GTG ACC AGA ATC AGC GG 3′ |
| NDM-5 probe | 5′ NH2-(CH2)6-TTT TTT TTT TGC CCA ATA TTA TGC ACC CG 3′ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maldonado, J.; González-Guerrero, A.B.; Fernández-Gavela, A.; González-López, J.J.; Lechuga, L.M. Ultrasensitive Label-Free Detection of Unamplified Multidrug-Resistance Bacteria Genes with a Bimodal Waveguide Interferometric Biosensor. Diagnostics 2020, 10, 845. https://doi.org/10.3390/diagnostics10100845
Maldonado J, González-Guerrero AB, Fernández-Gavela A, González-López JJ, Lechuga LM. Ultrasensitive Label-Free Detection of Unamplified Multidrug-Resistance Bacteria Genes with a Bimodal Waveguide Interferometric Biosensor. Diagnostics. 2020; 10(10):845. https://doi.org/10.3390/diagnostics10100845
Chicago/Turabian StyleMaldonado, Jesús, Ana Belén González-Guerrero, Adrián Fernández-Gavela, Juan José González-López, and Laura M. Lechuga. 2020. "Ultrasensitive Label-Free Detection of Unamplified Multidrug-Resistance Bacteria Genes with a Bimodal Waveguide Interferometric Biosensor" Diagnostics 10, no. 10: 845. https://doi.org/10.3390/diagnostics10100845
APA StyleMaldonado, J., González-Guerrero, A. B., Fernández-Gavela, A., González-López, J. J., & Lechuga, L. M. (2020). Ultrasensitive Label-Free Detection of Unamplified Multidrug-Resistance Bacteria Genes with a Bimodal Waveguide Interferometric Biosensor. Diagnostics, 10(10), 845. https://doi.org/10.3390/diagnostics10100845