PSA Based Biomarkers, Imagistic Techniques and Combined Tests for a Better Diagnostic of Localized Prostate Cancer
Abstract
1. Introduction
2. Classic PCa Diagnosis Pathways
2.1. DRE (Digital Rectal Examination)
2.2. PSA (Prostate-Specific Antigen) and fPSA (freePSA)
2.3. PSA Density (PSAD)
2.4. MpMRI (Multiparametric Magnetic Resonance imaging)
3. Non-Invasive Biomarkers in PCa Diagnosis
3.1. Prostate Health Index (PHI) and Prostate Health Index Density (PHID)
3.2. 4 Kscore (4 Kallikreins Score)
3.3. The Stockholm-3 Model for Prostate Cancer Detection (STHLM3)
4. Imagistic Techniques
4.1. Mp-MRI (Multiparametric Magnetic Resonance Imaging)
4.2. Mp-US (Multiparametric Ultrasound)
5. Combined Tests
6. Other Perspectives
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| 4K | 4 kalikrein score |
| 68 Ga-PET-CT | Gallium 68 positron emission tomography- computer tomography |
| ACS | American Cancer Society |
| ADC | apparent diffusion coefficient |
| AS | active surveillance |
| AUA | American Urology Association |
| AUC | area under the curve |
| BMI | body mass index |
| BpMRI | bi parametric magnetic resonance imaging |
| BPH | benign prostatic hyperplasia |
| BRCA | breast cancer gene |
| CAP | The Cluster Randomized Trial of PSA Testing for Prostate Cancer |
| CDUS | color Doppler ultrasound |
| CEUS | contrast-enhanced ultrasound |
| csPCa | clinically significant PCa |
| DCE | dynamic contrast enhancement |
| DRE | digital rectal examination |
| DWI | diffusion-weighted imaging |
| EAU | European Association of Urology |
| ERSPC | European Randomized Study of Screening for Prostate Cancer trial |
| ESMO | European Society of Medical Oncology |
| FDA | Food and Drug Administration |
| fPSA | freePSA |
| GG | Gleason Grade |
| HG | high grade |
| ISUP | International Society of Urological Pathology |
| MIC1 | macrophage inhibitory cytokine-1 |
| MicroRNAs | MiRNA |
| Mp MRI | multiparametric magnetic resonance imaging |
| Mp US | multiparametric ultrasound |
| MRGB | magnetic resonance guided biopsy |
| MSMB | microseminoprotein beta |
| NPV | negative predictive value |
| PCa | prostate cancer |
| PCA3 | prostate cancer antigen 3 |
| PCPT | Prostate Cancer Prevention Trial |
| PET-CT | positron emission tomography- computer tomography |
| PHID | prostate health index density |
| PHI | prostate health index |
| PI-RADS | Prostate Imaging–Reporting and Data System |
| PLCO | The Prostate, Lung, Colorectal and Ovarian |
| PPV | positive predictive value |
| PRI-MUS | Prostate risk identification-micro ultrasound |
| PSA | prostatic specific antigen |
| PSAD | PSA density |
| ROC | receiver operating characteristics |
| RPCRC | Rotterdam Prostate Cancer Risk Calculator |
| SB | systematic biopsy |
| SE | strain elastography |
| SMI | spectacular micro-vascular imaging |
| STHLM3 (S3M) | Stockholm 3 test |
| SWE | shear wave elastography |
| T2W | T2 weighted image |
| TB | targeted biopsy |
| TP | transperineal |
| TPM | template prostate mapping |
| tPSA | Total PSA |
| TR | transrectal |
| TRES | transrectal elastography |
| TRUS | TransRectal UltraSound |
| TRUSGB | transrectal ultrasound-guided biopsy |
References
- Nassir, A.M. A Piece in Prostate Cancer Puzzle: Future Perspective of Novel Molecular Signatures. Saudi J. Biol. Sci. 2020, 27, 1148–1154. [Google Scholar] [CrossRef]
- Barsouk, A.; Padala, S.A.; Vakiti, A.; Mohammed, A.; Saginala, K.; Thandra, K.C.; Rawla, P.; Barsouk, A. Epidemiology, Staging and Management of Prostate Cancer. Med. Sci. 2020, 8, 28. [Google Scholar] [CrossRef]
- Pilleron, S.; Soto-Perez-de-Celis, E.; Vignat, J.; Ferlay, J.; Soerjomataram, I.; Bray, F.; Sarfati, D. Estimated Global Cancer Incidence in the Oldest Adults in 2018 and Projections to 2050. Int. J. Cancer 2020, ijc.33232. [Google Scholar] [CrossRef] [PubMed]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global Cancer Statistics 2018: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA. Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Merriel, S.W.D.; Funston, G.; Hamilton, W. Prostate Cancer in Primary Care. Adv. Ther. 2018, 35, 1285–1294. [Google Scholar] [CrossRef] [PubMed]
- Mottet, N.; van den Bergh, R.C.N.; Briers, E.; Cornford, P.; de Santis, M.; Fanti, S.; Gillessen, S.; Grummet, J.; Henry, A.M.; Lam, T.B.; et al. EAU-EANM-ESTRO-ESUR-SIOG Guidelines on Prostate Cancer. Eur. Assoc. Urol. 2019, 75, 889–890. [Google Scholar]
- McDougal, W.S.; Wein, J.A.; Kavoussi, R.L.; Partin, W.A.; Peters, C. Prostate Cancer Tumor Markers. In Campbell-Walsh Urology; Elsevier: Philadelphia, PA, USA, 2016; pp. 2568–2569. [Google Scholar]
- Mottet, N.; Cornford, P.; van den Bergh, R.C.N.; Briers, E.; De Santis, M.; Fanti, S.; Gillessen, S.; Grummet, J.; Henry, A.M.; Lam, T.B.; et al. EAU Guidelines. Edn. presented at the EAU Annual Congress Amsterdam; EAU Guidelines Office: Arnhem, The Netherlands, 2020. [Google Scholar]
- Thomsen, F.B.; Brasso, K.; Klotz, L.H.; Røder, M.A.; Berg, K.D.; Iversen, P. Active Surveillance for Clinically Localized Prostate Cancer--A Systematic Review. J. Surg. Oncol. 2014, 109, 830–835. [Google Scholar] [CrossRef]
- Heidenreich, A.; Bastian, P.J.; Bellmunt, J.; Bolla, M.; Joniau, S.; van der Kwast, T.; Mason, M.; Matveev, V.; Wiegel, T.; Zattoni, F.; et al. EAU Guidelines on Prostate Cancer. Part 1: Screening, Diagnosis, and Local Treatment with Curative Intent—Update 2013. Eur. Urol. 2014, 65, 124–137. [Google Scholar] [CrossRef]
- Wilt, T.J.; Brawer, M.K.; Jones, K.M.; Barry, M.J.; Aronson, W.J.; Fox, S.; Gingrich, J.R.; Wei, J.T.; Gilhooly, P.; Grob, B.M.; et al. Radical Prostatectomy versus Observation for Localized Prostate Cancer. N. Engl. J. Med. 2012, 367, 203–213. [Google Scholar] [CrossRef]
- Hamdy, F.C.; Donovan, J.L.; Lane, J.A.; Mason, M.; Metcalfe, C.; Holding, P.; Wade, J.; Noble, S.; Garfield, K.; Young, G.; et al. Active Monitoring, Radical Prostatectomy and Radical Radiotherapy in PSA-Detected Clinically Localised Prostate Cancer: The ProtecT Three-Arm RCT. Health Technol. Assess. 2020, 24, 1–176. [Google Scholar] [CrossRef]
- Houédé, N.; Rébillard, X.; Bouvet, S.; Kabani, S.; Fabbro-Peray, P.; Trétarre, B.; Ménégaux, F. Impact on Quality of Life 3 Years after Diagnosis of Prostate Cancer Patients below 75 at Diagnosis: An Observational Case-Control Study. BMC Cancer 2020, 20, 757. [Google Scholar] [CrossRef] [PubMed]
- Tan, G.H.; Nason, G.; Ajib, K.; Woon, D.T.S.; Herrera-Caceres, J.; Alhunaidi, O.; Perlis, N. Smarter Screening for Prostate Cancer. World J. Urol. 2019, 37, 991–999. [Google Scholar] [CrossRef] [PubMed]
- Osses, D.; Roobol, M.; Schoots, I. Prediction Medicine: Biomarkers, Risk Calculators and Magnetic Resonance Imaging as Risk Stratification Tools in Prostate Cancer Diagnosis. Int. J. Mol. Sci. 2019, 20, 1637. [Google Scholar] [CrossRef]
- Schoots, I.G.; Roobol, M.J. Multivariate Risk Prediction Tools Including MRI for Individualized Biopsy Decision in Prostate Cancer Diagnosis: Current Status and Future Directions. World J. Urol. 2020, 38, 517–529. [Google Scholar] [CrossRef] [PubMed]
- Gomez Gomez, E.; Salamanca Bustos, J.J.; Carrasco Valiente, J.; Fernandez Rueda, J.L.; Blanca, A.; Valero Rosa, J.; Bravo Arrebola, I.; Marquez López, J.; Jimenez Vacas, J.M.; Luque, R.; et al. Observational Study Comparing the Accuracy/Variability between the ERSPC and the PCPT Risk Calculators for the Prediction of Significant Prostate Cancer in Patients with PSA <10 ng/mL. BMJ open 2019, 9, e031032. [Google Scholar] [CrossRef]
- Naji, L.; Randhawa, H.; Sohani, Z.; Dennis, B.; Lautenbach, D.; Kavanagh, O.; Bawor, M.; Banfield, L.; Profetto, J. Digital Rectal Examination for Prostate Cancer Screening in Primary Care: A Systematic Review and Meta-Analysis. Ann. Fam. Med. 2018, 16, 149–154. [Google Scholar] [CrossRef]
- Herrera-Caceres, J.O.; Wettstein, M.S.; Goldberg, H.; Toi, A.; Chandrasekar, T.; Woon, D.T.S.; Ahmad, A.E.; Sanmamed-Salgado, N.; Alhunaidi, O.; Ajib, K.; et al. Utility of Digital Rectal Examination in a Population with Prostate Cancer Treated with Active Surveillance. Can. Urol. Assoc. J. 2020, 14. [Google Scholar] [CrossRef]
- Gosselaar, C.; Roobol, M.J.; Roemeling, S.; Schröder, F.H. The Role of the Digital Rectal Examination in Subsequent Screening Visits in the European Randomized Study of Screening for Prostate Cancer (ERSPC), Rotterdam. Eur. Urol. 2008, 54, 581–588. [Google Scholar] [CrossRef]
- Eckersberger, E.; Finkelstein, J.; Sadri, H.; Margreiter, M.; Taneja, S.S.; Lepor, H.; Djavan, B. Screening for Prostate Cancer: A Review of the ERSPC and PLCO Trials. Rev. Urol. 2009, 11, 127–133. [Google Scholar]
- Gupta, A.; Roobol, M.J.; Savage, C.J.; Peltola, M.; Pettersson, K.; Scardino, P.T.; Vickers, A.J.; Schröder, F.H.; Lilja, H. A Four-Kallikrein Panel for the Prediction of Repeat Prostate Biopsy: Data from the European Randomized Study of Prostate Cancer Screening in Rotterdam, Netherlands. Br. J. Cancer 2010, 103, 708–714. [Google Scholar] [CrossRef]
- Vickers, A.J.; Cronin, A.M.; Roobol, M.J.; Savage, C.J.; Peltola, M.; Pettersson, K.; Scardino, P.T.; Schroder, F.H.; Lilja, H. A Four-Kallikrein Panel Predicts Prostate Cancer in Men with Recent Screening: Data from the European Randomized Study of Screening for Prostate Cancer, Rotterdam. Clin. Cancer Res. 2010, 16, 3232–3239. [Google Scholar] [CrossRef] [PubMed]
- Tikkinen, K.A.O.; Dahm, P.; Lytvyn, L.; Heen, A.F.; Vernooij, R.W.M.; Siemieniuk, R.A.C.; Wheeler, R.; Vaughan, B.; Fobuzi, A.C.; Blanker, M.H.; et al. Prostate Cancer Screening with Prostate-Specific Antigen (PSA) Test: A Clinical Practice Guideline. BMJ 2018, k3581. [Google Scholar] [CrossRef] [PubMed]
- Schröder, F.H.; Hugosson, J.; Roobol, M.J.; Tammela, T.L.J.; Zappa, M.; Nelen, V.; Kwiatkowski, M.; Lujan, M.; Määttänen, L.; Lilja, H.; et al. Screening and Prostate Cancer Mortality: Results of the European Randomised Study of Screening for Prostate Cancer (ERSPC) at 13 Years of Follow-Up. Lancet 2014, 384, 2027–2035. [Google Scholar] [CrossRef]
- Tohi, Y.; Kato, T.; Matsumoto, R.; Shinohara, N.; Shiga, K.; Yokomizo, A.; Nakamura, M.; Kume, H.; Mitsuzuka, K.; Sasaki, H.; et al. The Impact of Complications after Initial Prostate Biopsy on Repeat Protocol Biopsy Acceptance Rate. Results from the Prostate Cancer Research International: Active Surveillance JAPAN Study. Int. J. Clin. Oncol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Roberts, M.J.; Macdonald, A.; Ranasinghe, S.; Bennett, H.; Teloken, P.E.; Harris, P.; Paterson, D.; Coughlin, G.; Dunglison, N.; Esler, R.; et al. Transrectal versus Transperineal Prostate Biopsy under Intravenous Anaesthesia: A Clinical, Microbiological and Cost Analysis of 2048 Cases over 11 Years at a Tertiary Institution. Prostate Cancer Prostatic Dis. 2020. [Google Scholar] [CrossRef] [PubMed]
- Moradi, A.; Srinivasan, S.; Clements, J.; Batra, J. Beyond the Biomarker Role: Prostate-Specific Antigen (PSA) in the Prostate Cancer Microenvironment. Cancer Metastasis Rev. 2019, 38, 333–346. [Google Scholar] [CrossRef]
- Perera, M.; Mirchandani, R.; Papa, N.; Breemer, G.; Effeindzourou, A.; Smith, L.; Swindle, P.; Smith, E. PSA-Based Machine Learning Model Improves Prostate Cancer Risk Stratification in a Screening Population. World J. Urol. 2020. [Google Scholar] [CrossRef]
- Birkeland, S.; Pedersen, S.S.; Haakonsson, A.K.; Barry, M.J.; Rottmann, N. Men’s View on Participation in Decisions about Prostate-Specific Antigen (PSA) Screening: Patient and Public Involvement in Development of a Survey. BMC Med. Inform. Decis. Mak. 2020, 20, 65. [Google Scholar] [CrossRef]
- Parker, C.; Castro, E.; Fizazi, K.; Heidenreich, A.; Ost, P.; Procopio, G.; Tombal, B.; Gillessen, S. Prostate Cancer: ESMO Clinical Practice Guidelines for Diagnosis, Treatment and Follow-Up. Ann. Oncol. 2020, 31, 1119–1134. [Google Scholar] [CrossRef]
- Smith, R.A.; Andrews, K.S.; Brooks, D.; Fedewa, S.A.; Manassaram-Baptiste, D.; Saslow, D.; Wender, R.C. Cancer Screening in the United States, 2019: A Review of Current American Cancer Society Guidelines and Current Issues in Cancer Screening. CA. Cancer J. Clin. 2019, 69, 184–210. [Google Scholar] [CrossRef]
- Loeb, S.; Bruinsma, S.M.; Nicholson, J.; Briganti, A.; Pickles, T.; Kakehi, Y.; Carlsson, S.V.; Roobol, M.J. Active Surveillance for Prostate Cancer: A Systematic Review of Clinicopathologic Variables and Biomarkers for Risk Stratification. Eur. Urol. 2015, 67, 619–626. [Google Scholar] [CrossRef] [PubMed]
- Schulze, A.; Christoph, F.; Sachs, M.; Schroeder, J.; Stephan, C.; Schostak, M.; Koenig, F. Use of the Prostate Health Index and Density in 3 Outpatient Centers to Avoid Unnecessary Prostate Biopsies. Urol. Int. 2020, 104, 181–186. [Google Scholar] [CrossRef] [PubMed]
- Takeshima, Y.; Tanaka, Y.; Takemura, K.; Nakazono, S.; Yamashita, E.; Kume, H. Evaluating the Efficacy of a Low-Cost Cognitive MRI-Targeted Prostate Biopsy Protocol: Is There Still a Role for Lower Volume Centers in the Prostate Imaging Reporting and Data System (PI-RADS) Version 2 Era? Int. Urol. Nephrol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Nordström, T.; Akre, O.; Aly, M.; Grönberg, H.; Eklund, M. Prostate-Specific Antigen (PSA) Density in the Diagnostic Algorithm of Prostate Cancer. Prostate Cancer Prostatic Dis. 2018, 21, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Vickers, A.; Carlsson, S.V.; Cooperberg, M. Routine Use of Magnetic Resonance Imaging for Early Detection of Prostate Cancer Is Not Justified by the Clinical Trial Evidence. Eur. Urol. 2020. [Google Scholar] [CrossRef]
- Jin, W.; Fei, X.; Wang, X.; Song, Y.; Chen, F. Detection and Prognosis of Prostate Cancer Using Blood-Based Biomarkers. Mediat. Inflamm. 2020, 2020, 1–11. [Google Scholar] [CrossRef]
- Brönimann, S.; Pradere, B.; Karakiewicz, P.; Abufaraj, M.; Briganti, A.; Shariat, S.F. An Overview of Current and Emerging Diagnostic, Staging and Prognostic Markers for Prostate Cancer. Expert Rev. Mol. Diagn. 2020, 1–10. [Google Scholar] [CrossRef]
- Heijnsdijk, E.A.M.; Denham, D.; de Koning, H.J. The Cost-Effectiveness of Prostate Cancer Detection with the Use of Prostate Health Index. Value Health 2016, 19, 153–157. [Google Scholar] [CrossRef]
- Kim, L.; Boxall, N.; George, A.; Burling, K.; Acher, P.; Aning, J.; McCracken, S.; Page, T.; Gnanapragasam, V.J. Clinical Utility and Cost Modelling of the Phi Test to Triage Referrals into Image-Based Diagnostic Services for Suspected Prostate Cancer: The PRIM (Phi to RefIne Mri) Study. BMC Med. 2020, 18, 95. [Google Scholar] [CrossRef]
- Schwen, Z.R.; Mamawala, M.; Tosoian, J.J.; Druskin, S.C.; Ross, A.E.; Sokoll, L.J.; Epstein, J.I.; Carter, H.B.; Gorin, M.A.; Pavlovich, C.P. Prostate Health Index and Multiparametric Magnetic Resonance Imaging to Predict Prostate Cancer Grade Reclassification in Active Surveillance. BJU Int. 2020. [Google Scholar] [CrossRef]
- Nassir, A.M.; Kamel, H.F.M. Explication of the Roles of Prostate Health Index (PHI) and Urokinase Plasminogen Activator (UPA) as Diagnostic and Predictor Tools for Prostate Cancer in Equivocal PSA Range of 4–10 Ng/ML. Saudi J. Biol. Sci. 2020, 27, 1975–1984. [Google Scholar] [CrossRef] [PubMed]
- Lin, D.W.; Newcomb, L.F.; Brown, M.D.; Sjoberg, D.D.; Dong, Y.; Brooks, J.D.; Carroll, P.R.; Cooperberg, M.; Dash, A.; Ellis, W.J.; et al. Evaluating the Four Kallikrein Panel of the 4Kscore for Prediction of High-Grade Prostate Cancer in Men in the Canary Prostate Active Surveillance Study. Eur. Urol. 2017, 72, 448–454. [Google Scholar] [CrossRef] [PubMed]
- Braun, K.; Sjoberg, D.D.; Vickers, A.J.; Lilja, H.; Bjartell, A.S. A Four-Kallikrein Panel Predicts High-Grade Cancer on Biopsy: Independent Validation in a Community Cohort. Eur. Urol. 2016, 69, 505–511. [Google Scholar] [CrossRef] [PubMed]
- Darst, B.F.; Chou, A.; Wan, P.; Pooler, L.; Sheng, X.; Vertosick, E.A.; Conti, D.V.; Wilkens, L.R.; Le Marchand, L.; Vickers, A.J.; et al. The Four-Kallikrein Panel Is Effective in Identifying Aggressive Prostate Cancer in a Multiethnic Population. Cancer Epidemiol. Biomark. Prev. 2020, 29, 1381–1388. [Google Scholar] [CrossRef]
- Verbeek, J.F.M.; Bangma, C.H.; Kweldam, C.F.; van der Kwast, T.H.; Kümmerlin, I.P.; van Leenders, G.J.L.H.; Roobol, M.J. Reducing Unnecessary Biopsies While Detecting Clinically Significant Prostate Cancer Including Cribriform Growth with the ERSPC Rotterdam Risk Calculator and 4Kscore. Urol. Oncol. Semin. Orig. Investig. 2019, 37, 138–144. [Google Scholar] [CrossRef]
- Vigneswaran, H.T.; Discacciati, A.; Gann, P.H.; Grönberg, H.; Eklund, M.; Abern, M.R. Ethnic Variation in Prostate Cancer Detection: A Feasibility Study for Use of the Stockholm3 Test in a Multiethnic U.S. Cohort. Prostate Cancer Prostatic Dis. 2020. [Google Scholar] [CrossRef]
- Grönberg, H.; Adolfsson, J.; Aly, M.; Nordström, T.; Wiklund, P.; Brandberg, Y.; Thompson, J.; Wiklund, F.; Lindberg, J.; Clements, M.; et al. Prostate Cancer Screening in Men Aged 50–69 Years (STHLM3): A Prospective Population-Based Diagnostic Study. Lancet Oncol. 2015, 16, 1667–1676. [Google Scholar] [CrossRef]
- Ström, P.; Nordström, T.; Aly, M.; Egevad, L.; Grönberg, H.; Eklund, M. The Stockholm-3 Model for Prostate Cancer Detection: Algorithm Update, Biomarker Contribution, and Reflex Test Potential. Eur. Urol. 2018, 74, 204–210. [Google Scholar] [CrossRef]
- Grönberg, H.; Eklund, M.; Picker, W.; Aly, M.; Jäderling, F.; Adolfsson, J.; Landquist, M.; Haug, E.S.; Ström, P.; Carlsson, S.; et al. Prostate Cancer Diagnostics Using a Combination of the Stockholm3 Blood Test and Multiparametric Magnetic Resonance Imaging. Eur. Urol. 2018, 74, 722–728. [Google Scholar] [CrossRef]
- Palsdottir, T.; Nordström, T.; Aly, M.; Jäderling, F.; Clements, M.; Grönberg, H.; Eklund, M. A Unified Prostate Cancer Risk Prediction Model Combining the Stockholm3 Test and Magnetic Resonance Imaging. Eur. Urol. Oncol. 2019, 2, 490–496. [Google Scholar] [CrossRef]
- Thestrup, K.C.D.; Logager, V.; Baslev, I.; Møller, J.M.; Hansen, R.H.; Thomsen, H.S. Biparametric versus Multiparametric MRI in the Diagnosis of Prostate Cancer. Acta Radiol. Open 2016, 5, 205846011666304. [Google Scholar] [CrossRef] [PubMed]
- Kasivisvanathan, V.; Rannikko, A.S.; Borghi, M.; Panebianco, V.; Mynderse, L.A.; Vaarala, M.H.; Briganti, A.; Budäus, L.; Hellawell, G.; Hindley, R.G.; et al. MRI-Targeted or Standard Biopsy for Prostate-Cancer Diagnosis. N. Engl. J. Med. 2018, 378, 1767–1777. [Google Scholar] [CrossRef] [PubMed]
- Labra, A.; González, F.; Silva, C.; Franz, G.; Pinochet, R.; Gupta, R.T. MRI/TRUS Fusion vs. Systematic Biopsy: Intra-Patient Comparison of Diagnostic Accuracy for Prostate Cancer Using PI-RADS V2. Abdom. Radiol. 2020, 45, 2235–2243. [Google Scholar] [CrossRef]
- Noh, T.I.; Tae, J.H.; Kim, H.K.; Shim, J.S.; Kang, S.G.; Sung, D.J.; Cheon, J.; Lee, J.G.; Kang, S.H. Diagnostic Accuracy and Value of Magnetic Resonance Imaging–Ultrasound Fusion Transperineal Targeted and Template Systematic Prostate Biopsy Based on Bi-Parametric Magnetic Resonance Imaging. Cancer Res. Treat. 2020, 52, 714–721. [Google Scholar] [CrossRef] [PubMed]
- Marzouk, K.; Ehdaie, B.; Vertosick, E.; Zappala, S.; Vickers, A. Developing an Effective Strategy to Improve the Detection of Significant Prostate Cancer by Combining the 4Kscore and Multiparametric MRI. Urol. Oncol. Semin. Orig. Investig. 2019, 37, 672–677. [Google Scholar] [CrossRef] [PubMed]
- Klotz, L.; Pond, G.; Loblaw, A.; Sugar, L.; Moussa, M.; Berman, D.; Van der Kwast, T.; Vesprini, D.; Milot, L.; Kebabdjian, M.; et al. Randomized Study of Systematic Biopsy Versus Magnetic Resonance Imaging and Targeted and Systematic Biopsy in Men on Active Surveillance (ASIST): 2-Year Postbiopsy Follow-Up. Eur. Urol. 2020, 77, 311–317. [Google Scholar] [CrossRef]
- Drudi, F.M.; Cantisani, V.; Angelini, F.; Ciccariello, M.; Messineo, D.; Ettorre, E.; Liberatore, M.; Scialpi, M. Multiparametric MRI Versus Multiparametric US in the Detection of Prostate Cancer. Anticancer Res. 2019, 39, 3101–3110. [Google Scholar] [CrossRef]
- Zhen, L.; Liu, X.; Yegang, C.; Yongjiao, Y.; Yawei, X.; Jiaqi, K.; Xianhao, W.; Yuxuan, S.; Rui, H.; Wei, Z.; et al. Accuracy of Multiparametric Magnetic Resonance Imaging for Diagnosing Prostate Cancer: A Systematic Review and Meta-Analysis. BMC Cancer 2019, 19, 1244. [Google Scholar] [CrossRef]
- Morote, J.; Celma, A.; Roche, S.; de Torres, I.M.; Mast, R.; Semedey, M.E.; Regis, L.; Planas, J. Who Benefits from Multiparametric Magnetic Resonance Imaging After Suspicion of Prostate Cancer? Eur. Urol. Oncol. 2019, 2, 664–669. [Google Scholar] [CrossRef]
- Porpiglia, F.; Russo, F.; Manfredi, M.; Mele, F.; Fiori, C.; Bollito, E.; Papotti, M.; Molineris, I.; Passera, R.; Regge, D. The Roles of Multiparametric Magnetic Resonance Imaging, PCA3 and Prostate Health Index—Which Is the Best Predictor of Prostate Cancer after a Negative Biopsy? J. Urol. 2014, 192, 60–66. [Google Scholar] [CrossRef]
- van der Leest, M.; Cornel, E.; Israël, B.; Hendriks, R.; Padhani, A.R.; Hoogenboom, M.; Zamecnik, P.; Bakker, D.; Setiasti, A.Y.; Veltman, J.; et al. Head-to-Head Comparison of Transrectal Ultrasound-Guided Prostate Biopsy Versus Multiparametric Prostate Resonance Imaging with Subsequent Magnetic Resonance-Guided Biopsy in Biopsy-Naïve Men with Elevated Prostate-Specific Antigen: A Large Prospective Mu. Eur. Urol. 2019, 75, 570–578. [Google Scholar] [CrossRef]
- Correas, J.-M.; Halpern, E.J.; Barr, R.G.; Ghai, S.; Walz, J.; Bodard, S.; Dariane, C.; de la Rosette, J. Advanced Ultrasound in the Diagnosis of Prostate Cancer. World J. Urol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Yunkai, Z.; Yaqing, C.; Jun, J.; Tingyue, Q.; Weiyong, L.; Yuehong, Q.; Wenbin, G.; Lifeng, W.; Jun, Q. Comparison of Contrast-Enhanced Ultrasound Targeted Biopsy versus Standard Systematic Biopsy for Clinically Significant Prostate Cancer Detection: Results of a Prospective Cohort Study with 1024 Patients. World J. Urol. 2019, 37, 805–811. [Google Scholar] [CrossRef] [PubMed]
- Ghai, S.; Eure, G.; Fradet, V.; Hyndman, M.E.; McGrath, T.; Wodlinger, B.; Pavlovich, C.P. Assessing Cancer Risk on Novel 29 MHz Micro-Ultrasound Images of the Prostate: Creation of the Micro-Ultrasound Protocol for Prostate Risk Identification. J. Urol. 2016, 196, 562–569. [Google Scholar] [CrossRef]
- Abouassaly, R.; Klein, E.A.; El-Shefai, A.; Stephenson, A. Impact of Using 29 MHz High-Resolution Micro-Ultrasound in Real-Time Targeting of Transrectal Prostate Biopsies: Initial Experience. World J. Urol. 2020, 38, 1201–1206. [Google Scholar] [CrossRef] [PubMed]
- Klotz, L.; Lughezzani, G.; Maffei, D.; Sanchez, A.; Pereira, J.G.; Staerman, F.; Cash, H.; Luger, F.; Lopez, L.; Sanchez-Salas, R.; et al. Comparison of Micro-Ultrasound and Multiparametric Magnetic Resonance Imaging for Prostate Cancer: A Multicenter, Prospective Analysis. Can. Urol. Assoc. J. 2020, 15. [Google Scholar] [CrossRef]
- Zhu, Y.-C.; Shan, J.; Zhang, Y.; Jiang, Q.; Wang, Y.-B.; Deng, S.-H.; Qu, Q.-H.; Li, Q. Prostate Cancer Vascularity: Superb Microvascular Imaging Ultrasonography with Histopathology Correlation. Med. Sci. Monit. 2019, 25, 8571–8578. [Google Scholar] [CrossRef]
- Lughezzani, G.; Saita, A.; Lazzeri, M.; Paciotti, M.; Maffei, D.; Lista, G.; Hurle, R.; Buffi, N.M.; Guazzoni, G.; Casale, P. Comparison of the Diagnostic Accuracy of Micro-Ultrasound and Magnetic Resonance Imaging/Ultrasound Fusion Targeted Biopsies for the Diagnosis of Clinically Significant Prostate Cancer. Eur. Urol. Oncol. 2019, 2, 329–332. [Google Scholar] [CrossRef]
- Grey, A.D.R.; Connor, M.J.; Tam, J.; Loch, T. Can Transrectal Prostate Ultrasound Compete with Multiparametric MRI in the Detection of Clinically Significant Prostate Cancer? Transl. Androl. Urol. 2020, 9, 1492–1500. [Google Scholar] [CrossRef]
- Falagario, U.G.; Martini, A.; Wajswol, E.; Treacy, P.-J.; Ratnani, P.; Jambor, I.; Anastos, H.; Lewis, S.; Haines, K.; Cormio, L.; et al. Avoiding Unnecessary Magnetic Resonance Imaging (MRI) and Biopsies: Negative and Positive Predictive Value of MRI According to Prostate-Specific Antigen Density, 4Kscore and Risk Calculators. Eur. Urol. Oncol. 2019. [Google Scholar] [CrossRef]
- Wysock, J.S.; Becher, E.; Persily, J.; Loeb, S.; Lepor, H. Concordance and Performance of 4Kscore and SelectMDx for Informing Decision to Perform Prostate Biopsy and Detection of Prostate Cancer. Urology 2020, 141, 119–124. [Google Scholar] [CrossRef]
- Wiemer, L.; Hollenbach, M.; Heckmann, R.; Kittner, B.; Plage, H.; Reimann, M.; Asbach, P.; Friedersdorff, F.; Schlomm, T.; Hofbauer, S.; et al. Evolution of Targeted Prostate Biopsy by Adding Micro-Ultrasound to the Magnetic Resonance Imaging Pathway. Eur. Urol. Focus 2020. [Google Scholar] [CrossRef] [PubMed]
- Gadzinski, A.J.; Cooperberg, M.R. Prostate Cancer Markers. U.S. Patent 6,673,545, 6 January 2018. [Google Scholar] [CrossRef]
- Ahmed, H.U.; El-Shater Bosaily, A.; Brown, L.C.; Gabe, R.; Kaplan, R.; Parmar, M.K.; Collaco-Moraes, Y.; Ward, K.; Hindley, R.G.; Freeman, A.; et al. Diagnostic Accuracy of Multi-Parametric MRI and TRUS Biopsy in Prostate Cancer (PROMIS): A Paired Validating Confirmatory Study. Lancet 2017, 389, 815–822. [Google Scholar] [CrossRef]
- Afaq, A.; Bomanji, J. Prostate-Specific Membrane Antigen Positron Emission Tomography in the Management of Recurrent Prostate Cancer. Br. Med. Bull. 2018, 128, 37–48. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.W.; Cheon, G.J. Prostate-Specific Membrane Antigen PET Imaging in Prostate Cancer: Opportunities and Challenges. Korean J. Radiol. 2018, 19, 819. [Google Scholar] [CrossRef] [PubMed]
- Munteanu, V.C.; Munteanu, R.A.; Onaciu, A.; Berindan-Neagoe, I.; Petrut, B.; Coman, I. MiRNA-Based Inspired Approach in Diagnosis of Prostate Cancer. Medicina 2020, 56, 94. [Google Scholar] [CrossRef]
- Fredsøe, J.; Rasmussen, A.K.I.; Mouritzen, P.; Bjerre, M.T.; Østergren, P.; Fode, M.; Borre, M.; Sørensen, K.D. Profiling of Circulating MicroRNAs in Prostate Cancer Reveals Diagnostic Biomarker Potential. Diagnostics 2020, 10, 188. [Google Scholar] [CrossRef]
- Mannaerts, C.K.; Gayet, M.; Verbeek, J.F.; Engelbrecht, M.R.W.; Savci-Heijink, C.D.; Jager, G.J.; Gielens, M.P.M.; van der Linden, H.; Beerlage, H.P.; de Reijke, T.M.; et al. Prostate Cancer Risk Assessment in Biopsy-Naïve Patients: The Rotterdam Prostate Cancer Risk Calculator in Multiparametric Magnetic Resonance Imaging-Transrectal Ultrasound (TRUS) Fusion Biopsy and Systematic TRUS Biopsy. Eur. Urol. Oncol. 2018, 1, 109–117. [Google Scholar] [CrossRef]
- Teoh, J.Y.-C.; Leung, C.-H.; Wang, M.H.; Chiu, P.K.-F.; Yee, C.-H.; Ng, C.-F.; Wong, M.C.-S. The Cost-Effectiveness of Prostate Health Index for Prostate Cancer Detection in Chinese Men. Prostate Cancer Prostatic Dis. 2020. [Google Scholar] [CrossRef]
- Sathianathen, N.J.; Kuntz, K.M.; Alarid-Escudero, F.; Lawrentschuk, N.L.; Bolton, D.M.; Murphy, D.G.; Weight, C.J.; Konety, B.R. Incorporating Biomarkers into the Primary Prostate Biopsy Setting: A Cost-Effectiveness Analysis. J. Urol. 2018, 200, 1215–1220. [Google Scholar] [CrossRef]
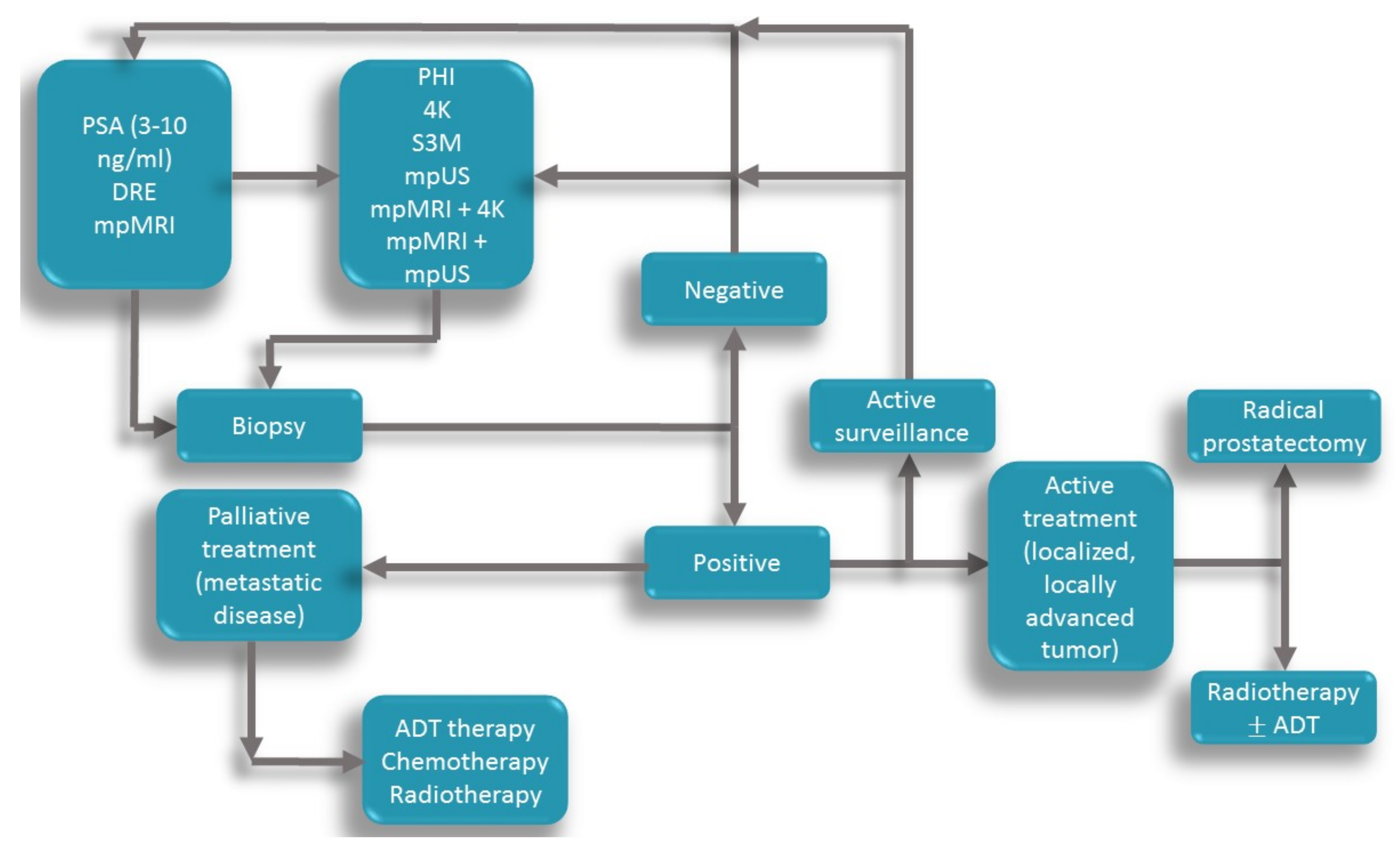
| Screening Recommendation Associations | Recommendations |
|---|---|
| AUA (American Association of Urology) [30] | PSA screening for men 55–70 years old Urinary, serum biomarkers, imaging, Risk Calculators can be used for men with a suspicious PSA level Screening at 2 years or more can be applied to reduce the harm of screening No screening if life expectancy is <15 years old, or men >70 years old |
| EAU (European Association of Urology) [8] | PSA screening for men over 50-year-old or over 45 if they had a family history of PCa, African descent or over 40 if carrying BRCA2 mutations Men with PSA level >1 ng/mL at 40-year-old or >2 ng/mL at 60-year-old are at risk Men with PSA 2–10ng/mL and normal DRE, prior to biopsy, use additional tests (PCA3, PHI, 4Kscore, Kallikreins, TMPRSS2-ERG or Risk Calculators, Imaging (mpMRI) PSA screening every 2 years for those at risk |
| ESMO (European Society of Medical Oncology) [31] | Subclinical PCa is common in men >50-year-old PSA screening for men 55–70 years old PSA level >1 ng/mL for men at 40 years old and >2 ng/mL for men at 60-year-old represents a risk Early PSA screening for men >50 years old, >45 with a family history of PCa, African-American, and BRCA 1/2 carriers Do not test if life expectancy is <10 years Use Risk Calculators or mpMRI before biopsy |
| ACS (American Cancer Society) [32] | Informed PSA screening for men >50 years old, >45 if African-American or men with first-degree relative diagnosed with PCa (under the age 65), >40 years old if they have more than one first degree relative with PCa Early screening for men with PSA level 2.5 ng/mL If the biopsy is negative, additional tests can help (PHI, 4Kscore, PCa3, ConfirmMDx) |
| Marker/Technique | Number of Patients | Study Design | Cancer Detection | Other Details |
|---|---|---|---|---|
| PHI [41] | 545 | Prospective, multicentric study | PHI AUC 0.82 PSAD AUC 0.79 PSA AUC 0.70 | If MRI is negative, PHI AUC for positive PCa 0.78 If PHI ≥ 30, 35% of MRIs could be avoided Spared 40% of biopsies Missed 8% PCas |
| 4Kscore [46] | 2 224 PCa 2 230 controls | Prospective, multicentric, case-control, multiethnic | 4K AUC 0.782 PSA+fPSA AUC 0.739 PSA AUC 0.685 | The AUCs represent the identification of aggressive PCas 4kscore can accurately differentiate between benign and malign cases, indolent and aggressive tumors |
| S3M [50] | 59 149 | Prospective, population baased, diagnostic trial | S3M AUC 0.75 PSA AUC 0.58 | 34% of biopsies spared |
| MRI [76] | 576 | Prospective, multicenter, paired-cohort | sensitivity 93%, specificity 41% | Could avoid 27% of biopsies TRUS directed by MRI, could diagnose 18% more csPCas |
| mpUS [68] | 1040 | Prospective, multicenter | mpUS sensitivity 94%, specificity 22% | Results were similar with mpMRI sensitivity 93%, specificity 23% |
| 4Kscore, MRI, PSAD [72] | 266 | Retrospective, unicentric | 4Kscore and MRI if 4K > 7.5 | 4kscore followed by MRI if 4K > 7.5 and biopsy if MRI is positive or biopsy if MRI negative and 4K > 18 |
| Country | Test | Costs |
|---|---|---|
| Germany [34] | PHID | 100 EUR |
| China, Hong Kong [82] | PHI | 370 USD |
| US [15] | PHI | 80 USD |
| 4Kscore | 500 USD | |
| PCA 3 | 300 USD | |
| MiPS | 700 USD | |
| SelectMDx | 300 USD | |
| ERSPC RPCRC Risk Calculator | 0 | |
| PCPT Risk Calculator | 0 | |
| MRI | 1000 USD | |
| Europe [15] | MRI | 300–500 EUR |
| UK [71] | US Scanner | 35,000–150,000 USD |
| MRI machine | Approx. 3 million USD | |
| MRI + biopsy | 965 GBP |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Munteanu, V.C.; Munteanu, R.A.; Gulei, D.; Schitcu, V.H.; Petrut, B.; Berindan Neagoe, I.; Achimas Cadariu, P.; Coman, I. PSA Based Biomarkers, Imagistic Techniques and Combined Tests for a Better Diagnostic of Localized Prostate Cancer. Diagnostics 2020, 10, 806. https://doi.org/10.3390/diagnostics10100806
Munteanu VC, Munteanu RA, Gulei D, Schitcu VH, Petrut B, Berindan Neagoe I, Achimas Cadariu P, Coman I. PSA Based Biomarkers, Imagistic Techniques and Combined Tests for a Better Diagnostic of Localized Prostate Cancer. Diagnostics. 2020; 10(10):806. https://doi.org/10.3390/diagnostics10100806
Chicago/Turabian StyleMunteanu, Vlad Cristian, Raluca Andrada Munteanu, Diana Gulei, Vlad Horia Schitcu, Bogdan Petrut, Ioana Berindan Neagoe, Patriciu Achimas Cadariu, and Ioan Coman. 2020. "PSA Based Biomarkers, Imagistic Techniques and Combined Tests for a Better Diagnostic of Localized Prostate Cancer" Diagnostics 10, no. 10: 806. https://doi.org/10.3390/diagnostics10100806
APA StyleMunteanu, V. C., Munteanu, R. A., Gulei, D., Schitcu, V. H., Petrut, B., Berindan Neagoe, I., Achimas Cadariu, P., & Coman, I. (2020). PSA Based Biomarkers, Imagistic Techniques and Combined Tests for a Better Diagnostic of Localized Prostate Cancer. Diagnostics, 10(10), 806. https://doi.org/10.3390/diagnostics10100806







