Application of Hepatic Venous Pressure Gradient to Predict Prognosis in Cirrhotic Patients with a Low Model for End-stage Liver Disease Score
Abstract
:1. Introduction
2. Method
2.1. Study Population
2.2. Hepatic Venous Pressure Gradient Measurement
2.3. Statistical Analysis
2.4. Ethical Consideration
3. Results
3.1. Baseline Characteristics
3.2. Development of a Novel Risk Scoring Model from The Derivation Cohort
3.3. Impact of The H6C Score In Predicting The Overall Survival (OS) Of Cirrhotic Patients
3.4. Prognostic Power Of The Models For Predicting OS
3.5. Prognostic Power of The H6C Score For Predicting OS In Patients With Viral Etiology
3.6. External Validation of The H6C Score in Predicting OS Of Cirrhotic Patients
4. Discussion
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Malinchoc, M.; Kamath, P.S.; Gordon, F.D.; Peine, C.J.; Rank, J.; Ter Borg, P.C. A model to predict poor survival in patients undergoing transjugular intrahepatic portosystemic shunts. Hepatology 2000, 31, 864–871. [Google Scholar] [CrossRef] [PubMed]
- Kamath, P.S.; Wiesner, R.H.; Malinchoc, M.; Kremers, W.; Therneau, T.M.; Kosberg, C.L.; D'Amico, G.; Dickson, E.R.; Kim, W.R. A model to predict survival in patients with end-stage liver disease. Hepatology 2001, 33, 464–470. [Google Scholar] [CrossRef] [PubMed]
- Wiesner, R.H.; McDiarmid, S.V.; Kamath, P.S.; Edwards, E.B.; Malinchoc, M.; Kremers, W.K.; Krom, R.A.; Kim, W.R. MELD and PELD: Application of survival models to liver allocation. Liver Transpl. 2001, 7, 567–580. [Google Scholar] [CrossRef] [PubMed]
- Sheth, M.; Riggs, M.; Patel, T. Utility of the Mayo End-Stage Liver Disease (MELD) score in assessing prognosis of patients with alcoholic hepatitis. BMC Gastroenterol. 2002, 2, 2. [Google Scholar] [CrossRef] [PubMed]
- Chalasani, N.; Kahi, C.; Francois, F.; Pinto, A.; Marathe, A.; Bini, E.J.; Pandya, P.; Sitaraman, S.; Shen, J. Model for end-stage liver disease (MELD) for predicting mortality in patients with acute variceal bleeding. Hepatology 2002, 35, 1282–1284. [Google Scholar] [CrossRef]
- Wiesner, R.; Edwards, E.; Freeman, R.; Harper, A.; Kim, R.; Kamath, P.; Kremers, W.; Lake, J.; Howard, T.; Merion, R.M.; et al. United network for organ sharing liver disease severity score, C. Model for end-stage liver disease (MELD) and allocation of donor livers. Gastroenterology 2003, 124, 91–96. [Google Scholar] [CrossRef] [Green Version]
- Sanyal, A.J.; Bosch, J.; Blei, A.; Arroyo, V. Portal hypertension and its complications. Gastroenterology 2008, 134, 1715–1728. [Google Scholar] [CrossRef] [Green Version]
- Bosch, J.; Berzigotti, A.; Garcia-Pagan, J.C.; Abraldes, J.G. The management of portal hypertension: Rational basis, available treatments and future options. J. Hepatol. 2008, 48, S68–S92. [Google Scholar] [CrossRef] [Green Version]
- Myers, J.D.; Taylor, W.J. Occlusive hepatic venous catheterization in the study of the normal liver, cirrhosis of the liver and noncirrhotic portal hypertension. Circulation 1956, 13, 368–380. [Google Scholar]
- Suk, K.T. Hepatic venous pressure gradient: Clinical use in chronic liver disease. Clin. Mol. Hepatol. 2014, 20, 6–14. [Google Scholar] [CrossRef]
- Ripoll, C.; Banares, R.; Rincon, D.; Catalina, M.V.; Lo Iacono, O.; Salcedo, M.; Clemente, G.; Nunez, O.; Matilla, A.; Molinero, L.M. Influence of hepatic venous pressure gradient on the prediction of survival of patients with cirrhosis in the MELD Era. Hepatology 2005, 42, 793–801. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.Y.; Suk, K.T.; Jeong, S.W.; Ryu, T.; Kim, D.J.; Baik, S.K.; Sohn, J.H.; Jeong, W.K.; Choi, E.; Jang, J.Y.; et al. The new cutoff value of the hepatic venous pressure gradient on predicting long-term survival in cirrhotic patients. J. Korean. Med. Sci. 2019, 34, e223. [Google Scholar] [CrossRef] [PubMed]
- Pagliaro, L. MELD: The end of Child-Pugh classification? J. Hepatol. 2002, 36, 141–142. [Google Scholar] [CrossRef]
- Garcia-Tsao, G.; Friedman, S.; Iredale, J.; Pinzani, M. Now there are many (stages) where before there was one: In search of a pathophysiological classification of cirrhosis. Hepatology 2010, 51, 1445–1449. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Said, A.; Williams, J.; Holden, J.; Remington, P.; Gangnon, R.; Musat, A.; Lucey, M.R. Model for end stage liver disease score predicts mortality across a broad spectrum of liver disease. J. Hepatol. 2004, 40, 897–903. [Google Scholar] [CrossRef] [PubMed]
- Groszmann, R.J.; Wongcharatrawee, S. The hepatic venous pressure gradient: Anything worth doing should be done right. Hepatology 2004, 39, 280–282. [Google Scholar] [CrossRef] [PubMed]
- Somsouk, M.; Kornfield, R.; Vittinghoff, E.; Inadomi, J.M.; Biggins, S.W. Moderate ascites identifies patients with low model for end-stage liver disease scores awaiting liver transplantation who have a high mortality risk. Liver Transpl. 2011, 17, 129–136. [Google Scholar] [CrossRef] [Green Version]
- Ripoll, C. Hepatic venous pressure gradient and outcomes in cirrhosis. J. Clin. Gastroenterol. 2007, 41, S330–S335. [Google Scholar] [CrossRef]
- Bosch, J.; Abraldes, J.G.; Berzigotti, A.; Garcia-Pagan, J.C. The clinical use of HVPG measurements in chronic liver disease. Nat. Rev. Gastroenterol. Hepatol. 2009, 6, 573–582. [Google Scholar] [CrossRef]
- Silva-Junior, G.; Baiges, A.; Turon, F.; Torres, F.; Hernandez-Gea, V.; Bosch, J.; Garcia-Pagan, J.C. The prognostic value of hepatic venous pressure gradient in patients with cirrhosis is highly dependent on the accuracy of the technique. Hepatology 2015, 62, 1584–1592. [Google Scholar] [CrossRef] [Green Version]
- Kwong, A.J.; Lai, J.C.; Dodge, J.L.; Roberts, J.P. Outcomes for liver transplant candidates listed with low model for end-stage liver disease score. Liver Transpl. 2015, 21, 1403–1409. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Atiemo, K.; Skaro, A.; Maddur, H.; Zhao, L.; Montag, S.; VanWagner, L.; Goel, S.; Kho, A.; Ho, B.; Kang, R.; et al. Mortality risk factors among patients with cirrhosis and a low model for end-stage liver disease sodium score (≤15): An Analysis of Liver Transplant Allocation Policy Using Aggregated Electronic Health Record Data. Am. J. Transplant. 2017, 17, 2410–2419. [Google Scholar] [CrossRef] [PubMed]
- Huo, T.I.; Lin, H.C.; Wu, J.C.; Hou, M.C.; Lee, F.Y.; Lee, P.C.; Chang, F.Y.; Lee, S.D. Limitation of the model for end-stage liver disease for outcome prediction in patients with cirrhosis-related complications. Clin. Transplant. 2006, 20, 188–194. [Google Scholar] [CrossRef] [PubMed]
- La Mura, V.; Garcia-Guix, M.; Berzigotti, A.; Abraldes, J.G.; Garcia-Pagan, J.C.; Villanueva, C.; Bosch, J. A prognostic strategy based on stage of cirrhosis and HVPG to improve risk stratification after variceal bleeding. Hepatology 2020. [Google Scholar] [CrossRef] [PubMed]
- Hall, A.; Germani, G.; Isgro, G.; Burroughs, A.K.; Dhillon, A.P. Fibrosis distribution in explanted cirrhotic livers. Histopathology 2012, 60, 270–277. [Google Scholar] [CrossRef] [PubMed]
- Michalak, S.; Rousselet, M.C.; Bedossa, P.; Pilette, C.; Chappard, D.; Oberti, F.; Gallois, Y.; Cales, P. Respective roles of porto-septal fibrosis and centrilobular fibrosis in alcoholic liver disease. J. Pathol. 2003, 201, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Bolognesi, M.; Verardo, A.; Di Pascoli, M. Peculiar characteristics of portal-hepatic hemodynamics of alcoholic cirrhosis. World J. Gastroenterol. 2014, 20, 8005–8010. [Google Scholar] [CrossRef]
- Mehta, G.; Mookerjee, R.P.; Sharma, V.; Jalan, R. Systemic inflammation is associated with increased intrahepatic resistance and mortality in alcohol-related acute-on-chronic liver failure. Liver Int. 2015, 35, 724–734. [Google Scholar] [CrossRef]
- Mookerjee, R.P.; Sen, S.; Davies, N.A.; Hodges, S.J.; Williams, R.; Jalan, R. Tumour necrosis factor alpha is an important mediator of portal and systemic haemodynamic derangements in alcoholic hepatitis. Gut 2003, 52, 1182–1187. [Google Scholar] [CrossRef]
- D'Amico, G.; Garcia-Pagan, J.C.; Luca, A.; Bosch, J. Hepatic vein pressure gradient reduction and prevention of variceal bleeding in cirrhosis: A systematic review. Gastroenterology 2006, 131, 1611–1624. [Google Scholar] [CrossRef]
- Augustin, S.; Gonzalez, A.; Badia, L.; Millan, L.; Gelabert, A.; Romero, A.; Segarra, A.; Martell, M.; Esteban, R.; Guardia, J.; et al. Long-term follow-up of hemodynamic responders to pharmacological therapy after variceal bleeding. Hepatology 2012, 56, 706–714. [Google Scholar] [CrossRef] [PubMed]
- Sauerbruch, T.; Mengel, M.; Dollinger, M.; Zipprich, A.; Rossle, M.; Panther, E.; Wiest, R.; Caca, K.; Hoffmeister, A.; Lutz, H.; et al. Prevention of Rebleeding From Esophageal Varices in Patients With Cirrhosis Receiving Small-Diameter Stents Versus Hemodynamically Controlled Medical Therapy. Gastroenterology 2015, 149, 660–668. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Villanueva, C.; Graupera, I.; Aracil, C.; Alvarado, E.; Minana, J.; Puente, A.; Hernandez-Gea, V.; Ardevol, A.; Pavel, O.; Colomo, A.; et al. A randomized trial to assess whether portal pressure guided therapy to prevent variceal rebleeding improves survival in cirrhosis. Hepatology 2017, 65, 1693–1707. [Google Scholar] [CrossRef] [PubMed] [Green Version]
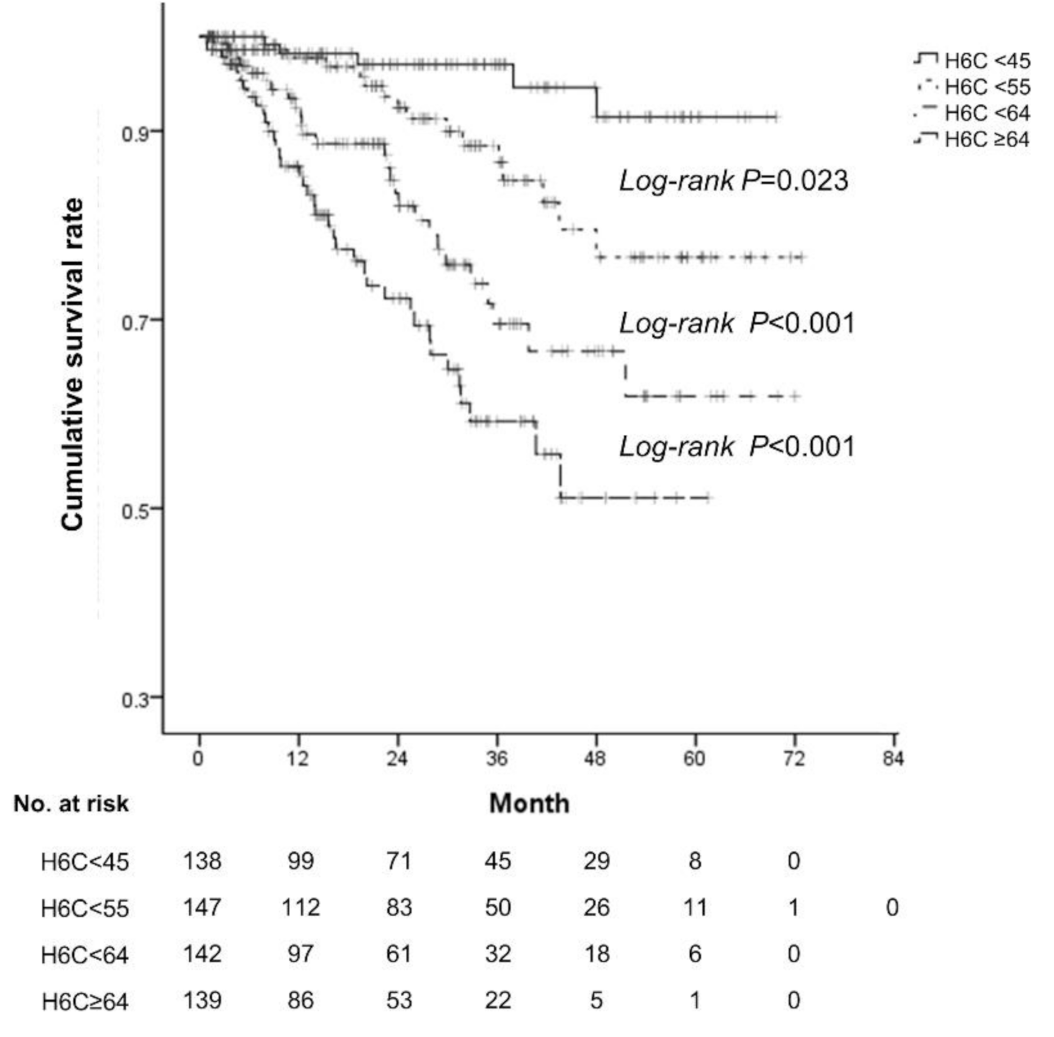



| Total (n =700) | Derivation Cohort (n =566) | Validation Cohort (n =134) | p-Value | |
|---|---|---|---|---|
| Age (year) | 52.55 ± 10.43 | 52.54 ± 9.71 | 52.59 ± 13.1 | 0.968 |
| Male, no. (%) | 521 (74.4) | 435 (76.9) | 86 (64.2) | <0.001 * |
| Platelet count | 120.07 ± 74.37 | 117.27 ± 72.65 | 131.89 ± 80.47 | 0.056 |
| Albumin (g/dL) | 3.3 (2.9, 3.8) | 3.3 (2.9, 3.7) | 3.4 (2.92, 4) | 0.059 |
| Total bilirubin (mg/dL) | 1.57 ± 1.25 | 1.61 ± 1.13 | 1.41 ± 1.66 | 0.194 |
| AST (U/L) | 52 (36, 76) | 51 (36, 75) | 55.5 (35, 84.75) | 0.539 |
| ALT (U/L) | 27 (17, 49.25) | 26 (17, 43.75) | 35.5 (20.25, 82.25) | <0.001 * |
| Prothrombin time (INR) | 1.21 ± 0.23 | 1.21 ± 0.24 | 1.21 ± 0.22 | 0.891 |
| Creatinine (mg/dL) | 0.78 ± 0.21 | 0.76 ± 0.21 | 0.83 ± 0.24 | 0.002 * |
| Ascites, no. (%) | 0.004 * | |||
| None | 376 (53.71%) | 300 (53%) | 76 (56.72%) | |
| Small | 231 (33%) | 200 (35.34%) | 31 (23.13%) | |
| Moderate | 93 (13.29%) | 66 (11.66%) | 27 (20.15%) | |
| Hepatic encephalopathy, no. (%) | <0.001 * | |||
| None | 648 (92.57%) | 528 (93.29%) | 120 (89.55%) | |
| Grade 1–2 | 32 (4.57%) | 29 (5.12%) | 3 (2.24%) | |
| Grade 3–4 | 20 (2.86%) | 9 (1.59%) | 11 (8.21%) | |
| Child-Pugh score | 7 (5, 8) | 7 (6, 8) | 6 (5, 8) | 0.213 |
| Child-Pugh class | 0.163 | |||
| A | 336 (48%) | 263 (46.47%) | 73 (54.48%) | |
| B | 291 (41.57%) | 245 (43.29%) | 46 (34.33%) | |
| C | 73 (10.43%) | 58 (10.25%) | 15 (11.19%) | |
| MELD | 10.12±3.24 | 10.2±3.25 | 9.82±3.17 | 0.217 |
| MELD-sodium | 10.56±4.65 | 10.71±4.67 | 9.91±4.55 | 0.069 |
| HVPG (mmHg) | 14.48±5.20 | 13.77±5.03 | 14.75±5.08 | 0.030 * |
| Univariate Analysis | Multivariate Analysis | |||
|---|---|---|---|---|
| Hazard Ratio (95% CI) | p-Value | Hazard Ratio (95% CI) | p-Value | |
| Sex | 0.75 (0.44–1.27) | 0.287 | ||
| Age | 1.01 (0.99–1.03) | 0.475 | ||
| Platelet count | 1.00 (0.996–1.003) | 0.790 | ||
| Albumin | 0.32 (0.22–0.47) | <0.001 * | ||
| Total bilirubin | 1.41 (1.20–1.67) | <0.001 * | ||
| AST | 0.998 (0.993–1.002) | 0.292 | ||
| ALT | 0.99 (0.985–1.000) | 0.042 * | ||
| Prothrombin time | 3.86 (1.83–8.12) | <0.001 * | ||
| Creatinine | 0.95 (0.34–2.67) | 0.916 | ||
| Child-Pugh score | 1.42 (1.28–1.57) | <0.001 * | 1.32 (1.18–1.48) | <0.001 * |
| MELD | 1.14 (1.08–1.21) | <0.001 * | ||
| HVPG | 1.12 (1.07–1.16) | <0.001 * | 1.07 (1.02–1.12) | 0.003 * |
| Subject | Time (months) | H6C | MELD | ||
|---|---|---|---|---|---|
| AUC | 95% CI | AUC | 95% CI | ||
| Derivation cohort | 7.43 | 0.773 | 0.682–0.865 | 0.636 | 0.473–0.798 |
| 11.865 | 0.744 | 0.655–0.833 | 0.614 | 0.497–0.731 | |
| 22.385 | 0.757 | 0.686–0.828 | 0.639 | 0.553–0.725 | |
| 33.465 | 0.761 | 0.698–0.824 | 0.672 | 0.592–0.752 | |
| 40.87 | 0.769 | 0.701–0.837 | 0.658 | 0.579–0.738 | |
| Viral etiology of derivation cohort | 9.09 | 0.776 | 0.632–0.920 | 0.612 | 0.308–0.916 |
| 13.939 | 0.842 | 0.727–0.958 | 0.732 | 0.500–0.964 | |
| 25.585 | 0.812 | 0.666–0.957 | 0.747 | 0.571–0.923 | |
| 36.658 | 0.880 | 0.764–0.996 | 0.799 | 0.644–0.954 | |
| 45.778 | 0.896 | 0.800–0.993 | 0.758 | 0.585–0.930 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, Y.; Suk, K.T.; Jeong, S.W.; Yoo, J.-J.; Kim, S.G.; Kim, Y.S.; Lee, S.H.; Kim, H.S.; Kang, S.H.; Baik, S.K.; et al. Application of Hepatic Venous Pressure Gradient to Predict Prognosis in Cirrhotic Patients with a Low Model for End-stage Liver Disease Score. Diagnostics 2020, 10, 805. https://doi.org/10.3390/diagnostics10100805
Chang Y, Suk KT, Jeong SW, Yoo J-J, Kim SG, Kim YS, Lee SH, Kim HS, Kang SH, Baik SK, et al. Application of Hepatic Venous Pressure Gradient to Predict Prognosis in Cirrhotic Patients with a Low Model for End-stage Liver Disease Score. Diagnostics. 2020; 10(10):805. https://doi.org/10.3390/diagnostics10100805
Chicago/Turabian StyleChang, Young, Ki Tae Suk, Soung Won Jeong, Jeong-Ju Yoo, Sang Gyune Kim, Young Seok Kim, Sae Hwan Lee, Hong Soo Kim, Seong Hee Kang, Soon Koo Baik, and et al. 2020. "Application of Hepatic Venous Pressure Gradient to Predict Prognosis in Cirrhotic Patients with a Low Model for End-stage Liver Disease Score" Diagnostics 10, no. 10: 805. https://doi.org/10.3390/diagnostics10100805
APA StyleChang, Y., Suk, K. T., Jeong, S. W., Yoo, J.-J., Kim, S. G., Kim, Y. S., Lee, S. H., Kim, H. S., Kang, S. H., Baik, S. K., Kim, D. J., Kim, M. Y., & Jang, J. Y. (2020). Application of Hepatic Venous Pressure Gradient to Predict Prognosis in Cirrhotic Patients with a Low Model for End-stage Liver Disease Score. Diagnostics, 10(10), 805. https://doi.org/10.3390/diagnostics10100805






