Diagnostic Ability of Radiofrequency Ultrasound in Parkinson’s Disease Compared to Conventional Transcranial Sonography and Magnetic Resonance Imaging
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients and Control Group
2.2. Radiofrequency Transcranial Sonography (RF TCS)
- Amplitude parameters:
- Strain parameter—Lagrangian strain as module of the derivative of amplitudes of a mean repeated movement calculated along the ultrasound scanning line’s direction.
- Morphology parameter—frequency of high-end spectra peak (shortly–FreqHP) was calculated from the entire displacement signal length (6-s). FreqHP was estimated at the peak of the power spectra observed in the frequency range from 1.5 × FreqD to 22.5 Hz, where FreqD is the dominant frequency in displacement low-end spectra (from 0.67 to 2.00 Hz interval) supposedly caused by heart beats.
2.3. Conventional TCS (cTCS)
2.4. MRI Acquisition
2.5. Statistical Analysis
3. Results
3.1. Demographic Data and Single TCS and MRI Quantitative Measures
3.2. Models of Logistic Regression (LR) Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AUC | area under a curve |
| FreqD | dominant frequency |
| FreqHP | frequency of high-end spectra peak |
| HC | healthy controls |
| IQR | interquartile range |
| LR | logistic regression |
| MRI | magnetic resonance imaging |
| PD | Parkinson’s disease |
| RF | radiofrequency |
| RMS | root mean square |
| ROI | region of interest |
| ROC | receiver operating characteristic |
| RMS | root mean square |
| SD | standard deviation |
| SN | substantia nigra |
| TCS | transcranial sonography |
| UPDRS | the Unified Parkinson’s disease rating scale |
| US | ultrasound |
References
- Fearnley, J.M.; Lees, A.J. Ageing and Parkinson’s disease: Substantia nigra regional selectivity. Brain 1991, 114, 2283–2301. [Google Scholar] [CrossRef] [PubMed]
- Hornykiewicz, O. Basic Research on Dopamine in Parkinson’s Disease and the Discovery of the Nigrostriatal Dopamine Pathway: The View of an Eyewitness. Neurodegener. Dis. 2008, 5, 114–117. [Google Scholar] [CrossRef] [PubMed]
- Braak, H.; Tredici, K.D.; Rüb, U.; de Vos, R.A.I.; Jansen Steur, E.N.H.; Braak, E. Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol. Aging 2003, 24, 197–211. [Google Scholar] [CrossRef]
- Berardelli, A.; Wenning, G.K.; Antonini, A.; Berg, D.; Bloem, B.R.; Bonifati, V.; Brooks, D.; Burn, D.J.; Colosimo, C.; Fanciulli, A.; et al. EFNS/MDS-ES recommendations for the diagnosis of Parkinson’s disease. Eur. J. Neurol. 2013, 20, 16–34. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, J.T.; Oertel, W.H.; McKeith, I.G.; Grosset, D.G.; Walker, Z.; Tatsch, K.; Tolosa, E.; Sherwin, P.F.; Grachev, I.D. Is ioflupane I123 injection diagnostically effective in patients with movement disorders and dementia? Pooled analysis of four clinical trials. BMJ Open 2014, 4, e005122. [Google Scholar] [CrossRef] [PubMed]
- Vlaar, A.M.; de Nijs, T.; van Kroonenburgh, M.J.; Mess, W.H.; Winogrodzka, A.; Tromp, S.C.; Weber, W.E. The predictive value of transcranial duplex sonography for the clinical diagnosis in undiagnosed parkinsonian syndromes: Comparison with SPECT scans. BMC Neurol. 2008, 8, 42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chau, M.T.; Todd, G.; Wilcox, R.; Agzarian, M.; Bezak, E. Diagnostic accuracy of the appearance of Nigrosome-1 on magnetic resonance imaging in Parkinson’s disease: A systematic review and meta-analysis. Parkinsonism Relat. Disord. 2020, 78, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Tuite, P. Magnetic resonance imaging as a potential biomarker for Parkinson’s disease. Transl. Res. 2016, 175, 4–16. [Google Scholar] [CrossRef]
- Berg, D.; Godau, J.; Walter, U. Transcranial sonography in movement disorders. Lancet Neurol. 2008, 7, 1044–1055. [Google Scholar] [CrossRef]
- Berg, D.; Behnke, S.; Walter, U. Application of Transcranial Sonography in Extrapyramidal Disorders: Updated Recommendations. Ultraschall Med. Eur. J. Ultrasound 2006, 27, 12–19. [Google Scholar] [CrossRef]
- Walter, U.; Behnke, S.; Eyding, J.; Niehaus, L.; Postert, T.; Seidel, G.; Berg, D. Transcranial brain parenchyma sonography in movement disorders: State of the art. Ultrasound Med. Biol. 2007, 33, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Ince, J.; Alharbi, M.; Minhas, J.S.; Chung, E.M. Ultrasound measurement of brain tissue movement in humans: A systematic review. Ultrasound 2020, 28, 70–81. [Google Scholar] [CrossRef] [PubMed]
- Kucewicz, J.C.; Dunmire, B.; Leotta, D.F.; Panagiotides, H.; Paun, M.; Beach, K.W. Functional Tissue Pulsatility Imaging of the Brain During Visual Stimulation. Ultrasound Med. Biol. 2007, 33, 681–690. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jurkonis, R.; Makūnaitė, M.; Baranauskas, M.; Lukoševičius, A.; Sakalauskas, A.; Matijošaitis, V.; Rastenytė, D. Quantification of Endogenous Brain Tissue Displacement Imaging by Radiofrequency Ultrasound. Diagnostics 2020, 10, 57. [Google Scholar] [CrossRef] [Green Version]
- Baranauskas, M.; Jurkonis, R.; Lukoševičius, A.; Makūnaitė, M.; Matijošaitis, V.; Gleiznienė, R.; Rastenytė, D. Ultrasonic Assessment of the Medial Temporal Lobe Tissue Displacements in Alzheimer’s Disease. Diagnostics 2020, 10, 452. [Google Scholar] [CrossRef]
- Hughes, A.J.; Daniel, S.E.; Kilford, L.; Lees, A.J. Accuracy of clinical diagnosis of idiopathic Parkinson’s disease: A clinico-pathological study of 100 cases. J. Neurol. Neurosurg. Psychiatry 1992, 55, 181–184. [Google Scholar] [CrossRef] [Green Version]
- Fahn, S.; Elton, R. Unified Parkinson’s Disease Rating Scale. In Recent Developments in Parkinson‘s Disease; Fahn, S., Marsden, C., Calne, D., Goldstein, M., Eds.; Macmillan Health Care Information: Florham Park, NJ, USA, 1987; pp. 153–164. [Google Scholar]
- Desmidt, T.; Brizard, B.; Dujardin, P.-A.; Ternifi, R.; Réméniéras, J.-P.; Patat, F.; Andersson, F.; Cottier, J.-P.; Vierron, E.; Gissot, V.; et al. Brain Tissue Pulsatility is Increased in Midlife Depression: A Comparative Study Using Ultrasound Tissue Pulsatility Imaging. Neuropsychopharmacology 2017, 42, 2575–2582. [Google Scholar] [CrossRef]
- Kucewicz, J.C.; Dunmire, B.; Giardino, N.D.; Leotta, D.F.; Paun, M.; Dager, S.R.; Beach, K.W. Tissue Pulsatility Imaging of Cerebral Vasoreactivity During Hyperventilation. Ultrasound Med. Biol. 2008, 34, 1200–1208. [Google Scholar] [CrossRef] [Green Version]
- Ternifi, R.; Cazals, X.; Desmidt, T.; Andersson, F.; Camus, V.; Cottier, J.-P.; Patat, F.; Remenieras, J.-P. Ultrasound Measurements of Brain Tissue Pulsatility Correlate with the Volume of MRI White-Matter Hyperintensity. J. Cereb. Blood Flow Metab. 2014, 34, 942–944. [Google Scholar] [CrossRef] [Green Version]
- Maurice, R.L.; Bertrand, M. Lagrangian speckle model and tissue-motion estimation-theory [ultrasonography]. IEEE Trans. Med. Imaging 1999, 18, 593–603. [Google Scholar] [CrossRef]
- Gibb, W.R.; Lees, A.J. The relevance of the Lewy body to the pathogenesis of idiopathic Parkinson’s disease. J. Neurol. Neurosurg. Psychiatry 1988, 51, 745–752. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marsili, L.; Rizzo, G.; Colosimo, C. Diagnostic Criteria for Parkinson’s Disease: From James Parkinson to the Concept of Prodromal Disease. Front. Neurol. 2018, 9, 156. [Google Scholar] [CrossRef] [PubMed]
- Lacouture, Y.; Cousineau, D. How to use MATLAB to fit the ex-Gaussian and other probability functions to a distribution of response times. Tutor. Quant. Methods Psychol. 2008, 4, 35–45. [Google Scholar] [CrossRef]
- Laučkaitė, K.; Rastenytė, D.; Šurkienė, D.; Vaitkus, A.; Sakalauskas, A.; Lukoševičius, A.; Gleiznienė, R. Specificity of transcranial sonography in parkinson spectrum disorders in comparison to degenerative cognitive syndromes. BMC Neurol. 2012, 12, 12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laučkaitė, K.; Rastenytė, D.; Šurkienė, D.; Vaidelytė, B.; Dambrauskaitė, G.; Sakalauskas, A.; Vaitkus, A.; Gleiznienė, R. Ultrasonographic (TCS) and clinical findings in overlapping phenotype of essential tremor and Parkinson’s disease (ET-PD). BMC Neurol. 2014, 14, 54. [Google Scholar] [CrossRef] [Green Version]
- Arbogast, K.B.; Margulies, S.S. Material characterization of the brainstem from oscillatory shear tests. J. Biomech. 1998, 31, 801–807. [Google Scholar] [CrossRef]
- Budday, S.; Nay, R.; de Rooij, R.; Steinmann, P.; Wyrobek, T.; Ovaert, T.C.; Kuhl, E. Mechanical properties of gray and white matter brain tissue by indentation. J. Mech. Behav. Biomed. Mater. 2015, 46, 318–330. [Google Scholar] [CrossRef] [Green Version]
- van Dommelen, J.A.W.; van der Sande, T.P.J.; Hrapko, M.; Peters, G.W.M. Mechanical properties of brain tissue by indentation: Interregional variation. J. Mech. Behav. Biomed. Mater. 2010, 3, 158–166. [Google Scholar] [CrossRef]
- Rektor, I.; Goldemund, D.; Sheardová, K.; Rektorová, I.; Michálková, Z.; Dufek, M. Vascular pathology in patients with idiopathic Parkinson’s disease. Parkinsonism Relat. Disord. 2009, 15, 24–29. [Google Scholar] [CrossRef]
- Vokatch, N.; Grötzsch, H.; Mermillod, B.; Burkhard, P.R.; Sztajzel, R. Is cerebral autoregulation impaired in Parkinson’s disease? A transcranial Doppler study. J. Neurol. Sci. 2007, 254, 49–53. [Google Scholar] [CrossRef]
- Feng, Y.; Okamoto, R.J.; Namani, R.; Genin, G.M.; Bayly, P.V. Measurements of mechanical anisotropy in brain tissue and implications for transversely isotropic material models of white matter. J. Mech. Behav. Biomed. Mater. 2013, 23, 117–132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Velardi, F.; Fraternali, F.; Angelillo, M. Anisotropic constitutive equations and experimental tensile behavior of brain tissue. Biomech. Model. Mechanobiol. 2006, 5, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Pyatigorskaya, N.; Gallea, C.; Garcia-Lorenzo, D.; Vidailhet, M.; Lehericy, S. A review of the use of magnetic resonance imaging in Parkinson’s disease. Ther. Adv. Neurol. Disord. 2014, 7, 206–220. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zeighami, Y.; Ulla, M.; Iturria-Medina, Y.; Dadar, M.; Zhang, Y.; Larcher, K.M.-H.; Fonov, V.; Evans, A.C.; Collins, D.L.; Dagher, A. Network structure of brain atrophy in de novo Parkinson’s disease. eLife 2015, 4, e08440. [Google Scholar] [CrossRef] [PubMed]
- Barry, R.L.; Coaster, M.; Rogers, B.P.; Newton, A.T.; Moore, J.; Anderson, A.W.; Zald, D.H.; Gore, J.C. On the Origins of Signal Variance in FMRI of the Human Midbrain at High Field. PLoS ONE 2013, 8, e62708. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reese, T.G.; Feinberg, D.A.; Dou, J.; Wedeen, V.J. Phase contrast MRI of myocardial 3D strain by encoding contiguous slices in a single shot. Magn. Reson. Med. 2002, 47, 665–676. [Google Scholar] [CrossRef] [PubMed]
- Tao, A.; Chen, G.; Deng, Y.; Xu, R. Accuracy of Transcranial Sonography of the Substantia Nigra for Detection of Parkinson’s Disease: A Systematic Review and Meta-analysis. Ultrasound Med. Biol. 2019, 45, 628–641. [Google Scholar] [CrossRef] [Green Version]
- Minati, L.; Grisoli, M.; Carella, F.; De Simone, T.; Bruzzone, M.G.; Savoiardo, M. Imaging degeneration of the substantia nigra in Parkinson disease with inversion-recovery MR imaging. AJNR Am. J. Neuroradiol. 2007, 28, 309–313. [Google Scholar]
- Oikawa, H.; Sasaki, M.; Tamakawa, Y.; Ehara, S.; Tohyama, K. The substantia nigra in Parkinson disease: Proton density-weighted spin-echo and fast short inversion time inversion-recovery MR findings. AJNR Am. J. Neuroradiol. 2002, 23, 1747–1756. [Google Scholar]
- Du, G.; Lewis, M.M.; Sica, C.; Kong, L.; Huang, X. Magnetic resonance T1w/T2w ratio: A parsimonious marker for Parkinson disease: Midbrain T1w/T2w Ratio in PD. Ann. Neurol. 2019, 85, 96–104. [Google Scholar] [CrossRef]
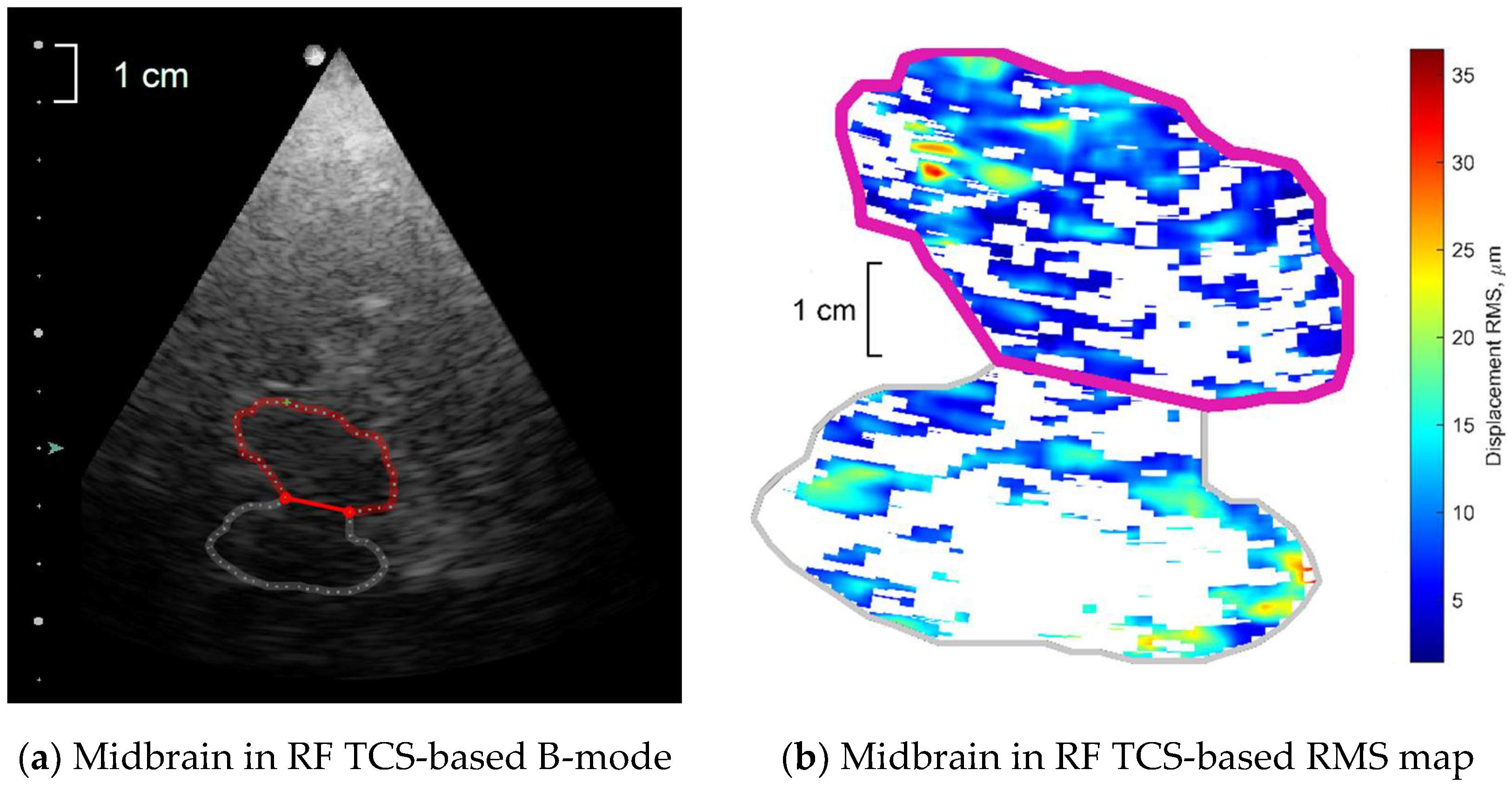
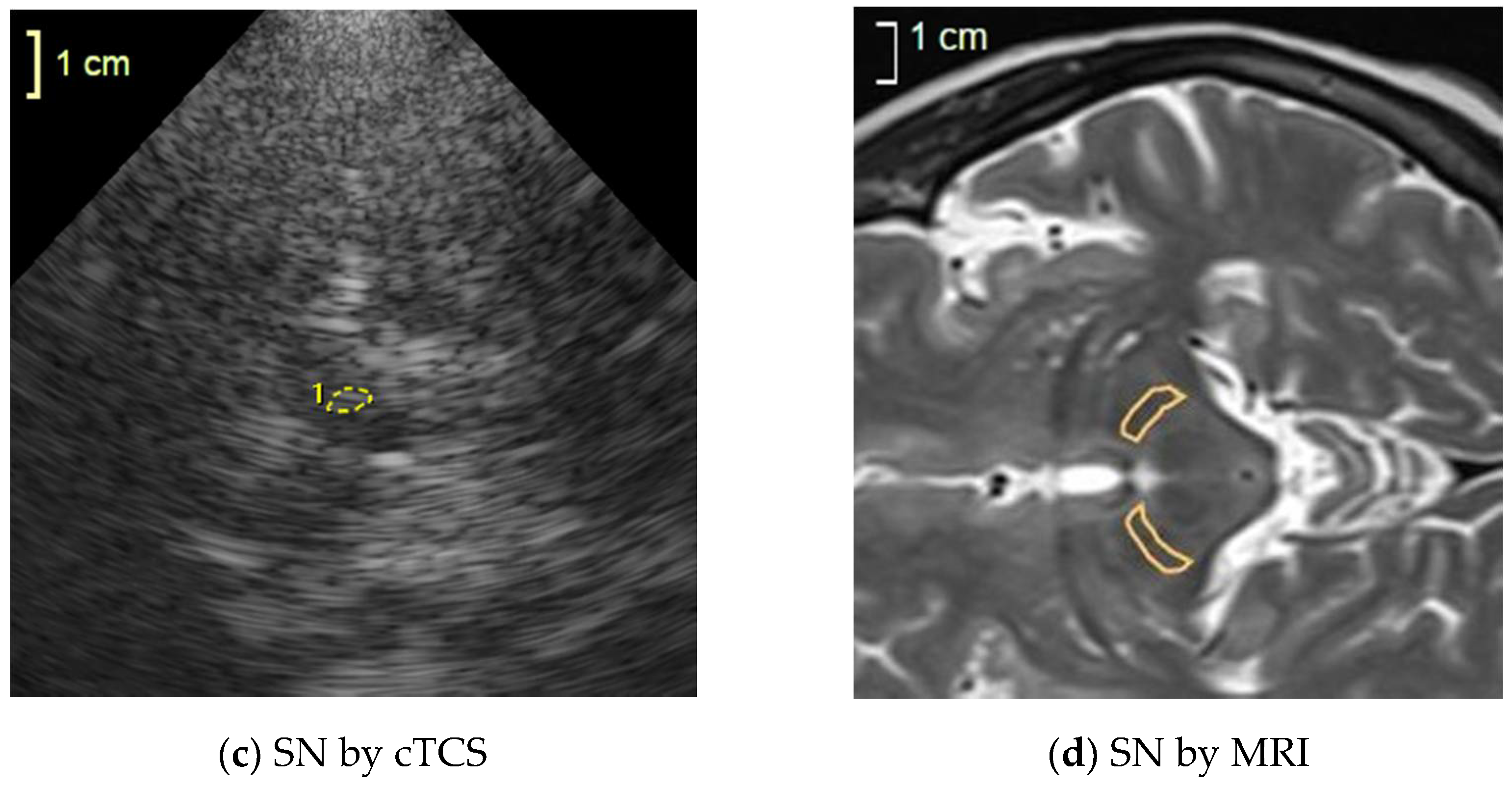

| Variable | Mean ± Standard Deviation | p Value | |
|---|---|---|---|
| HC | PD | ||
| All subjects | |||
| Age, years | 68.5 ± 6.8 | 64.1 ± 10.1 | 0.17 |
| Education, years | 15.2 ± 3.2 | 14.7 ± 2.9 | 0.61 |
| Motor section of UPDRS | – | 33.2 ± 12.9 | – |
| Subjects with repeatable waveforms in RF TCS recordings | |||
| Age, years | 68.1 ± 6.8 | 62.9 ± 10.7 | 0.123 |
| Education, years | 15.0 ± 3.2 | 14.9 ± 3.2 | 0.99 |
| Motor section of UPDRS | – | 31.6 ± 10.7 | – |
| SN dorsal width by MRI, mm | 4.29 ± 1.29 | 3.08 ± 1.77 | 0.007 |
| SN ventral width by MRI, mm | 5.35 ± 1.31 | 4.05 ± 1.44 | 0.002 |
| SN area by MRI, mm2 | 55.4 ± 7.9 | 33.1 ± 10.9 | <0.001 |
| SN area by cTCS, mm2 | 10.8 ± 2.74 | 24.4 ± 7.49 | <0.001 |
| Width of 3rd ventricle by MRI, mm | 6.19 ± 2.80 | 6.53 ± 1.98 | 0.70 |
| Width of 3rd ventricle by cTCS, mm | 5.89 ± 1.89 | 6.58 ± 2.38 | 0.36 |
| Parameter Estimate | β | Exp(β) | Exp(β) 95% CI | p Value |
|---|---|---|---|---|
| RFTCS 1st Model | ||||
| RMS_exG_SD | −3.490 | 0.030 | [0.001, 0.684] | 0.028 |
| FreqHP_Q3 | −4.392 | 0.012 | [0.000, 0.857] | 0.042 |
| FreqHP_Q3 × RMS_exG_SD | 1.806 | 6.084 | [1.352, 27.37] | 0.019 |
| Constant | 7.235 | 1387.2 | - | 0.112 |
| RF TCS 2nd Model | ||||
| RMS_exG_SD | −2.807 | 0.060 | [0.004, 0.917] | 0.043 |
| FreqHP_Q3 | −3.627 | 0.027 | [0.001, 1.364] | 0.071 |
| FreqHP_Q3 × RMS_exG_SD | 1.527 | 4.606 | [1.212, 17.50] | 0.025 |
| Age | −0.120 | 0.887 | [0.811, 0.971] | 0.010 |
| Constant | 12.873 | 3.898 × 105 | – | 0.012 |
| RF TCS 3rd Model | ||||
| RMS_exG_SD | −2.895 | 0.055 | [0.002, 1.433] | 0.081 |
| FreqHP_Q3 | −3.915 | 0.020 | [0.000, 2.249] | 0.104 |
| FreqHP_Q3 × RMS_exG_SD | 1.599 | 4.946 | [0.986, 24.806] | 0.052 |
| Age | −0.135 | 0.873 | [0.791, 0.964] | 0.007 |
| Relative energy in 4–6 Hz | 163.25 | 7.917 × 1070 | [0.000, 1.083 × 10180] | 0.203 |
| Constant | 13.448 | 6.923 × 105 | – | 0.018 |
| cTCS 1st Model | ||||
| TCS_SN_area | 0.731 | 2.076 | [1.319, 3.267] | 0.002 |
| Constant | −11.868 | 0.000 | – | 0.001 |
| cTCS 2nd Model | ||||
| TCS_SN_area | 0.808 | 2.243 | [1.239, 4.063] | 0.008 |
| Age | −0.119 | 0.888 | [0.694, 1.136] | 0.345 |
| Constant | −5.084 | 0.006 | – | 0.463 |
| MRI1st Model | ||||
| MRI_SN_area | −0.225 | 0.798 | [0.706, 0.902] | <0.001 |
| Constant | 9.633 | 1.526 × 104 | – | 0.001 |
| MRI 2nd Model | ||||
| MRI_SN_area | −0.264 | 0.768 | [0.652, 0.905] | 0.002 |
| Age | −0.202 | 0.817 | [0.680, 0.981] | 0.031 |
| Constant | 23.973 | 2.578 × 1010 | – | 0.006 |
| Model | AUC, % | 95% CI | p Value | Cut-Off, % | Sensitivity, % | Specificity, % | Overall Correct Classification, % |
|---|---|---|---|---|---|---|---|
| RF TCS 1st | 80.9 | [69.1, 92.7] | <0.001 | 29.9 | 85.0 | 69.0 | 75.5 |
| RF TCS 2nd | 86.2 | [74.9, 97.6] | <0.001 | 44.6 | 80.0 | 86.2 | 83.7 |
| RF TCS 3rd | 88.3 | [78.6, 97.9] | <0.001 | 42.7 | 80.0 | 86.2 | 83.7 |
| cTCS 1st | 98.2 | [94.9, 100.0] | <0.001 | 77.7 | 90.0 | 100.0 | 95.9 |
| cTCS 2nd | 98.7 | [96.5, 100.0] | <0.001 | 70.8 | 90.0 | 100.0 | 95.9 |
| MRI 1st | 94.1 | [86.5, 100.0] | 0.039 | 54.1 | 90.0 | 96.6 | 93.9 |
| MRI 2nd | 97.8 | [94.3, 100.0] | 0.018 | 43.3 | 95.0 | 96.6 | 95.9 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baranauskas, M.; Jurkonis, R.; Lukoševičius, A.; Matijošaitis, V.; Gleiznienė, R.; Rastenytė, D. Diagnostic Ability of Radiofrequency Ultrasound in Parkinson’s Disease Compared to Conventional Transcranial Sonography and Magnetic Resonance Imaging. Diagnostics 2020, 10, 778. https://doi.org/10.3390/diagnostics10100778
Baranauskas M, Jurkonis R, Lukoševičius A, Matijošaitis V, Gleiznienė R, Rastenytė D. Diagnostic Ability of Radiofrequency Ultrasound in Parkinson’s Disease Compared to Conventional Transcranial Sonography and Magnetic Resonance Imaging. Diagnostics. 2020; 10(10):778. https://doi.org/10.3390/diagnostics10100778
Chicago/Turabian StyleBaranauskas, Mindaugas, Rytis Jurkonis, Arūnas Lukoševičius, Vaidas Matijošaitis, Rymantė Gleiznienė, and Daiva Rastenytė. 2020. "Diagnostic Ability of Radiofrequency Ultrasound in Parkinson’s Disease Compared to Conventional Transcranial Sonography and Magnetic Resonance Imaging" Diagnostics 10, no. 10: 778. https://doi.org/10.3390/diagnostics10100778





