Proteomics in Deaths by Drowning: Diagnostic Efficacy of Apolipoprotein A1 and α-1 Antitrypsin, Pilot Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Procedure for Extraction of Biological Fluids
2.3. Protein Extraction
2.4. Database Searching
2.5. Criteria for Protein
2.6. Validation Experiment
2.7. Statistical Analysis
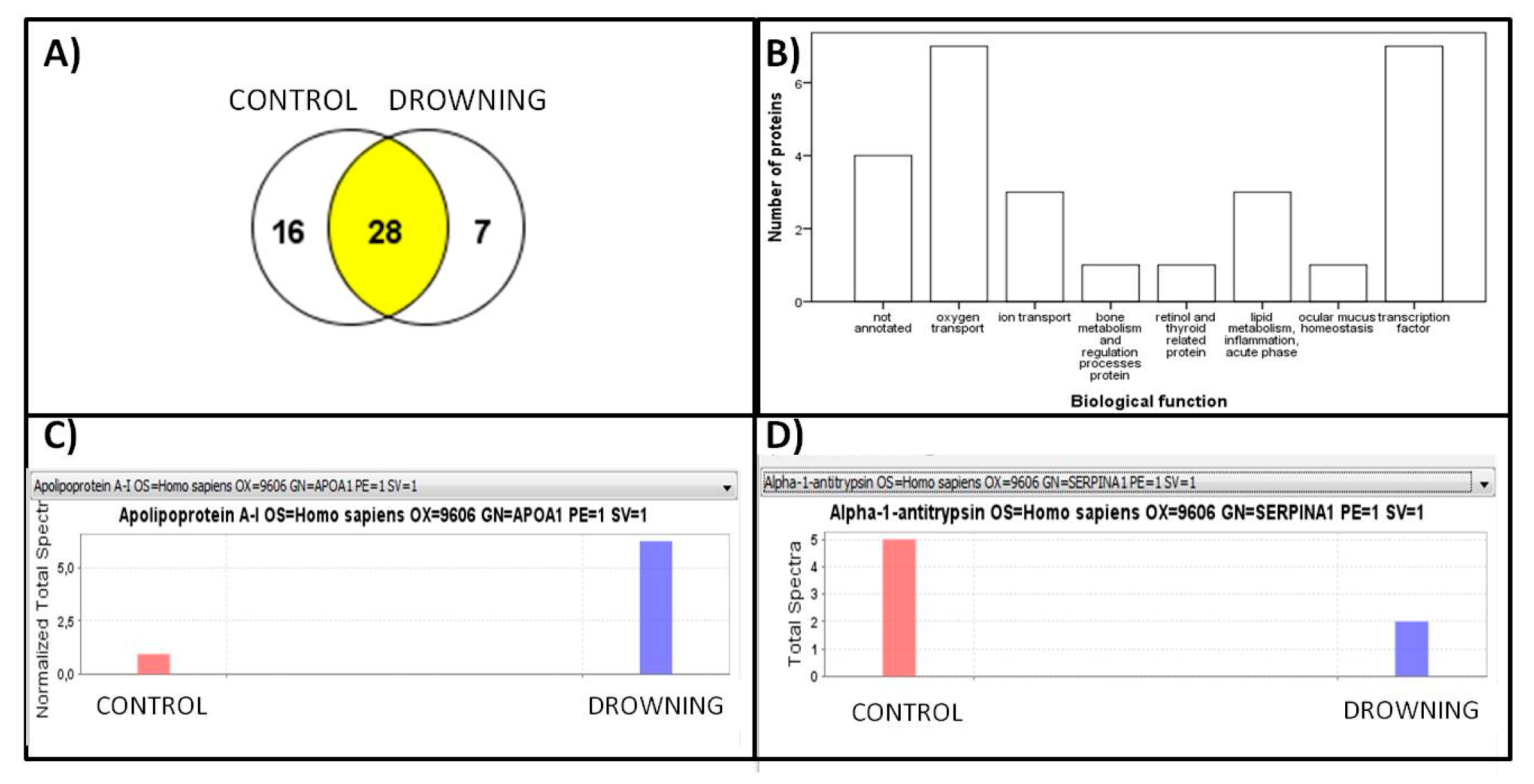
3. Results
Proteomic Approach
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- McCall, J.D.; Sternard, B.T. Drowning. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2020; p. 25. [Google Scholar]
- O’Connor, P.J.; O’Connor, N. Work-related maritime fatalities. Accid. Anal. Prev. 2006, 38, 737–741. [Google Scholar] [CrossRef] [PubMed]
- Diaz, J.H. Global climate changes, natural disasters, and travel health risks. Travel. Med. 2006, 13, 361–372. [Google Scholar] [CrossRef] [PubMed]
- Prasartritha, T.; Tungsiripat, R.; Warachit, P. The revisit of 2004 tsunami in Thailand: Characteristics of wounds. Int. Wound J. 2008, 5, 8–19. [Google Scholar] [CrossRef] [PubMed]
- Idris, A.; Berg, R.; Bierens, J.; Bossaert, L.; Branche, C.; Gabrielli, A.; Graves, S.; Handley, A.; Hoelle, R.; Morley, P.; et al. American Heart Association. Recommended guidelines for uniform reporting of data from drowning: The “Utstein style”. Circulation 2003, 108, 2565–2574. [Google Scholar] [PubMed] [Green Version]
- Linnan, M.; Anh, L.V.; Cuong, P.V. Special Series on Child Injury: Child Mortality and Injury in Asia: Survey Results and Evidence; UNICEF Innocenti Research Center: Florence, Italy, 2007. [Google Scholar]
- Web-Based Injury Statistics Query and Reporting System (WISQARS), Atlanta: Centers for Disease Control and Prevention. 2009. Available online: http://www.cdc.gov/injury/wisqars (accessed on 23 September 2020).
- Piette, M.H.; De Letter, E.A. Drowning: Still a difficult autopsy diagnosis. Forensic Sci. Int. 2006, 163, 1–9. [Google Scholar] [CrossRef]
- Armstrong, E.J.; Erskine, K.L. Investigation of Drowning Deaths: A Practical Review. Acad. Forensic Pathol. 2018, 8, 8–43. [Google Scholar] [CrossRef] [Green Version]
- Yajima, D.; Inokuchi, G.; Makino, Y.; Motomura, A.; Chiba, F.; Torimitsu, S.; Yamaguchi, R.; Hoshioka, Y.; Malakiene, D.; Raudys, R.; et al. Diagnosis of drowning by summation of sodium, potassium, and chloride ion levels in sphenoidal sinus fluid: Differentiating between freshwater and seawater drowning and its application to brackish water and bathtub deaths. Forensic Sci. Int. 2018, 284, 219–225. [Google Scholar] [CrossRef]
- Barranco, R.; Castiglioni, C.; Ventura, F.; Fracasso, T. A comparative digital morphometric study of lung tissue in saltwater and freshwater drowning. Forensic Sci. Int. 2019, 298, 157–160. [Google Scholar] [CrossRef]
- Barranco, R.; Ventura, F.; Fracasso, T. Immunohistochemical renal expression of aquaporin 2, arginine-vasopressin, vasopressin receptor 2, and renin in saltwater drowning and freshwater drowning. Int. J. Leg. Med. 2020, 134, 1733–1740. [Google Scholar] [CrossRef]
- Pérez-Cárceles, M.D.; Del Pozo, S.; Sibón, A.; Noguera-Velasco, J.A.; Osuna, E.; Vizcaya, M.; Luna, A. Serum biochemical markers in drowning: Diagnostic efficacy of Strontium and other trace elements. Forensic Sci. Int. 2011, 214, 159–166. [Google Scholar] [CrossRef]
- Barranco, R.; Castiglioni, C.; Ventura, F.; Fracasso, T. Immunohistochemical expression of P-selectin, SP-A, HSP70, aquaporin 5, and fibronectin in saltwater drowning and freshwater drowning. Int. J. Leg. Med. 2019, 133, 1461–1467. [Google Scholar] [CrossRef] [PubMed]
- Han, L.J.; Xu, H.M.; Chen, L. Proteomics and Its Application in Forensic Pathology. Fa Yi Xue Za Zhi 2019, 35, 78–83. [Google Scholar] [PubMed]
- Procopio, N.; Williams, A.; Chamberlain, A.T.; Buckley, M. Forensic proteomics for the evaluation of the post-mortem decay in bones. J. Proteom. 2018, 177, 21–30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, K.-M.; Zissler, A.; Kim, E.; Ehrenfellner, B.; Cho, E.; Lee, S.-I.; Steinbacher, P.; Na Yun, K.; Shin, J.H.; Kim, J.Y.; et al. Postmortem proteomics to discover biomarkers for forensic PMI estimation. Int. J. Leg. Med. 2019, 133, 899–908. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prieto-Bonete, G.; Pérez-Cárceles, M.D.; López, A.M.; Pérez-Martínez, C.; Luna, A. Association between protein profile and postmortem interval in human bone remains. J. Proteom. 2019, 192, 54–63. [Google Scholar] [CrossRef]
- Kay, R.; Barton, C.; Ratcliffe, L.; Matharoo-Ball, B.; Brown, P.; Roberts, J.; Teale, P.; Creaser, C.S. Enrichment of low molecular weight serum proteins using acetonitrile precipitation for mass spectrometry based proteomic analysis. Rapid Commun. Mass Spectrom. 2008, 22, 3255–3260. [Google Scholar] [CrossRef]
- Laemmli, U.K. Cleavage of Structural Proteins during the Assembly of the Head of Bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef]
- Shevchenko, A.; Tomas, H.; Havli, J.; Olsen, J.V.; Mann, M. In-gel digestion for mass spectrometric characterization of proteins and proteomes. Nat. Protoc. 2006, 1, 2856–2860. [Google Scholar] [CrossRef]
- Keller, A.; Nesvizhskii, A.I.; Kolker, A.E.; Aebersold, R. Empirical Statistical Model To Estimate the Accuracy of Peptide Identifications Made by MS/MS and Database Search. Anal. Chem. 2002, 74, 5383–5392. [Google Scholar] [CrossRef]
- Nesvizhskii, A.I.; Keller, A.; Kolker, E.; Aebersold, R. A Statistical Model for Identifying Proteins by Tandem Mass Spectrometry. Anal. Chem. 2003, 75, 4646–4658. [Google Scholar] [CrossRef]
- Wortham, M.; Benthuysen, J.R.; Wallace, M.; Savas, J.N.; Mulas, F.; Divakaruni, A.S.; Liu, F.; Albert, V.; Taylor, B.L.; Sui, Y.; et al. Integrated in Vivo Quantitative Proteomics and Nutrient Tracing Reveals Age-Related Metabolic Rewiring of Pancreatic β Cell Function. Cell Rep. 2018, 25, 2904–2918.e8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gianazza, E.; Miller, I.; Guerrini, U.; Palazzolo, L.; Parravicini, C.; Eberini, I. Gender proteomics I. Which proteins in non-sexual organs. J. Proteom. 2018, 178, 7–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gregorakos, L.; Markou, N.; Psalida, V.; Kanakaki, M.; Alexopoulou, A.; Sotiriou, E.; Damianos, A.; Myrianthefs, P. Near-Drowning: Clinical Course of Lung Injury in Adults. Lung 2009, 187, 93–97. [Google Scholar] [CrossRef] [PubMed]
- Leman, L.J.; Maryanoff, B.E.; Ghadiri, M.R. Molecules That Mimic Apolipoprotein A-I: Potential Agents for Treating Atherosclerosis. J. Med. Chem. 2013, 57, 2169–2196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Borna, H.; Noe, S.H.H.Q.; Harchegani, A.B.; Talatappe, N.R.; Ghatrehsamani, M.; Ghanei, M.; Shahriary, A. A review on proteomics analysis to reveal biological pathways and predictive proteins in sulfur mustard exposed patients: Roles of inflammation and oxidative stress. Inhal. Toxicol. 2019, 31, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Gauthier, M.; Ray, A.; Wenzel, S.E. Evolving Concepts of Asthma. Am. J. Respir. Crit. Care Med. 2015, 192, 660–668. [Google Scholar] [CrossRef] [Green Version]
- Yao, X.; Gordon, E.M.; Figueroa, D.M.; Barochia, A.V.; Levine, S.J. Emerging Roles of Apolipoprotein E and Apolipoprotein A-I in the Pathogenesis and Treatment of Lung Disease. Am. J. Respir. Cell Mol. Boil. 2016, 55, 159–169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tiniakou, I.; Drakos, E.; Sinatkas, V.; Van Eck, M.; Zannis, V.I.; Boumpas, D.; Verginis, P.; Kardassis, D. High-density lipoprotein attenuates Th1 and th17 autoimmune responses by modulating dendritic cell maturation and function. J. Immunol. 2015, 194, 4676–4687. [Google Scholar] [CrossRef] [Green Version]
- De Serres, F.; Blanco, I. Role of alpha-1 antitrypsin in human health and disease. J. Intern. Med. 2014, 276, 311–335. [Google Scholar] [CrossRef]
- Lewis, E.; Shapiro, L.; Bowers, O.; Dinarello, C. Alpha1-antitrypsin monotherapy prolongs islet allograft survival in mice. Proc. Natl. Acad. Sci. USA 2005, 102, 12153–12158. [Google Scholar] [CrossRef] [Green Version]
- Eden, E.; Holbrook, J.T.; Brantly, M.L.; Turino, G.M.A.; Wise, R. Prevalence of Alpha-1 Antitrypsin Deficiency in Poorly Controlled Asthma—Results from the ALA-ACRC Low-Dose Theophylline Trial. J. Asthma 2007, 44, 605–608. [Google Scholar] [CrossRef]
- Blanco, I.; Lara, B.; De Serres, F. Efficacy of alpha1-antitrypsin augmentation therapy in conditions other than pulmonary emphysema. Orphanet J. Rare Dis. 2011, 6, 14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clément, R.; Redpath, M.; Sauvageau, A. Mechanism of Death in Hanging: A Historical Review of the Evolution of Pathophysiological Hypotheses. J. Forensic Sci. 2010, 55, 1268–1271. [Google Scholar] [CrossRef]
- Szpilman, D.; Bierens, J.J.; Handley, A.J.; Orlowski, J.P. Drowning. N. Engl. J. Med. 2012, 366, 2102–2110. [Google Scholar] [CrossRef] [PubMed]
- Orlowski, J.P.; Abulleil, M.M.; Phillips, J.M. The hemodynamic and cardiovascular effects of near-drowning in hypotonic, isotonic, or hypertonic solutions. Ann. Emerg. Med. 1989, 18, 1044–1049. [Google Scholar] [CrossRef]
- Szpilman, D. Near-Drowning and Drowning Classification. Chest 1997, 112, 660–665. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lunetta, P.; Modell, J.H. Macroscopical, Microscopical, and Laboratory Findings in Drowning Victims. In Forensic Pathology Reviews; Tsokos, M., Ed.; Humana Press: Totowa, NJ, USA, 2005; Volume 3. [Google Scholar] [CrossRef]
- Bierens, J.; Lunetta, P.; Tipton, M.; Warner, D.S. Physiology of Drowning: A Review. Physiology 2016, 31, 147–166. [Google Scholar] [CrossRef] [Green Version]
- Santurro, A.; Vullo, A.M.; Borro, M.; Gentile, G.; La Russa, R.; Simmaco, M.; Frati, P.; Fineschi, V. Personalized Medicine applied to Forensic Sciences: New advances and perspectives for a tailored forensic approach. Curr. Pharm. Biotechnol. 2017, 18, 1. [Google Scholar] [CrossRef]
- Tavichakorntrakool, R.; Prasongwattana, V.; Sriboonlue, P.; Puapairoj, A.; Pongskul, J.; Khuntikeo, N.; Hanpanich, W.; Yenchitsomanus, P.-T.; Wongkham, C.; Thongboonkerd, V. Serial analyses of postmortem changes in human skeletal muscle: A case study of alterations in proteome profile, histology, electrolyte contents, water composition, and enzyme activity. Proteom. Clin. Appl. 2008, 2, 1255–1264. [Google Scholar] [CrossRef]
- Mizukami, H.; Hathway, B.; Procopio, N. Aquatic Decomposition of Mammalian Corpses: A Forensic Proteomic Approach. J. Proteome Res. 2020, 19, 2122–2135. [Google Scholar] [CrossRef]
- Aquila, I.; Sacco, M.A.; Gratteri, S.; Raffaele, R.; Ricci, P. The Forensic Application of Proteomics for the Study of the Time of Death: An Operative Experimental Model for Pmi Estimation. J. Integr. OMICS 2018, 8, 56. [Google Scholar] [CrossRef]
- Alkass, K.; Buchholz, B.A.; Ohtani, S.; Yamamoto, T.; Druid, H.; Spalding, K.L. Age estimation in forensic sciences: Application of combined aspartic acid racemization and radiocarbon analysis. Mol. Cell Prot. 2010, 9, 1022–1030. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hunt, N.J.; Phillips, L.; Waters, K.A.; Machaalani, R. Proteomic MALDI-TOF/TOF-IMS examination of peptide expression in the formalin fixed brainstem and changes in sudden infant death syndrome infants. J. Proteom. 2016, 138, 48–60. [Google Scholar] [CrossRef] [PubMed]
- Kakimoto, Y.; Ito, S.; Abiru, H.; Kotani, H.; Ozeki, M.; Tamaki, K.; Tsuruyama, T. Sorbin and SH3 Domain-Containing Protein 2 Is Released from Infarcted Heart in the Very Early Phase: Proteomic Analysis of Cardiac Tissues from Patients. J. Am. Hear. Assoc. 2013, 2, e000565. [Google Scholar] [CrossRef] [Green Version]
- Madea, B.; Saukko, P.; Oliva, A.; Musshoff, F. Molecular pathology in forensic medicine—Introduction. Forensic Sci. Int. 2010, 203, 3–14. [Google Scholar] [CrossRef]


| (a) | |||||
| Dependent Variable | Unstandardized Coefficients | Standardized Coefficients | t | p-Value | |
| Univariate | B | 95%CI for B | β | ||
| Drowning | −53.75 | −90.0–(−17.49) | −0.56 | −3.08 | 0.006 |
| Multivariate | |||||
| Drowning | −54.69 | −3.82–(−14.26) | −0.56 | −2.84 | 0.010 |
| Age | 0.13 | −0.96–(1.21) | 0.05 | 0.24 | 0.811 |
| Gender | −9.40 | −49.89–31.18 | −0.09 | −0.48 | 0.633 |
| (b) | |||||
| Dependent Variable | Unstandardized Coefficients | Standardized Coefficients | t | p-Value | |
| B | 95%CI for B | β | |||
| Drowning | 64.90 | 9.13–120.66 | 0.48 | 2.43 | 0.025 |
| Multivariate | |||||
| Drowning | −68.54 | −10.88–126.19 | 0.50 | 2.45 | 0.022 |
| Age | −0.69 | −2.36–0.98 | −0.18 | −0.87 | 0.395 |
| Gender | 30.62 | −30.0–91.22 | 0.21 | −1.06 | 0.303 |
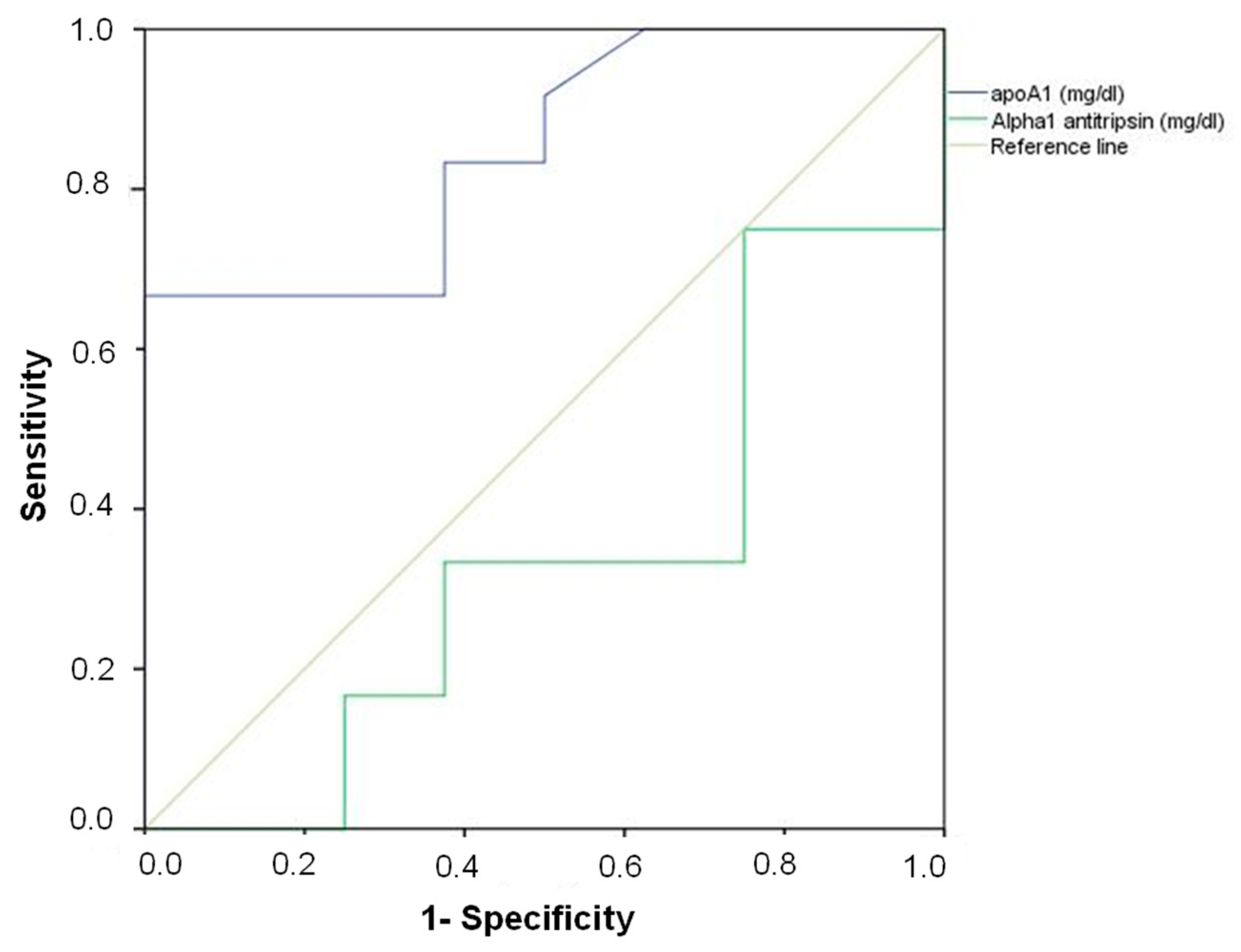
| Variable | Area | Standard Error | p-Value | Lower Limit | Upper Limit | Cut-Off Point | Sensitivity of Cut-Off Point | 1-Specificity of Cut-Off Point | Drowning (% Correct Classification) | Non Drowning (% Correct Classification) | Total (% Correct Classification) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| ApoA1 | 0.85 | 0.09 | 0.010 | 0.68 | 1.0 | 100 | 0.89 | 0.50 | 86.7 | 50.0 | 73.9 |
| α1-antitrypsin | 0.33 | 0.13 | 0.217 | 0.08 | 0.58 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hernández-Romero, D.; Sánchez-Rodríguez, E.; Osuna, E.; Sibón, A.; Martínez-Villanueva, M.; Noguera-Velasco, J.A.; Pérez-Cárceles, M.D. Proteomics in Deaths by Drowning: Diagnostic Efficacy of Apolipoprotein A1 and α-1 Antitrypsin, Pilot Study. Diagnostics 2020, 10, 747. https://doi.org/10.3390/diagnostics10100747
Hernández-Romero D, Sánchez-Rodríguez E, Osuna E, Sibón A, Martínez-Villanueva M, Noguera-Velasco JA, Pérez-Cárceles MD. Proteomics in Deaths by Drowning: Diagnostic Efficacy of Apolipoprotein A1 and α-1 Antitrypsin, Pilot Study. Diagnostics. 2020; 10(10):747. https://doi.org/10.3390/diagnostics10100747
Chicago/Turabian StyleHernández-Romero, Diana, Encarnación Sánchez-Rodríguez, Eduardo Osuna, Agustín Sibón, Miriam Martínez-Villanueva, José A. Noguera-Velasco, and María D. Pérez-Cárceles. 2020. "Proteomics in Deaths by Drowning: Diagnostic Efficacy of Apolipoprotein A1 and α-1 Antitrypsin, Pilot Study" Diagnostics 10, no. 10: 747. https://doi.org/10.3390/diagnostics10100747
APA StyleHernández-Romero, D., Sánchez-Rodríguez, E., Osuna, E., Sibón, A., Martínez-Villanueva, M., Noguera-Velasco, J. A., & Pérez-Cárceles, M. D. (2020). Proteomics in Deaths by Drowning: Diagnostic Efficacy of Apolipoprotein A1 and α-1 Antitrypsin, Pilot Study. Diagnostics, 10(10), 747. https://doi.org/10.3390/diagnostics10100747