Utility of Contrast-Enhanced Harmonic Endoscopic Ultrasonography for Early Diagnosis of Small Pancreatic Cancer
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Study Design
2.3. EUS Procedure
2.4. MDCT
2.5. MRI
2.6. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- American Cancer Society: Cancer Facts & Figures 2017; American Cancer Society: Atlanta, GA, USA, 2017.
- The Editorial Board of the Cancer Statics in Japan (Ed.) Foundation for Promotion of Cancer Research. In Cancer Statics in Japan-2015; FPCR c/o National Cancer Center: Tokyo, Japan, 2016; pp. 1–129. [Google Scholar]
- Agarwal, B.; Correa, A.M.; Ho, L. Survival in pancreatic carcinoma based on tumor size. Pancreas 2008, 36, 15–20. [Google Scholar] [CrossRef] [PubMed]
- Egawa, S.; Toma, H.; Ohigashi, H.; Okusaka, T.; Nakao, A.; Hatori, T.; Maguchi, H.; Yanagisawa, A.; Tanaka, M. Japan Pancreatic Cancer Registry; 30th year anniversary: Japan Pancreas Society. Pancreas 2012, 41, 985–992. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, N.A.; Kochman, M.L.; Lewis, J.D.; Kadish, S.; Morris, J.B.; Rosato, E.F.; Ginsberg, G.G. Endosonography is superior to angiography in the preoperative assessment of vascular involvement among patients with pancreatic carcinoma. J. Clin. Gastroenterol. 2001, 32, 54–58. [Google Scholar] [CrossRef] [PubMed]
- DeWitt, J.; Devereaux, B.; Chriswell, M.; McGreevy, K.; Howard, T.; Imperiale, T.F.; Ciaccia, D.; Lane, K.A.; Maglinte, D.; Kopecky, K.; et al. Comparison of endoscopic ultrasonography and multidetector computed tomography for detecting and staging pancreatic cancer. Ann. Intern. Med. 2004, 141, 753–763. [Google Scholar] [CrossRef] [PubMed]
- Kitano, M.; Kudo, M.; Yamao, K.; Takagi, T.; Sakamoto, H.; Komaki, T.; Kamata, K.; Imai, H.; Chiba, Y.; Okada, M.; et al. Characterization of small solid tumors in the pancreas: The value of contrast-enhanced harmonic endoscopic ultrasonography. Am. J. Gastroenterol. 2012, 107, 303–310. [Google Scholar] [CrossRef] [PubMed]
- Sontum, P.C.; Ostensen, J.; Dyrstad, K.; Hoff, L. Acoustic properties of NC100100 and their relation with the microbubble size distribution. Investig. Radiol. 1999, 34, 268–275. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.Y.; Cheon, Y.K.; Shim, C.S. Clinical role of contrast-enhanced harmonic endoscopic ultrasound in differentiating solid lesions of pancreas: A single-center experience in Korea. Gut. Liver 2013, 7, 559–604. [Google Scholar] [CrossRef]
- Napoleon, B.; Alvarez-Sanchez, M.V.; Gincoul, R.; Pujol, B.; Lefort, C.; Lepilliez, V.; Labadie, M.; Souquet, J.C.; Queneau, P.E.; Scoazec, J.Y.; et al. Contrast-enhanced harmonic endoscopic ultrasound in solid lesions of the pancreas: Results of a pilot study. Endoscopy 2010, 42, 564–570. [Google Scholar] [CrossRef]
- Fusaroli, P.; Spada, A.; Mancino, M.G.; Caletti, G. Contrast harmonic echo-endoscopic ultrasound improves accuracy in diagnosis of solid pancreatic masses. Clin. Gastroenterol. Hepatol. 2010, 8, 629–634. [Google Scholar] [CrossRef]
- Gincul, R.; Palazzo, M.; Pujol, B.; Tubach, F.; Palazzo, L.; Lefort, C.; Fumex, F.; Lombard, A.; Ribeiro, D.; Fabre, M.; et al. Contrast-harmonic endoscopic ultrasound for the diagnosis of pancreatic adenocarcinoma: A prospective multicenter trial. Endoscopy 2014, 46, 373–379. [Google Scholar] [CrossRef]
- Park, J.S.; Kim, H.K.; Bang, B.W.; Kim, S.G.; Jeong, S.; Lee, D.H. Effectiveness of contrast-enhanced harmonic endoscopic ultrasound for the evaluation of solid pancreatic masses. World J. Gastroenterol. 2014, 20, 518–524. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, Y.; Kato, J.; Ueda, K.; Nakamura, Y.; Kawaji, Y.; Abe, H.; Nuta, J.; Tamura, T.; Itonaga, M.; Yoshida, T.; et al. Contrast-enhanced endoscopic ultrasonography for pancreatic tumors. Biomed. Res. Int. 2015, 2015, 491782. [Google Scholar] [CrossRef] [PubMed]
- Iglesias-Garcia, J.; Lindkvist, B.; Lariño-Noia, J.; Abdulkader-Nallib, I.; Dominguez-Muñoz, J.E. Differential diagnosis of solid pancreatic masses: Contrast-enhanced harmonic (CEH-EUS), quantitative-elastography (QE-EUS), or both? United Eur. Gastroenterol. J. 2017, 5, 236–246. [Google Scholar] [CrossRef] [PubMed]
- Uekitani, K.; Kaino, S.; Harima, H.; Suenaga, S.; Sen-yo, M.; Sakaida, I. Efficacy of contrast-enhanced harmonic endoscopic ultrasonography in the diagnosis of pancreatic ductal carcinoma. Saudi J. Gastoroenterol. 2016, 22, 198–202. [Google Scholar] [CrossRef]
- Romagnuolo, J.; Hoffman, B.; Vela, S.; Hawes, R.; Vignesh, S. Accuracy of contrast-enhanced harmonic EUS with a second-generation perflutren lipid microsphere contrast agent (with video). Gastrointest. Endosc. 2011, 73, 52–63. [Google Scholar] [CrossRef]
- Matsubara, H.; Itoh, A.; Kawashima, H.; Matsubara, H.; Itoh, A.; Kawashima, H.; Kasugai, T.; Ohno, E.; Ishikawa, T.; Itoh, Y.; et al. Dynamic quantitative evaluation of contrast-enhanced endoscopic ultrasonography in the diagnosis of pancreatic disease. Pancreas 2011, 7, 1073–1079. [Google Scholar] [CrossRef]
- Seicean, A.; Badea, R.; Stan-Iuga, R.; Mocan, T.; Gulei, I.; Pascu, O. Quantitative contrast-enhanced harmonic endoscopic ultrasonography for the discrimination of solid pancreatic masses. Ultraschall. Med. 2010, 31, 571–576. [Google Scholar] [CrossRef]
- Săftoiu, A.; Vilmann, P.; Dietrich, C.F.; Iglesias-Garcia, J.; Hocke, M.; Seicean, A.; Ignee, A.; Hassan, H.; Streba, C.T.; Ioncică, A.M.; et al. Quantitative contrast-enhanced harmonic EUS in differential diagnosis of focal pancreatic masses (with videos). Gastrointest. Endosc. 2015, 82, 59–69. [Google Scholar] [CrossRef]
- Imazu, H.; Kanazawa, K.; Mori, N.; Ikeda, K.; Kakutani, H.; Sumiyama, K.; Hino, S.; Ang, T.L.; Omar, S.; Tajiri, H. Novel quantitative perfusion analysis with contrast-enhanced harmonic EUS for differentiation of autoimmune pancreatitis from pancreatic carcinoma. Scand. J. Gastroenterol. 2012, 47, 853–860. [Google Scholar] [CrossRef]
- Gheonea, D.; Streba, C.T.; Ciurea, T.; Săftoiu, A. Quantitative low mechanical index contrast-enhanced endoscopic ultrasound for the differential diagnosis of chronic pseudotumoral pancreatitis and pancreatic cancer. BMC Gastroenterol. 2013, 13, 2. [Google Scholar] [CrossRef]
- Leem, G.; Chung, M.J.; Park, J.Y.; Bang, S.; Song, S.Y.; Chung, J.B.; Park, S.W. Clinical value of contrast-enhanced harmonic endoscopic ultrasonography in the differential diagnosis of pancreatic and gallbladder masses. Clin. Endosc. 2018, 51, 80–88. [Google Scholar] [CrossRef] [PubMed]
- Omoto, S.; Takenaka, M.; Kitano, M.; Miyata, T.; Kamata, K.; Minaga, K.; Arizumi, T.; Yamao, K.; Imai, H.; Sakamoto, H.; et al. Characterization of pancreatic tumors with quantitative perfusion analysis in contrast-enhanced harmonic endoscopic ultrasonography. Oncology 2017, 93, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, T.; Hirooka, Y.; Kawashima, H.; Ohno, E.; Hashizume, K.; Funasaka, K.; Nakamura, M.; Miyahara, R.; Watanabe, O.; Ishigami, M.; et al. Multiphase evaluation of contrast-enhanced endoscopic ultrasonography in the diagnosis of pancreatic solid lesions. Pancreatology 2018, 18, 291–297. [Google Scholar] [CrossRef] [PubMed]
- Yasuda, K.; Mukai, H.; Nakajima, M.; Kawai, K. Staging of pancreatic carcinoma by endoscopic ultrasonography. Endoscopy 1993, 25, 151–155. [Google Scholar] [CrossRef]
- Legmann, P.; Vignaux, O.; Dousset, B.; Baraza, A.J.; Palazzo, L.; Dumontier, I.; Coste, J.; Louvel, A.; Roseau, G.; Couturier, D.; et al. Pancreatic tumors: Comparison of dual-phase helical CT and endoscopic sonography. Am. J. Roentgenol. 1998, 170, 1315–1322. [Google Scholar] [CrossRef]
- Ardengh, J.C.; de Paulo, G.A.; Ferrari, A.P. Pancreatic carcinomas smaller than 3.0 cm: Endosonography (EUS) in diagnosis, staging and prediction of resectability. HPB 2003, 5, 226–230. [Google Scholar] [CrossRef]
- Sakamoto, H.; Kitano, M.; Suetomi, Y.; Maekawa, K.; Takeyama, Y.; Kudo, M. Utility of contrast-enhanced endoscopic ultrasonography for diagnosis of small pancreatic carcinomas. Ultrasound Med. Biol. 2008, 34, 525–532. [Google Scholar] [CrossRef]
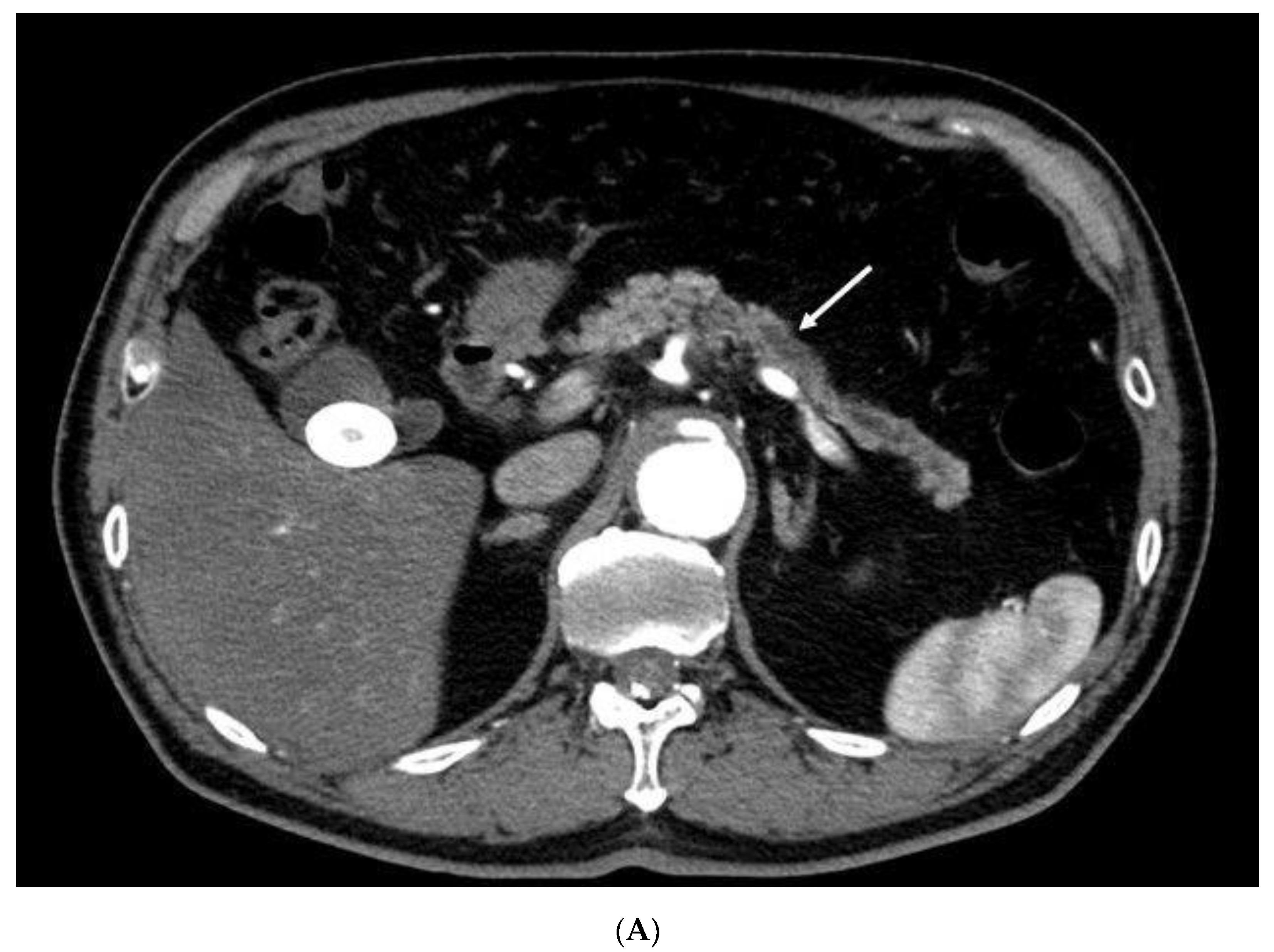
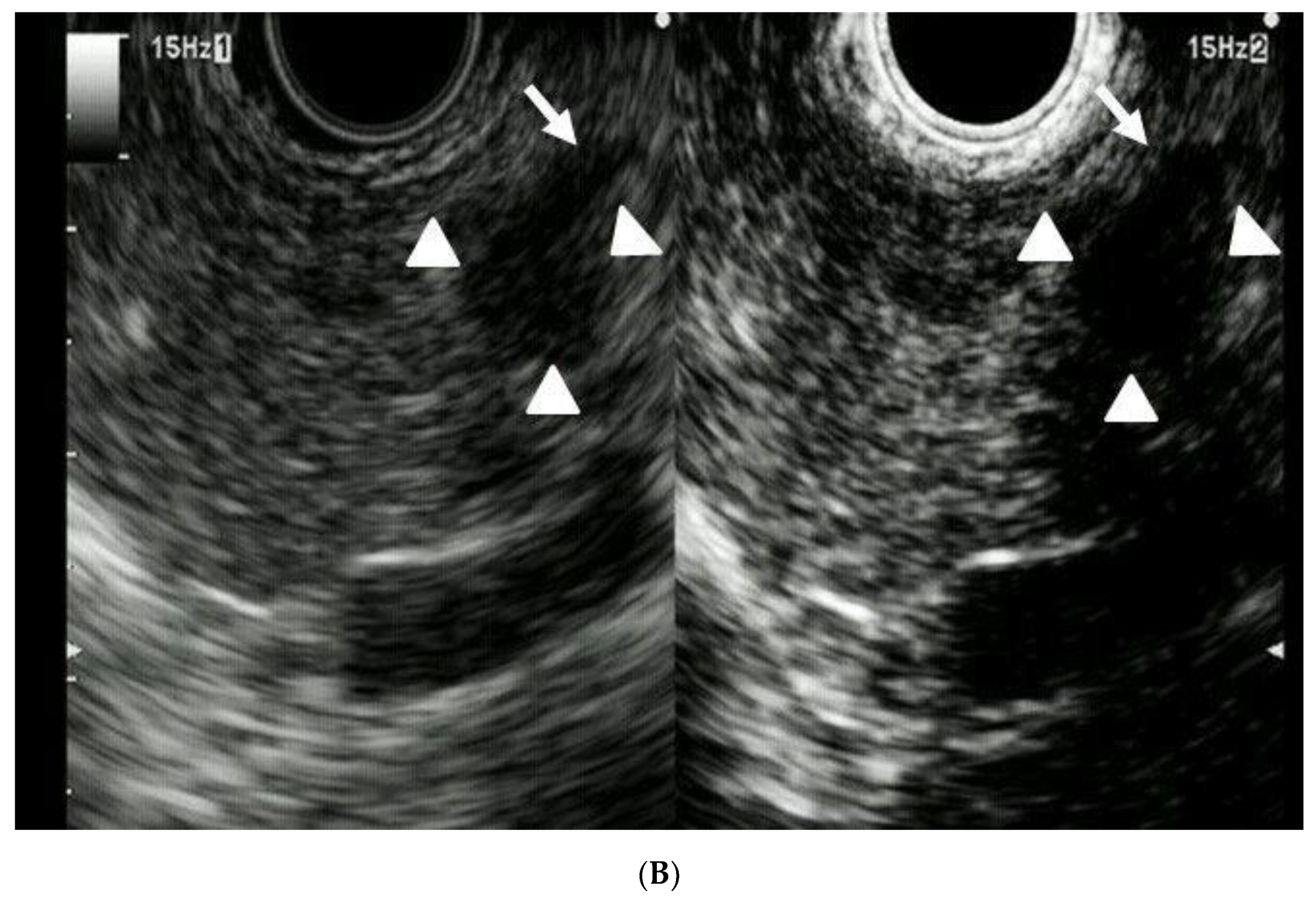
| Total Number of Patients | 41 |
|---|---|
| Sex (male/female) | 22/19 |
| Age, years (range) | 70 (43–88) |
| Lesion size, mm (mean ± SD) | 17.1 ± 2.6 |
| Lesion location (head/body-tail) | 28/13 |
| Symptoms (present/absent) | 24/17 |
| Pancreatic duct dilation (present/absent) | 26/15 |
| Lesion Size (11–20 mm) (n = 47) | ||||
|---|---|---|---|---|
| Sensitivity | Specificity | Accuracy | p-Value | |
| CH-EUS vs. MDCT | 95% vs. 78% | 83% vs. 83% | 94% vs. 79% | 0.02 |
| CH-EUS vs. MRI | 95% vs. 73% | 83% vs. 33% | 94% vs. 68% | 0.007 |
| MDCT vs. MRI | 78% vs. 73% | 83% vs. 33% | 79% vs. 68% | 0.41 |
| CH-EUS Findings | Final Diagnosis (Pathology) | |
|---|---|---|
| Pancreatic Cancer (n = 41) | Non-Pancreatic Cancer (n = 6) | |
| Pancreatic cancer | 39 | 1 |
| Non-pancreatic cancer | 2 | 5 |
| Total Number of Patients | 10 |
|---|---|
| Sex (male/female) | 4/6 |
| Age, years (range) | 73 (44–84) |
| Lesion size, mm (mean ± SD) | 5.1 ± 3.9 |
| Lesion location (head/body-tail) | 4/6 |
| Symptoms (present/absent) | 1/9 |
| Pancreatic duct dilation (present/absent) | 9/1 |
| Lesion Size ≤10 mm (n = 13) | ||||
|---|---|---|---|---|
| Sensitivity | Specificity | Accuracy | p-Value | |
| CH-EUS vs. MDCT | 70% vs. 20% | 100% vs. 100% | 77% vs. 39% | 0.025 |
| CH-EUS vs. MRI | 70% vs. 50% | 100% vs. 100% | 77% vs. 62% | 0.16 |
| MDCT vs. MRI | 20% vs. 50% | 100% vs. 100% | 39% vs. 62% | 0.08 |
| CH-EUS Findings | Final Diagnosis (Pathology) | |
|---|---|---|
| Pancreatic Cancer (n = 10) | Non-Pancreatic Cancer (n = 3) | |
| Pancreatic cancer | 7 | 0 |
| Non-pancreatic cancer | 3 | 3 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yamashita, Y.; Tanioka, K.; Kawaji, Y.; Tamura, T.; Nuta, J.; Hatamaru, K.; Itonaga, M.; Yoshida, T.; Ida, Y.; Maekita, T.; et al. Utility of Contrast-Enhanced Harmonic Endoscopic Ultrasonography for Early Diagnosis of Small Pancreatic Cancer. Diagnostics 2020, 10, 23. https://doi.org/10.3390/diagnostics10010023
Yamashita Y, Tanioka K, Kawaji Y, Tamura T, Nuta J, Hatamaru K, Itonaga M, Yoshida T, Ida Y, Maekita T, et al. Utility of Contrast-Enhanced Harmonic Endoscopic Ultrasonography for Early Diagnosis of Small Pancreatic Cancer. Diagnostics. 2020; 10(1):23. https://doi.org/10.3390/diagnostics10010023
Chicago/Turabian StyleYamashita, Yasunobu, Kensuke Tanioka, Yuki Kawaji, Takashi Tamura, Junya Nuta, Keiichi Hatamaru, Masahiro Itonaga, Takeichi Yoshida, Yoshiyuki Ida, Takao Maekita, and et al. 2020. "Utility of Contrast-Enhanced Harmonic Endoscopic Ultrasonography for Early Diagnosis of Small Pancreatic Cancer" Diagnostics 10, no. 1: 23. https://doi.org/10.3390/diagnostics10010023
APA StyleYamashita, Y., Tanioka, K., Kawaji, Y., Tamura, T., Nuta, J., Hatamaru, K., Itonaga, M., Yoshida, T., Ida, Y., Maekita, T., Iguchi, M., Terada, M., Sonomura, T., Hirono, S., Okada, K.-I., Kawai, M., Yamaue, H., & Kitano, M. (2020). Utility of Contrast-Enhanced Harmonic Endoscopic Ultrasonography for Early Diagnosis of Small Pancreatic Cancer. Diagnostics, 10(1), 23. https://doi.org/10.3390/diagnostics10010023







