Detection Rate of Culprit Tumors Causing Osteomalacia Using Somatostatin Receptor PET/CT: Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Methods
2.1. Search Strategy
2.2. Study Selection
2.3. Data Extraction
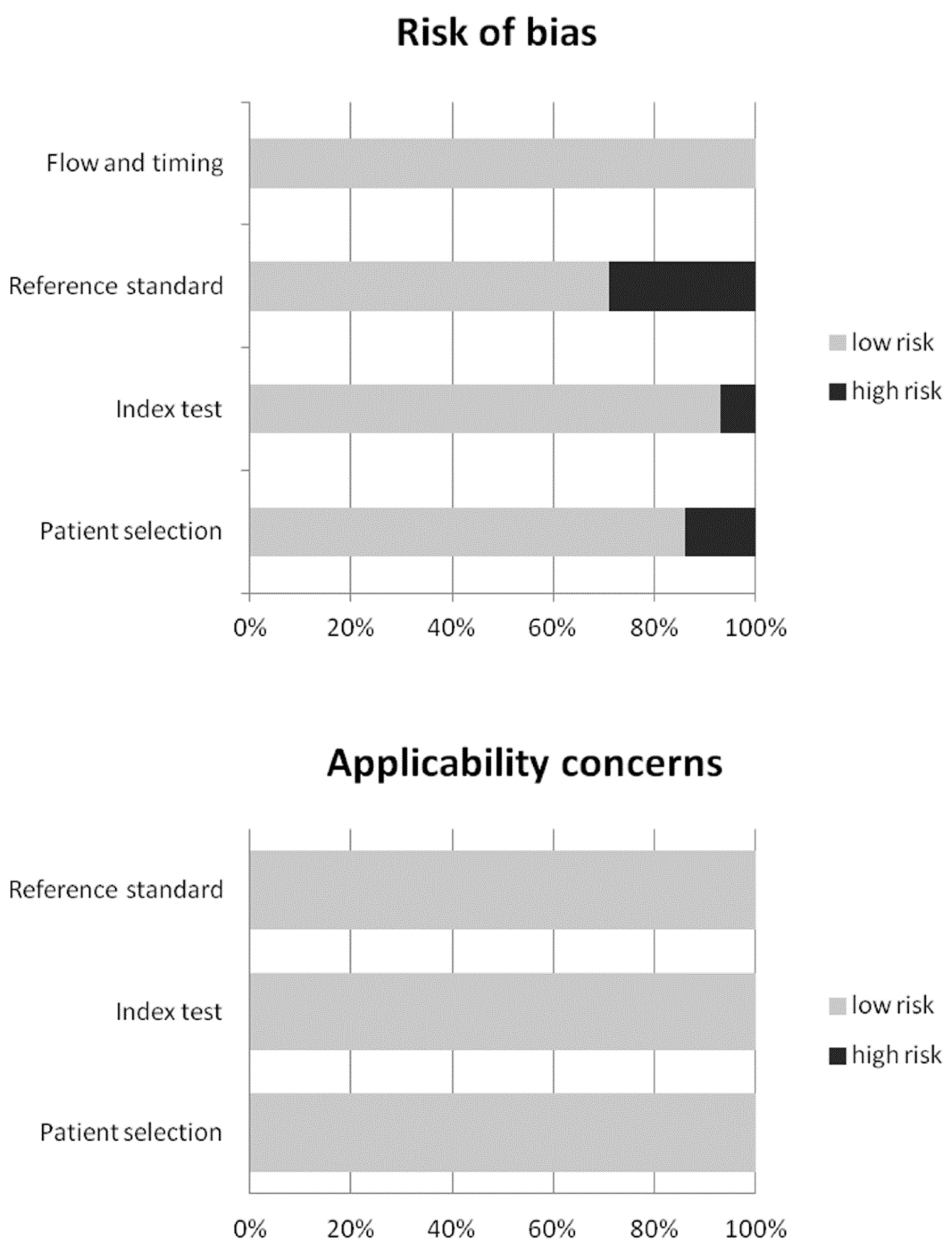
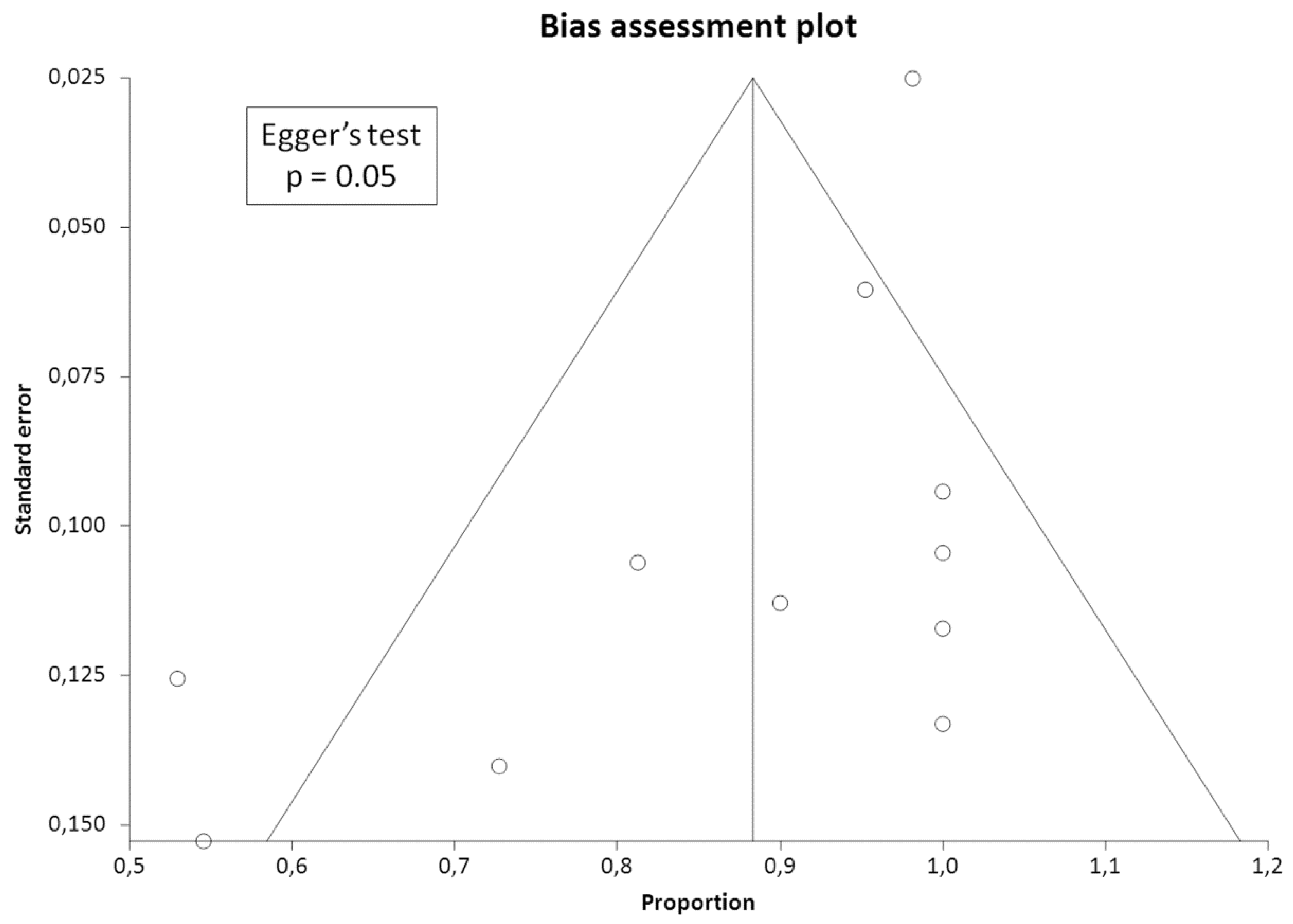
2.4. Quality Assessment
2.5. Statistical Analysis
3. Results
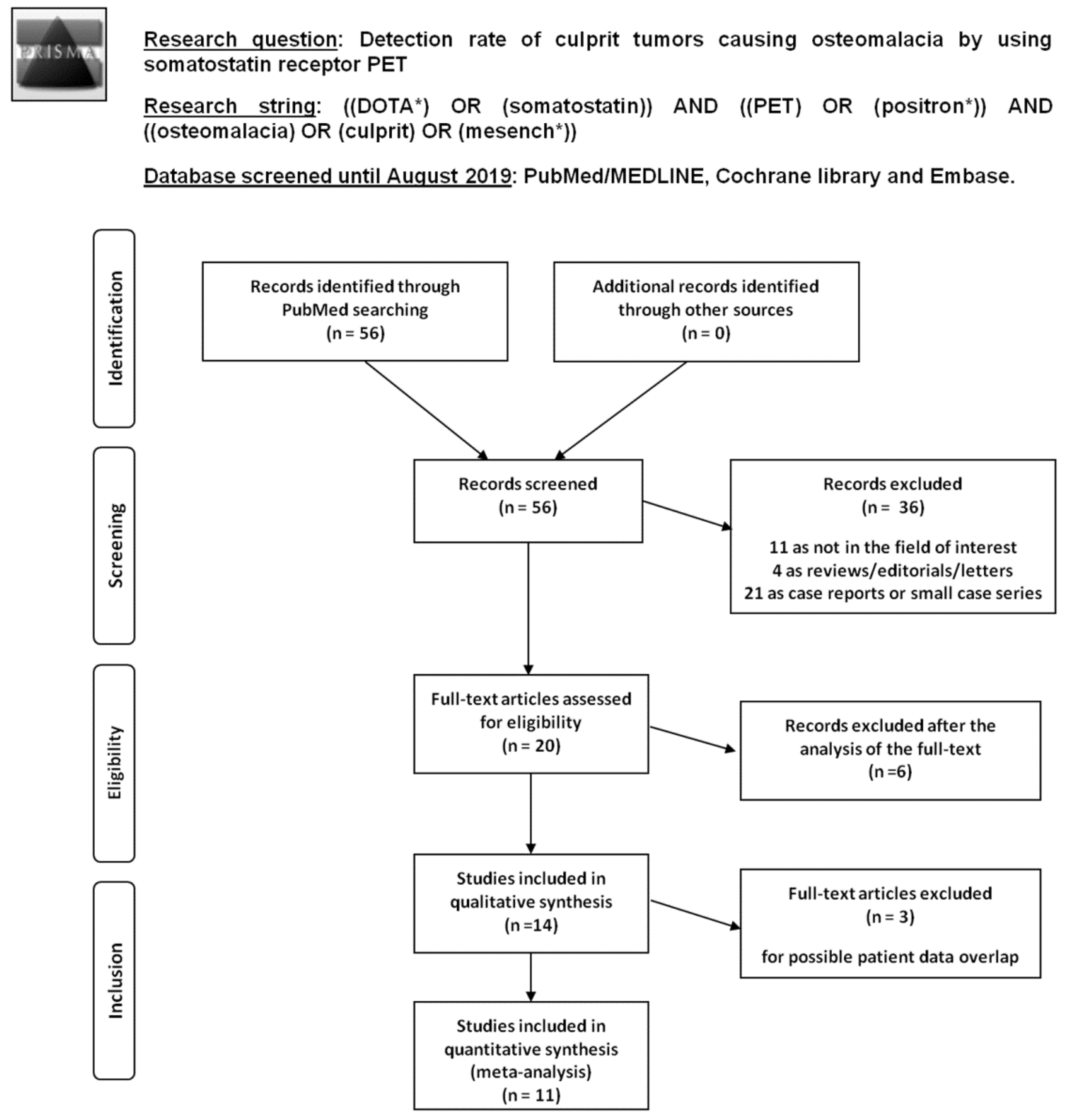
3.1. Literature Search
3.2. Qualitative Analysis (Systematic Review)
3.2.1. Basic Study and Patient Characteristics
3.2.2. Technical Aspects
3.2.3. Main Findings
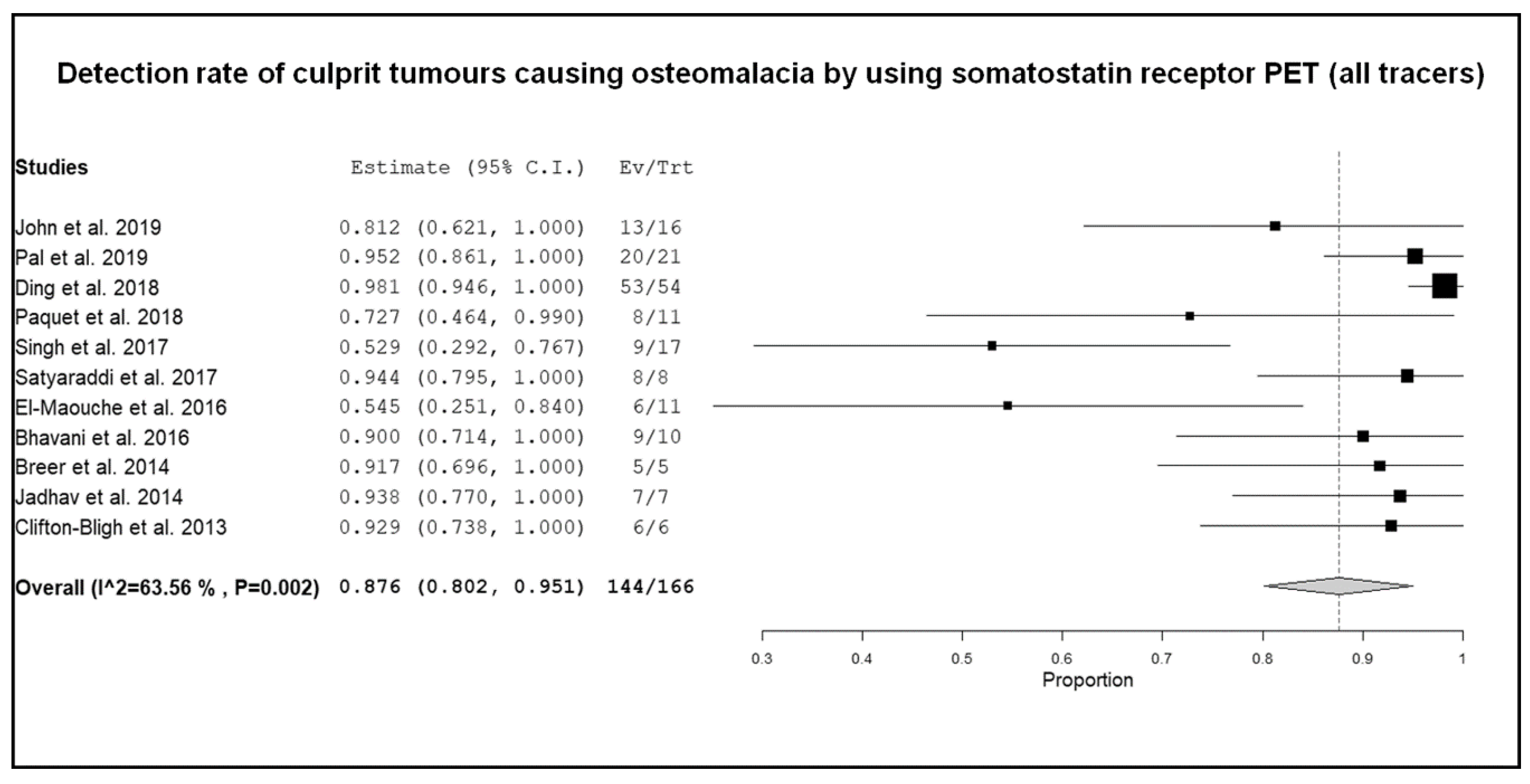
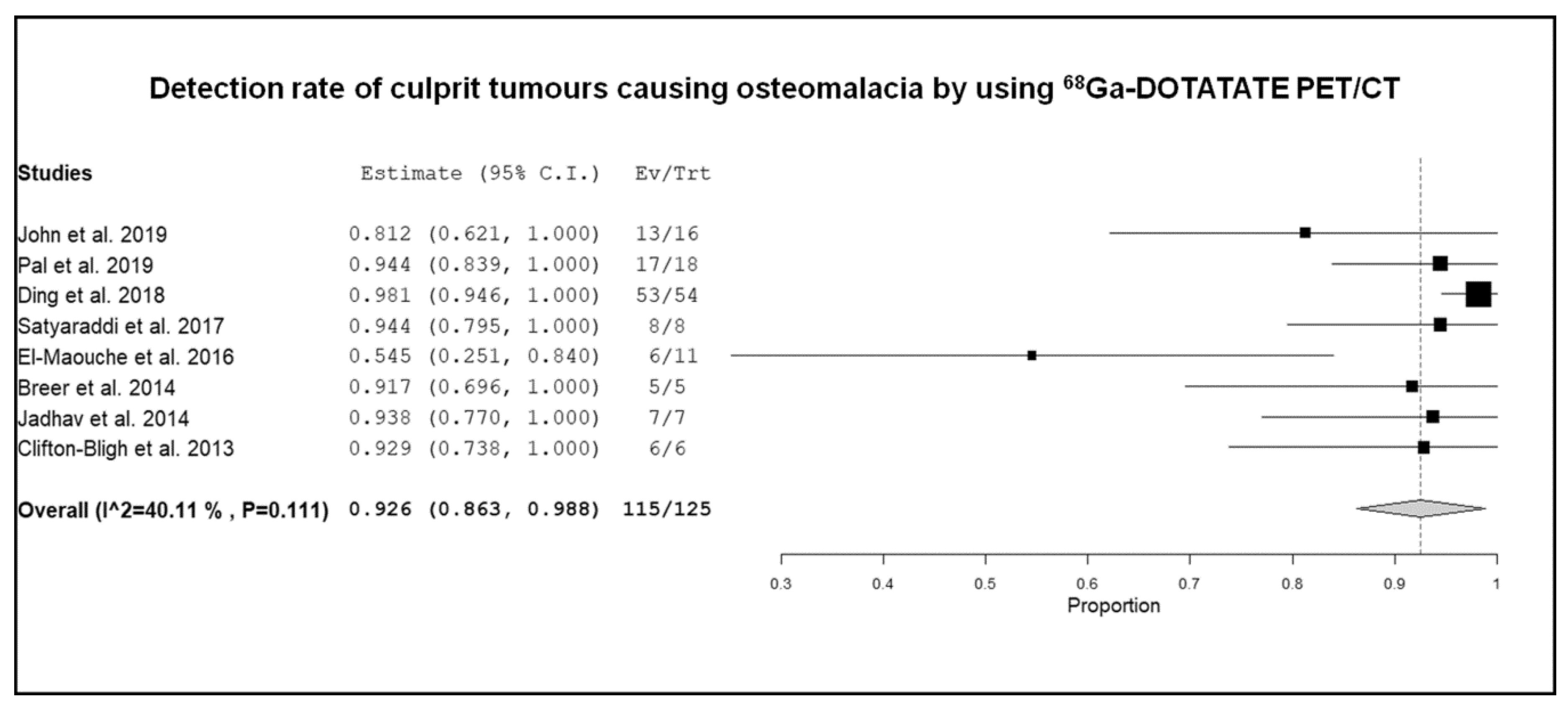
3.3. Quantitative Analysis (Meta-Analysis)
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Yin, Z.; Du, J.; Yu, F.; Xia, W. Tumor-induced osteomalacia. Osteoporos. Sarcopenia 2018, 4, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Imel, E.A.; Biggin, A.; Schindeler, A.; Munns, C.F. FGF23, Hypophosphatemia, and Emerging Treatments. JBMR Plus 2019, 3, e10190. [Google Scholar] [CrossRef] [PubMed]
- Folpe, A.L. Phosphaturic mesenchymal tumors: A review and update. Semin. Diagn. Pathol. 2019, 36, 260–268. [Google Scholar] [CrossRef] [PubMed]
- Rayamajhi, S.J.; Yeh, R.; Wong, T.; Dumeer, S.; Mittal, B.R.; Remotti, F.; Chikeka, I.; Reddy, A.K. Tumor-induced osteomalacia—Current imaging modalities and a systematic approach for tumor localization. Clin. Imaging. 2019, 56, 114–123. [Google Scholar] [CrossRef]
- Yang, M.; Doshi, K.B.; Roarke, M.C.; Nguyen, B.D. Molecular Imaging in Diagnosis of Tumor-induced Osteomalacia. Curr. Probl. Diagn. Radiol. 2019, 48, 379–386. [Google Scholar] [CrossRef]
- De Dosso, S.; Treglia, G.; Pascale, M.; Tamburello, A.; Santhanam, P.; Kroiss, A.S.; Pereira Mestre, R.; Saletti, P.; Giovanella, L. Detection rate of unknown primary tumour by using somatostatin receptor PET/CT in patients with metastatic neuroendocrine tumours: A meta-analysis. Endocrine 2019, 64, 456–468. [Google Scholar] [CrossRef]
- Treglia, G.; Kroiss, A.S.; Piccardo, A.; Lococo, F.; Santhanam, P.; Imperiale, A. Role of positron emission tomography in thyroid and neuroendocrine tumors. Minerva Endocrinol. 2018, 43, 341–355. [Google Scholar] [CrossRef]
- Treglia, G.; Castaldi, P.; Rindi, G.; Giordano, A.; Rufini, V. Diagnostic performance of Gallium-68 somatostatin receptor PET and PET/CT in patients with thoracic and gastroenteropancreatic neuroendocrine tumours: A meta-analysis. Endocrine 2012, 42, 80–87. [Google Scholar] [CrossRef]
- Treglia, G.; Rindi, G.; Rufini, V. Expression of somatostatin receptors may guide the use of somatostatin receptor imaging and therapy in differentiated thyroid cancer. Hormones 2012, 11, 230–232. [Google Scholar] [CrossRef]
- McInnes, M.D.F.; Moher, D.; Thombs, B.D.; McGrath, T.A.; Bossuyt, P.M.; The PRISMA-DTA Group; Clifford, T.; Cohen, J.F.; Deeks, J.J.; Gatsonis, C.; et al. Preferred Reporting Items for a Systematic Review and Meta-analysis of Diagnostic Test Accuracy Studies: The PRISMA-DTA Statement. JAMA 2018, 319, 388–396. [Google Scholar] [CrossRef]
- Sadeghi, R.; Treglia, G. Systematic reviews and meta-analyses of diagnostic studies: A practical guideline. Clin. Transl. Imaging. 2017, 5, 83–87. [Google Scholar] [CrossRef]
- Treglia, G.; Sadeghi, R. Meta-analyses and systematic reviews on PET and PET/CT in oncology: The state of the art. Clin. Transl. Imaging. 2013, 1, 73–75. [Google Scholar] [CrossRef][Green Version]
- Whiting, P.F.; Rutjes, A.W.; Westwood, M.E.; Mallett, S.; Deeks, J.J.; Reitsma, J.B.; Leeflang, M.M.; Sterne, J.A.; Bossuyt, P.M. QUADAS-2 Group. QUADAS-2: A revised tool for the quality assessment of diagnostic accuracy studies. Ann. Int. Med. 2011, 155, 529–536. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.; Thompson, S.G. Quantifying heterogeneity in a meta-analysis. Stat. Med. 2002, 21, 1539–1558. [Google Scholar] [CrossRef]
- Harbord, R.M.; Egger, M.; Sterne, J.A. A modified test for small-study effects in meta-analyses of controlled trials with binary endpoints. Stat. Med. 2006, 25, 3443–3457. [Google Scholar] [CrossRef]
- John, J.R.; Hephzibah, J.; Oommen, R.; Shanthly, N.; Mathew, D. Ga-68 DOTATATE Positron Emission Tomography-Computed Tomography Imaging in Oncogenic Osteomalacia: Experience from a Tertiary Level Hospital in South India. Indian J. Nucl. Med. 2019, 34, 188–193. [Google Scholar] [CrossRef]
- Pal, R.; Bhadada, S.K.; Shingare, A.; Bhansali, A.; Kamalanathan, S.; Chadha, M.; Chauhan, P.; Sood, A.; Dhiman, V.; Sharma, D.C.; et al. Tumor-induced osteomalacia: Experience from three tertiary care centres In India. Endocr. Connect. 2019, 8, 266–267. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, L.; Wang, T.; Xing, H.Q.; Huo, L.; Li, F. Value of (68)Ga-DOTA-TATE Positron Emission Tomography/Computed Tomography in the Localization of Culprit Tumors Causing Osteomalacia with Negative (99m) Tc-HYNIC-TOC Single Photo Emission Computed Tomography. Zhongguo Yi Xue Ke Xue Yuan Xue Bao 2018, 40, 757–764. [Google Scholar] [CrossRef]
- Ding, J.; Hu, G.; Wang, L.; Li, F.; Huo, L. Increased Activity Due to Fractures Does Not Significantly Affect the Accuracy of 68Ga-DOTATATE PET/CT in the Detection of Culprit Tumor in the Evaluation of Tumor-Induced Osteomalacia. Clin. Nucl. Med. 2018, 43, 880–886. [Google Scholar] [CrossRef]
- Paquet, M.; Gauthé, M.; Zhang, Y.J.; Nataf, V.; Bélissant, O.; Orcel, P.; Roux, C.; Talbot, J.N.; Montravers, F. Diagnostic performance and impact on patient management of (68)Ga-DOTA-TOC PET/CT for detecting osteomalacia-associated tumours. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 1710–1720. [Google Scholar] [CrossRef]
- Singh, D.; Chopra, A.; Ravina, M.; Kongara, S.; Bhatia, E.; Kumar, N.; Gupta, S.; Yadav, S.; Dabadghao, P.; Yadav, R.; et al. Oncogenic osteomalacia: Role of Ga-68 DOTANOC PET/CT scan in identifying the culprit lesion and its management. Br. J. Radiol. 2017, 90, 20160811. [Google Scholar] [CrossRef] [PubMed]
- Satyaraddi, A.; Cherian, K.E.; Shetty, S.; Kapoor, N.; Jebasingh, F.K.; Cherian, V.M.; Hephzibah, J.; Prabhu, A.J.; Thomas, N.; Paul, T.V. Musculoskeletal oncogenic osteomalacia-An experience from a single centre in South India. J. Orthop. 2017, 14, 184–188. [Google Scholar] [CrossRef] [PubMed]
- El-Maouche, D.; Sadowski, S.M.; Papadakis, G.Z.; Guthrie, L.; Cottle-Delisle, C.; Merkel, R.; Millo, C.; Chen, C.C.; Kebebew, E.; Collins, M.T. (68)Ga-DOTATATE for Tumor Localization in Tumor-Induced Osteomalacia. J. Clin. Endocrinol. Metab. 2016, 101, 3575–3581. [Google Scholar] [CrossRef] [PubMed]
- Bhavani, N.; Reena Asirvatham, A.; Kallur, K.; Menon, A.S.; Pavithran, P.V.; Nair, V.; Vasukutty, J.R.; Menon, U.; Kumar, H. Utility of Gallium-68 DOTANOC PET/CT in the localization of Tumour-induced osteomalacia. Clin. Endocrinol. 2016, 84, 134–140. [Google Scholar] [CrossRef]
- Zhang, J.; Zhu, Z.; Zhong, D.; Dang, Y.; Xing, H.; Du, Y.; Jing, H.; Qiao, Z.; Xing, X.; Zhuang, H.; et al. 68Ga DOTATATE PET/CT is an Accurate Imaging Modality in the Detection of Culprit Tumors Causing Osteomalacia. Clin. Nucl. Med. 2015, 40, 642–646. [Google Scholar] [CrossRef]
- Agrawal, K.; Bhadada, S.; Mittal, B.R.; Shukla, J.; Sood, A.; Bhattacharya, A.; Bhansali, A. Comparison of 18F-FDG and 68Ga DOTATATE PET/CT in localization of tumor causing oncogenic osteomalacia. Clin. Nucl. Med. 2015, 40, e6–e10. [Google Scholar] [CrossRef]
- Breer, S.; Brunkhorst, T.; Beil, F.T.; Peldschus, K.; Heiland, M.; Klutmann, S.; Barvencik, F.; Zustin, J.; Gratz, K.F.; Amling, M. 68Ga DOTA-TATE PET/CT allows tumor localization in patients with tumor-induced osteomalacia but negative 111In-octreotide SPECT/CT. Bone 2014, 64, 222–227. [Google Scholar] [CrossRef]
- Jadhav, S.; Kasaliwal, R.; Lele, V.; Rangarajan, V.; Chandra, P.; Shah, H.; Malhotra, G.; Jagtap, V.S.; Budyal, S.; Lila, A.R.; et al. Functional imaging in primary tumour-induced osteomalacia: Relative performance of FDG PET/CT vs somatostatin receptor-based functional scans: A series of nine patients. Clin. Endocrinol. 2014, 81, 31–37. [Google Scholar] [CrossRef]
- Clifton-Bligh, R.J.; Hofman, M.S.; Duncan, E.; Sim, I.e.W.; Darnell, D.; Clarkson, A.; Wong, T.; Walsh, J.P.; Gill, A.J.; Ebeling, P.R.; et al. Improving diagnosis of tumor-induced osteomalacia with Gallium-68 DOTATATE PET/CT. J. Clin. Endocrinol. Metab. 2013, 98, 687–694. [Google Scholar] [CrossRef]
- Anzola, L.K.; Glaudemans, A.W.J.M.; Dierckx, R.A.J.O.; Martinez, F.A.; Moreno, S.; Signore, A. Somatostatin receptor imaging by SPECT and PET in patients with chronic inflammatory disorders: A systematic review. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 2496–2513. [Google Scholar] [CrossRef]
- Treglia, G. Diagnostic Performance of (18) F-FDG PET/CT in Infectious and Inflammatory Diseases according to Published Meta-Analyses. Contrast Media Mol. Imaging 2019, 2019, 3018349. [Google Scholar] [CrossRef] [PubMed]
- Hope, T.A.; Pampaloni, M.H.; Flavell, R.R.; Nakakura, E.C.; Bergsland, E.K. Somatostatin receptor PET/MRI for the evaluation of neuroendocrine tumors. Clin. Transl. Imaging 2017, 5, 63–69. [Google Scholar] [CrossRef]
- Fuchs, S.; Grössmann, N.; Ferch, M.; Busse, R.; Wild, C. Evidence-based indications for the planning of PET or PET/CT capacities are needed. Clin. Transl. Imaging 2019, 7, 65–81. [Google Scholar] [CrossRef]
| Authors | Year | Country | Study Design | Type of Patients Evaluated | Number of Patients with TIO Referred for SSTR-PET/CT | Age (years) | %Male | FGF23 Serum Level |
|---|---|---|---|---|---|---|---|---|
| John et al. [16] | 2019 | India | Retrospective single centre | Patients with clinical and biochemical diagnosis of TIO | 16 | Mean: 45 (18–61) | 75% | 112–1500 RU/mL |
| Pal et al. [17] | 2019 | India | Retrospective multicentre | Patients with clinical and biochemical diagnosis of TIO | 21 | Mean: 40.2 (19–58) | 38% | 102–6435 RU/mL |
| Zhang et al. [18] | 2018 | China | Retrospective single centre | Patients with clinical and biochemical diagnosis of TIO and negative 99mTc-octreotide SPECT | 37 | Mean: 44 (17–75) | 59% | NR |
| Ding et al. [19] | 2018 | China | Retrospective single centre | Patients with clinical and biochemical diagnosis of TIO | 54 | Mean: 41.2 (15–82) | 63% | NR |
| Paquet et al. [20] | 2018 | France | Retrospective single centre | Patients with clinical and biochemical diagnosis of TIO | 15 | Mean: 53 (23–83) | 67% | 29–1916 RU/mL |
| Singh et al. [21] | 2017 | India | Retrospective single centre | Patients with suspected TIO | 17 | Mean: 42.4 (18–70) | 47% | 59–12000 RU/mL |
| Satyaraddi et al. [22] | 2017 | India | Retrospective single centre | Patients with clinical and biochemical diagnosis of TIO | 8 | Mean: 46.6 (18–74) | 50% | 202–3556 RU/mL |
| El-Maouche et al. [23] | 2016 | USA | Prospective single centre | Patients with clinical and biochemical diagnosis of TIO | 11 | Mean: 38 (19–60) | 45% | 105–5939 pg/mL |
| Bhavani et al. [24] | 2016 | India | Retrospective single centre | Patients with clinical and biochemical diagnosis of TIO | 10 | Mean: 40 (13–53) | 80% | 152–2323 RU/mL |
| Zhang et al. [25] | 2015 | China | Retrospective single centre | Patients with suspected TIO | 54 | Mean: 42.2 (19–68) | 48% | NR |
| Agrawal et al. [26] | 2015 | India | Retrospective single centre | Patients with suspected TIO | 6 | Mean: 37.5 (26–55) | 17% | 148–6685 RU/mL |
| Breer et al. [27] | 2014 | Germany | Retrospective single centre | Patients with suspected TIO | 5 | Mean: 50.2 (41–62) | 40% | <9.9–78.3 pg/nL |
| Jadhav et al. [28] | 2014 | India | Retrospective single centre | Patients with clinical and biochemical diagnosis of TIO | 7 | Mean: 35.7 (22–49) | 71% | 109–6000 RU/mL |
| Clifton-Bligh et al. [29] | 2013 | Australia | Retrospective multicentre | Patients with clinical and biochemical diagnosis of TIO | 6 | Mean: 43.5 (28–65) | 50% | 59–1940 ng/L |
| Authors | Hybrid Imaging Modality | Tracer Used | Injected Activity | Time Interval between Radiotracer Injection and Image Acquisition | Image Analysis | Other Functional Imaging Modalities Performed for Comparison |
|---|---|---|---|---|---|---|
| John et al. [16] | PET/CT (contrast enhanced CT) | 68Ga-DOTATATE | 75–185 MBq | 30–45 min | Visual | bone scintigraphy 18F-FDG PET/CT |
| Pal et al. [17] | PET/CT (low-dose CT) | 68Ga-DOTATATE, 68Ga-DOTANOC | NR | NR | Visual and semi-quantitative (SUVmax) | 99mTc-octreotide SPECT/CT 18F-FDG PET/CT |
| Zhang et al. [18] | PET/CT (low-dose CT) | 68Ga-DOTATATE | 44–111 MBq | 40–60 min | Visual and semi-quantitative (SUVmax) | 99mTc-octreotide SPECT/CT |
| Ding et al. [19] | PET/CT (low-dose CT) | 68Ga-DOTATATE | NR | NR | Visual and semi-quantitative (SUVmax) | |
| Paquet et al. [20] | PET/CT (low-dose CT) | 68Ga-DOTATOC | 1.6 MBq/kg | 60 min | Visual and semi-quantitative (SUVmax, BTV) | 111In-octreotide SPECT/CT 18F-FDG PET/CT |
| Singh et al. [21] | PET/CT (low-dose CT) | 68Ga-DOTANOC | 111–148 MBq | 45 ± 15 min | Visual and semi-quantitative (SUVmax) | |
| Satyaraddi et al. [22] | PET/CT (low-dose CT) | 68Ga-DOTATATE | NR | NR | Visual | 18F-FDG PET/CT 99mTc-red blood cells scintigraphy |
| El-Maouche et al. [23] | PET/CT (low-dose CT) | 68Ga-DOTATATE | 185 MBq | 60 min | Visual and semi-quantitative (SUVmax) | 111In-octreotide SPECT/CT 18F-FDG PET/CT |
| Bhavani et al. [24] | PET/CT (low-dose CT) | 68Ga-DOTANOC | 111–185 MBq | 60 min | Visual and semi-quantitative (SUVmax) | bone scintigraphy 99mTc-sestamibi scintigraphy |
| Zhang et al. [25] | PET/CT (low-dose CT) | 68Ga-DOTATATE | 111–148 MBq | 45 min | Visual and semi-quantitative (SUVmax) | |
| Agrawal et al. [26] | PET/CT (low-dose CT) | 68Ga-DOTATATE | 1.5 MBq/kg | 45–60 min | Visual | 18F-FDG PET/CT |
| Breer et al. [27] | PET/CT (low-dose CT) | 68Ga-DOTATATE | 58–110 MBq | 20 min | Visual and semi-quantitative (SUVmax) | 111In-octreotide SPECT/CT |
| Jadhav et al. [28] | PET/CT (low-dose CT) | 68Ga-DOTATATE | 74–111 MBq | 60–90 min | Visual | 99mTc-octreotide SPECT/CT 18F-FDG PET/CT |
| Clifton-Bligh et al. [29] | PET/CT (low-dose CT) | 68Ga-DOTATATE | 103–226 MBq | 45–60 min | Visual | bone scintigraphy 99mTc-sestamibi scintigraphy 111In-octreotide scintigraphy 18F-FDG PET/CT |
| Authors | Detection Rate (Patient-Based Analysis) | Site of Culprit Lesion Detected by SSTR-PET/CT | Number of Tumors Detected by SSTR-PET/CT with Histopathology | Histological Type of Culprit Tumors Detected by SSTR-PET/CT | ||||
|---|---|---|---|---|---|---|---|---|
| Cranio-Facial | Trunk | Upper Limbs | Lower Limbs | Metastatic | ||||
| John et al. [16] | 13/16 (81.3%) | 2 | 1 | 10 | 10/13 | 10 PMT | ||
| Pal et al. [17] | 20/21 (95.2%) | 5 | 3 | 12 | 15/20 | 11 PMT,2 HP, 1 GCT, 1 HE | ||
| Zhang et al. [18] * | 37/37 (100%) | 5 | 11 | 2 | 19 | 37/37 | 35 PMT, 2 SCT | |
| Ding et al. [19] | 53/54 (98.1%) | NR | NR | NR | NR | NR | 52/53 | NR |
| Paquet et al. [20] | 8/11 (72.7%) | 1 | 4 | 3 | 8/8 | 6 PMT, 1 HE, 1 NR | ||
| Singh et al. [21] | 9/17 (52.9%) | 2 | 2 | 2 | 3 | 7/9 | 7 PMT | |
| Satyaraddi et al. [22] | 8/8 (100%) | 1 | 7 | 5/8 | 5 PMT | |||
| El-Maouche et al. [23] | 6/11 (54.5%) | 1 | 1 | 3 | 1 | 5/6 | 5 PMT | |
| Bhavani et al. [24] | 9/10 (90%) | 3 | 1 | 5 | 8/9 | 6 PMT, 1 HP, 1 SCT | ||
| Zhang et al. [25] * | 32/32 (100%) | 7 | 5 | 2 | 17 | 32/32 | 31 PMT, 1 OT | |
| Agrawal et al. [26] * | 5/6 (83.3%) | 2 | 3 | 5/5 | 2 PMT, 2 HP, 1 OT | |||
| Breer et al. [27] | 5/5 (100%) | 2 | 1 | 1 | 1 | 5/5 | 3 PMT, 2 OT | |
| Jadhav et al. [28] | 7/7 (100%) | 1 | 6 | 4/7 | NR | |||
| Clifton-Bligh et al. [29] | 6/6 (100%) | 1 | 5 | 6/6 | 6 PMT | |||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meyer, M.; Nicod Lalonde, M.; Testart, N.; Jreige, M.; Kamani, C.; Boughdad, S.; Muoio, B.; Becce, F.; Schaefer, N.; Candrian, C.; et al. Detection Rate of Culprit Tumors Causing Osteomalacia Using Somatostatin Receptor PET/CT: Systematic Review and Meta-Analysis. Diagnostics 2020, 10, 2. https://doi.org/10.3390/diagnostics10010002
Meyer M, Nicod Lalonde M, Testart N, Jreige M, Kamani C, Boughdad S, Muoio B, Becce F, Schaefer N, Candrian C, et al. Detection Rate of Culprit Tumors Causing Osteomalacia Using Somatostatin Receptor PET/CT: Systematic Review and Meta-Analysis. Diagnostics. 2020; 10(1):2. https://doi.org/10.3390/diagnostics10010002
Chicago/Turabian StyleMeyer, Marie, Marie Nicod Lalonde, Nathalie Testart, Mario Jreige, Christel Kamani, Sarah Boughdad, Barbara Muoio, Fabio Becce, Niklaus Schaefer, Christian Candrian, and et al. 2020. "Detection Rate of Culprit Tumors Causing Osteomalacia Using Somatostatin Receptor PET/CT: Systematic Review and Meta-Analysis" Diagnostics 10, no. 1: 2. https://doi.org/10.3390/diagnostics10010002
APA StyleMeyer, M., Nicod Lalonde, M., Testart, N., Jreige, M., Kamani, C., Boughdad, S., Muoio, B., Becce, F., Schaefer, N., Candrian, C., Giovanella, L., Prior, J. O., Treglia, G., & Riegger, M. (2020). Detection Rate of Culprit Tumors Causing Osteomalacia Using Somatostatin Receptor PET/CT: Systematic Review and Meta-Analysis. Diagnostics, 10(1), 2. https://doi.org/10.3390/diagnostics10010002








