Ultrasound Imaging of the Abdominal Wall and Trunk Muscles in Patients with Achilles Tendinopathy versus Healthy Participants
Abstract
1. Introduction
2. Materials and Methods
2.1. Design
2.2. Sample Size Calculation
2.3. Participants
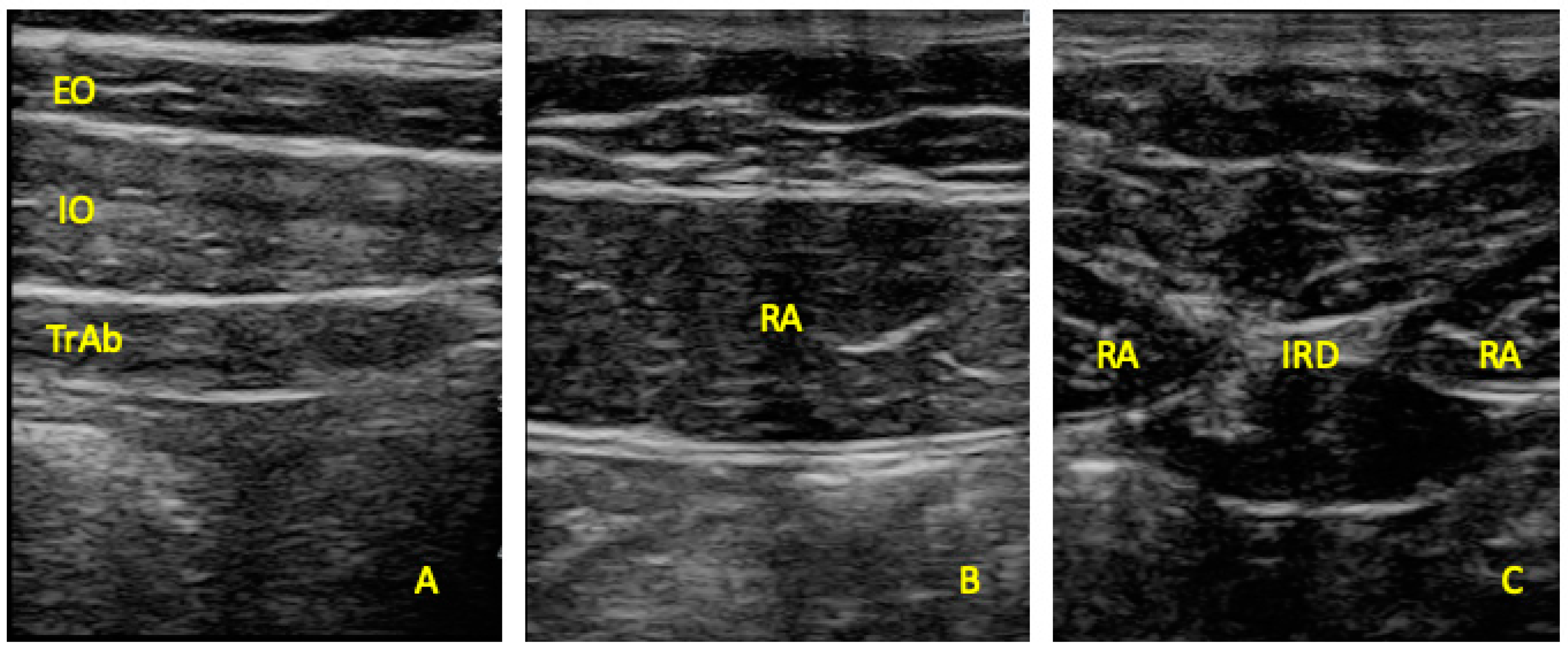
2.4. Ultrasonography Imaging Measurements
2.5. Statistics
3. Results
4. Discussion
4.1. Inter-Recti Distance and Rectus Abdominis Thickness
4.2. External Oblique, Internal Oblique, and Transversus Abdominis Thickness
4.3. Multifidus Muscles
4.4. Clinical Implications
4.5. Limitations
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Alfredson, H. Chronic midportion Achilles tendinopathy: An update on research and treatment. Clin. Sports Med. 2003, 22, 727–741. [Google Scholar] [CrossRef]
- Albers, I.S.; Zwerver, J.; Diercks, R.L.; Dekker, J.H.; Van den Akker-Scheek, I. Incidence and prevalence of lower extremity tendinopathy in a Dutch general practice population: A cross sectional study. BMC Musculoskelet. Disord. 2016, 17, 16. [Google Scholar] [CrossRef] [PubMed]
- Alfredson, H.; Lorentzon, R. Chronic Achilles tendinosis: Recommendations for treatment and prevention. Sports Med. 2000, 29, 135–146. [Google Scholar] [CrossRef] [PubMed]
- Cook, J.L.; Khan, K.M.; Purdam, C. Achilles tendinopathy. Man. Ther. 2002, 7, 121–130. [Google Scholar] [CrossRef]
- Li, H.-Y.; Hua, Y.-H. Achilles Tendinopathy: Current Concepts about the Basic Science and Clinical Treatments. Biomed Res. Int. 2016, 2016, 6492597. [Google Scholar] [CrossRef]
- Boesen, A.P.; Boesen, M.I.; Koenig, M.J.; Bliddal, H.; Torp-Pedersen, S.; Langberg, H. Evidence of accumulated stress in Achilles and anterior knee tendons in elite badminton players. Knee Surg. Sports Traumatol. Arthrosc. 2011, 19, 30–37. [Google Scholar] [CrossRef]
- Richardson, C.A.; Snijders, C.J.; Hides, J.A.; Damen, L.; Pas, M.S.; Storm, J. The relation between the transversus abdominis muscles, sacroiliac joint mechanics, and low back pain. Spine 2002, 27, 399–405. [Google Scholar] [CrossRef]
- Brown, S.H.M.; Ward, S.R.; Cook, M.S.; Lieber, R.L. Architectural analysis of human abdominal wall muscles: Implications for mechanical function. Spine 2011, 36, 355–362. [Google Scholar] [CrossRef]
- Whittaker, J.L.; Warner, M.B.; Stokes, M. Comparison of the Sonographic Features of the Abdominal Wall Muscles and Connective Tissues in Individuals With and Without Lumbopelvic Pain. J. Orthop. Sports Phys. Ther. 2013, 43, 11–19. [Google Scholar] [CrossRef]
- Hodges, P.W.; Moseley, G.L. Pain and motor control of the lumbopelvic region: Effect and possible mechanisms. J. Electromyogr. Kinesiol. 2003, 13, 361–370. [Google Scholar] [CrossRef]
- Radebold, A.; Cholewicki, J.; Panjabi, M.M.; Patel, T.C. Muscle response pattern to sudden trunk loading in healthy individuals and in patients with chronic low back pain. Spine 2000, 25, 947–954. [Google Scholar] [CrossRef] [PubMed]
- Hides, J.; Gilmore, C.; Stanton, W.; Bohlscheid, E. Multifidus size and symmetry among chronic LBP and healthy asymptomatic subjects. Man. Ther. 2008, 13, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Stokes, M.; Hides, J.; Elliott, J.; Kiesel, K.; Hodges, P. Rehabilitative ultrasound imaging of the posterior paraspinal muscles. J. Orthop. Sports Phys. Ther. 2007, 37, 581–595. [Google Scholar] [CrossRef] [PubMed]
- Chang, K.-V.; Yang, K.-C.; Wu, W.-T.; Huang, K.-C.; Han, D.-S. Association between metabolic syndrome and limb muscle quantity and quality in older adults: A pilot ultrasound study. Diabetes Metab. Syndr. Obes. Targets Ther. 2019, 12, 1821–1830. [Google Scholar] [CrossRef] [PubMed]
- Chang, K.-V.; Wu, W.-T.; Huang, K.-C.; Jan, W.H.; Han, D.-S. Limb muscle quality and quantity in elderly adults with dynapenia but not sarcopenia: An ultrasound imaging study. Exp. Gerontol. 2018, 108, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Romero-Morales, C.; Martin-Llantino, P.J.; Calvo-Lobo, C.; Sanchez-Gomez, R.; Lopez-Lopez, D.; Pareja-Galeano, H.; Rodriguez-Sanz, D. Ultrasound evaluation of extrinsic foot muscles in patients with chronic non-insertional Achilles tendinopathy: A case-control study. Phys. Ther. Sport 2019, 37, 44–48. [Google Scholar] [CrossRef] [PubMed]
- Romero-Morales, C.; Martin-Llantino, P.J.; Calvo-Lobo, C.; Almazan-Polo, J.; Lopez-Lopez, D.; de la Cruz-Torres, B.; Palomo-Lopez, P.; Rodriguez-Sanz, D. Intrinsic foot muscles morphological modifications in patients with Achilles tendinopathy: A novel case-control research study. Phys. Ther. Sport 2019, 40, 208–212. [Google Scholar] [CrossRef]
- Romero-Morales, C.; Martín-Llantino, P.J.; Calvo-Lobo, C.; López-López, D.; Sánchez-Gómez, R.; De-La-Cruz-Torres, B.; Rodríguez-Sanz, D. Ultrasonography Features of the Plantar Fascia Complex in Patients with Chronic Non-Insertional Achilles Tendinopathy: A Case-Control Study. Sensors 2019, 19, 2052. [Google Scholar] [CrossRef]
- Lobo, C.C.; Morales, C.R.; Sanz, D.R.; Corbalan, I.S.; Marin, A.G.; Lopez, D.L. Ultrasonography Comparison of Peroneus Muscle Cross-sectional Area in Subjects With or Without Lateral Ankle Sprains. J. Manip. Physiol. Ther. 2016, 39, 635–644. [Google Scholar] [CrossRef]
- Lobo, C.C.; Marin, A.G.; Sanz, D.R.; Lopez, D.L.; Lopez, P.P.; Morales, C.R.; Corbalan, I.S. Ultrasound evaluation of intrinsic plantar muscles and fascia in hallux valgus: A case-control study. Medicine 2016, 95, e5243. [Google Scholar] [CrossRef]
- Romero-Morales, C.; Almazán-Polo, J.; Rodríguez-Sanz, D.; Palomo-López, P.; López-López, D.; Vázquez-González, S.; Calvo-Lobo, C. Rehabilitative Ultrasound Imaging Features of the Abdominal Wall Muscles in Elite and Amateur Basketball Players. Appl. Sci. 2018, 8, 909. [Google Scholar] [CrossRef]
- Morales, C.R.; Polo, J.A.; Sanz, D.R.; Lopez, D.L.; Gonzalez, S.V.; Buria, J.L.A.; Lobo, C.C. Ultrasonography features of abdominal perimuscular connective tissue in elite and amateur basketball players: An observational study. Rev. Assoc. Med. Bras. 2018, 64, 936–941. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Zheng, Y.P.; Chen, X.; Huang, Q.H. Assessment of muscle fatigue using sonomyography: Muscle thickness change detected from ultrasound images. Med. Eng. Phys. 2007, 29, 472–479. [Google Scholar] [CrossRef]
- Chang, K.-V.; Kara, M.; Su, D.C.-J.; Gurcay, E.; Kaymak, B.; Wu, W.-T.; Ozcakar, L. Sonoanatomy of the spine: A comprehensive scanning protocol from cervical to sacral region. Med. Ultrason. 2019, 21, 474–482. [Google Scholar] [CrossRef]
- Hides, J.A.; Belavy, D.L.; Stanton, W.; Wilson, S.J.; Rittweger, J.; Felsenberg, D.; Richardson, C.A. Magnetic resonance imaging assessment of trunk muscles during prolonged bed rest. Spine 2007, 32, 1687–1692. [Google Scholar] [CrossRef] [PubMed]
- Hides, J.A.; Boughen, C.L.; Stanton, W.R.; Strudwick, M.W.; Wilson, S.J. A magnetic resonance imaging investigation of the transversus abdominis muscle during drawing-in of the abdominal wall in elite Australian Football League players with and without low back pain. J. Orthop. Sports Phys. Ther. 2010, 40, 4–10. [Google Scholar] [CrossRef][Green Version]
- Kiesel, K.B.; Uhl, T.L.; Underwood, F.B.; Rodd, D.W.; Nitz, A.J. Measurement of lumbar multifidus muscle contraction with rehabilitative ultrasound imaging. Man. Ther. 2007, 12, 161–166. [Google Scholar] [CrossRef]
- Kim, J.-S.; Kang, M.-H.; Jang, J.-H.; Oh, J.-S. Comparison of selective electromyographic activity of the superficial lumbar multifidus between prone trunk extension and four-point kneeling arm and leg lift exercises. J. Phys. Ther. Sci. 2015, 27, 1037–1039. [Google Scholar] [CrossRef]
- Teyhen, D.S.; Gill, N.W.; Whittaker, J.L.; Henry, S.M.; Hides, J.A.; Hodges, P. Rehabilitative ultrasound imaging of the abdominal muscles. J. Orthop. Sports Phys. Ther. 2007, 37, 450–466. [Google Scholar] [CrossRef]
- Romero-Morales, C.; Martin-Llantino, P.J.; Calvo-Lobo, C.; Palomo-Lopez, P.; Lopez-Lopez, D.; Pareja-Galeano, H.; Rodriguez-Sanz, D. Comparison of the sonographic features of the Achilles Tendon complex in patients with and without achilles tendinopathy: A case-control study. Phys. Ther. Sport 2019, 35, 122–126. [Google Scholar] [CrossRef]
- von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gotzsche, P.C.; Vandenbroucke, J.P. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: Guidelines for reporting observational studies. Int. J. Surg. 2014, 12, 1495–1499. [Google Scholar] [CrossRef] [PubMed]
- Holt, G.R. Declaration of Helsinki-the world’s document of conscience and responsibility. South. Med. J. 2014, 107, 407. [Google Scholar] [CrossRef] [PubMed]
- Faul, F.; Erdfelder, E.; Lang, A.-G.; Buchner, A. G * Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods 2007, 39, 175–191. [Google Scholar] [CrossRef] [PubMed]
- Habets, B.; van Cingel, R.E.H.; Backx, F.J.G.; Huisstede, B.M.A. Alfredson versus Silbernagel exercise therapy in chronic midportion Achilles tendinopathy: Study protocol for a randomized controlled trial. BMC Musculoskelet. Disord. 2017, 18, 296. [Google Scholar] [CrossRef]
- Alfredson, H.; Cook, J. A treatment algorithm for managing Achilles tendinopathy: New treatment options. Br. J. Sports Med. 2007, 41, 211–216. [Google Scholar] [CrossRef]
- Wallwork, T.L.; Hides, J.A.; Stanton, W.R. Intrarater and interrater reliability of assessment of lumbar multifidus muscle thickness using rehabilitative ultrasound imaging. J. Orthop. Sports Phys. Ther. 2007, 37, 608–612. [Google Scholar] [CrossRef]
- Huang, Q.; Li, D.; Zhang, Y.; Hu, A.; Huo, M.; Maruyama, H. The Reliability of Rehabilitative Ultrasound Imaging of the Cross-sectional Area of the Lumbar Multifidus Muscles in the PNF Pattern. J. Phys. Ther. Sci. 2014, 26, 1539–1541. [Google Scholar] [CrossRef]
- Park, S. doo Reliability of Ultrasound Imaging of the Transversus Deep Abdominial, Internal Oblique and External Oblique Muscles of Patients with Low Back Pain Performing the Drawing-in Maneuver. J. Phys. Ther. Sci. 2013, 25, 845–847. [Google Scholar] [CrossRef]
- Keshwani, N.; Hills, N.; McLean, L. Inter-Rectus Distance Measurement Using Ultrasound Imaging: Does the Rater Matter? Physiother. Can. 2016, 68, 223–229. [Google Scholar] [CrossRef]
- Sions, J.M.; Velasco, T.O.; Teyhen, D.S.; Hicks, G.E. Ultrasound imaging: Intraexaminer and interexaminer reliability for multifidus muscle thickness assessment in adults aged 60 to 85 years versus younger adults. J. Orthop. Sports Phys. Ther. 2014, 44, 425–434. [Google Scholar] [CrossRef]
- Nabavi, N.; Mosallanezhad, Z.; Haghighatkhah, H.R.; Mohseni Bandpeid, M.A. Reliability of rehabilitative ultrasonography to measure transverse abdominis and multifidus muscle dimensions. Iran. J. Radiol. 2014, 11, e21008. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671. [Google Scholar] [CrossRef]
- Ghasemi, A.; Zahediasl, S. Normality tests for statistical analysis: A guide for non-statisticians. Int. J. Endocrinol. Metab. 2012, 10, 486–489. [Google Scholar] [CrossRef]
- Jansen, J.; Weir, A.; Denis, R.; Mens, J.; Backx, F.; Stam, H. Resting thickness of transversus abdominis is decreased in athletes with longstanding adduction-related groin pain. Man. Ther. 2010, 15, 200–205. [Google Scholar] [CrossRef]
- Stuge, B.; Morkved, S.; Dahl, H.H.; Vollestad, N. Abdominal and pelvic floor muscle function in women with and without long lasting pelvic girdle pain. Man. Ther. 2006, 11, 287–296. [Google Scholar] [CrossRef]
- Coldron, Y.; Stokes, M.J.; Newham, D.J.; Cook, K. Postpartum characteristics of rectus abdominis on ultrasound imaging. Man. Ther. 2008, 13, 112–121. [Google Scholar] [CrossRef]
- Rankin, G.; Stokes, M.; Newham, D.J. Abdominal muscle size and symmetry in normal subjects. Muscle Nerve 2006, 34, 320–326. [Google Scholar] [CrossRef]
- Mota, P.; Pascoal, A.G.; Sancho, F.; Carita, A.I.; Bo, K. Reliability of the inter-rectus distance measured by palpation. Comparison of palpation and ultrasound measurements. Man. Ther. 2013, 18, 294–298. [Google Scholar] [CrossRef]
- Rostami, M.; Ansari, M.; Noormohammadpour, P.; Mansournia, M.A.; Kordi, R. Ultrasound assessment of trunk muscles and back flexibility, strength and endurance in off-road cyclists with and without low back pain. J. Back Musculoskelet. Rehabil. 2015, 28, 635–644. [Google Scholar] [CrossRef]
- Sutherlin, M.A.; Gage, M.; Mangum, L.C.; Hertel, J.; Russell, S.; Saliba, S.A.; Hart, J.M. Changes in Muscle Thickness Across Positions on Ultrasound Imaging in Participants With or Without a History of Low Back Pain. J. Athl. Train. 2018, 53, 553–559. [Google Scholar] [CrossRef]
- Whittaker, J.L. Ultrasound imaging of the lateral abdominal wall muscles in individuals with lumbopelvic pain and signs of concurrent hypocapnia. Man. Ther. 2008, 13, 404–410. [Google Scholar] [CrossRef] [PubMed]
- Hides, J.A.; Saide, M.; Stokes, M.J.; Jull, G.A.; Cooper, D.H. Evidence of lumbar multifidus muscle wasting ipsilateral to symptoms in patients with acute/subacute low back pain. Spine 1994, 19, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Kliziene, I.; Sipaviciene, S.; Klizas, S.; Imbrasiene, D. Effects of core stability exercises on multifidus muscles in healthy women and women with chronic low-back pain. J. Back Musculoskelet. Rehabil. 2015, 28, 841–847. [Google Scholar] [CrossRef] [PubMed]
- Garrow, J.S.; Webster, J. Quetelet’s index (W/H2) as a measure of fatness. Int. J. Obes. 1985, 9, 147–153. [Google Scholar] [PubMed]

| Data | Tendinopathy (n = 71) | Controls (n = 70) | p-Value Cases vs. Controls |
|---|---|---|---|
| Age, y | 45.11 ± 12.75 * | 37.61 ± 11.91 * | 0.200 ** |
| Weight, kg | 76.00 ± 12.00 † | 75.00 ± 18.50 † | 0.412 ‡ |
| Height, m | 1.76 ± 0.11 † | 1.76 ± 0.12 † | 0.566 ‡ |
| BMI, kg/m2 | 24.81 ± 2.13 † | 23.88 ± 3.67 † | 0.012 ‡ |
| VAS | 2.00 ± 3.00 † | N/A | N/A |
| VISA-A | 56.00 ± 14.00 † | N/A | N/A |
| Measurement | Tendinopathy (n = 71) | Health (n = 72) | p-Value |
|---|---|---|---|
| IRD (mm) | 17.21 ± 8.37 (10.29–22.63) † | 9.83 ± 7.75 (2.01–22.05) † | 0.001 ‡ |
| RA Thickness (mm2) | 10.19 ± 2.31 (5.21–16.49) * | 11.33 ± 2.15 (6.22–21.55) † | 0.001 ‡ |
| EO Thickness (mm) | 3.99 ± 2.30 (2.10–8.71) † | 5.56 ± 1.55 (2.63–9.00) * | 0.001 ‡ |
| IO Thickness (mm) | 8.36 ± 3.47 (2.23–15.57) † | 11.08 ± 5.04 (4.65–19.50) † | 0.001 ‡ |
| TrAb Thickness (mm) | 3.74 ± 2.43 (13.38–26.89) † | 4.70 ± 1.98 (2.35–9.70) † | 0.041 ‡ |
| Multifidus Thickness (mm) | 24.07 ± 5.67 (18.94–31.63) † | 12.17 ± 8.48 (8.08–25.13) † | 0.001 ‡ |
| Multifidus CSA (mm2) | 1068.58 ± 175.48 (773.79–1610.19) † | 960.72 ± 437.52 (665.19–1641.28) | 0.001 ‡ |
| Parameter | Model | P Value | Model R2 |
|---|---|---|---|
| IRD (mm) | −207.961 | 0.494 | |
| −1.686 * Weight 112.29 * Height 6.019 * BMI 5.506 * Group | 0.006 0.028 0.001 0.001 | ||
| RA Thickness (mm) | 65.252 | 0.303 | |
| −1.740 * Age | 0.001 | ||
| EO Thickness (mm) | 19.754 | 0.387 | |
| −0.025 * Age 1.312 * Sex −1.132 * Group | 0.013 0.006 0.001 | ||
| IO Thickness (mm) | −29.138 | 0.380 | |
| 4.129 * Sex −2.892 * Group | 0.001 0.001 | ||
| TrAb (mm) | −15.813 | 0.260 | |
| −1.061 * Group | 0.001 | ||
| Multifidus CSA (mm2) | −882.850 | 0.341 | |
| −2.729 * Age | 0.048 | ||
| Multifidus Thickness (mm) | −103.879 | 0.643 | |
| −3.653 * Sex 10.116 * Group | 0.013 0.001 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Romero-Morales, C.; Martín-Llantino, P.; Calvo-Lobo, C.; San-Antolín, M.; López-López, D.; Blanco-Morales, M.; Rodríguez-Sanz, D. Ultrasound Imaging of the Abdominal Wall and Trunk Muscles in Patients with Achilles Tendinopathy versus Healthy Participants. Diagnostics 2020, 10, 17. https://doi.org/10.3390/diagnostics10010017
Romero-Morales C, Martín-Llantino P, Calvo-Lobo C, San-Antolín M, López-López D, Blanco-Morales M, Rodríguez-Sanz D. Ultrasound Imaging of the Abdominal Wall and Trunk Muscles in Patients with Achilles Tendinopathy versus Healthy Participants. Diagnostics. 2020; 10(1):17. https://doi.org/10.3390/diagnostics10010017
Chicago/Turabian StyleRomero-Morales, Carlos, Pedro Martín-Llantino, César Calvo-Lobo, Marta San-Antolín, Daniel López-López, María Blanco-Morales, and David Rodríguez-Sanz. 2020. "Ultrasound Imaging of the Abdominal Wall and Trunk Muscles in Patients with Achilles Tendinopathy versus Healthy Participants" Diagnostics 10, no. 1: 17. https://doi.org/10.3390/diagnostics10010017
APA StyleRomero-Morales, C., Martín-Llantino, P., Calvo-Lobo, C., San-Antolín, M., López-López, D., Blanco-Morales, M., & Rodríguez-Sanz, D. (2020). Ultrasound Imaging of the Abdominal Wall and Trunk Muscles in Patients with Achilles Tendinopathy versus Healthy Participants. Diagnostics, 10(1), 17. https://doi.org/10.3390/diagnostics10010017









