The VNTR 48 bp Polymorphism in the DRD4 Gene Is Associated with Higher Tobacco Smoking in Male Mexican Mestizo Smokers with and without COPD
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Sample Size
2.3. DNA Extraction
2.4. Genotyping
2.5. Statistical Analysis
3. Results
3.1. Clinical and Demographical Variables
3.2. Allele Association and Genotype Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| DRD4 | Dopamine receptor D4 gene |
| VNTR | Variable number tandem repeat |
| L | Long allele |
| COPD | Chronic obstructive pulmonary disease |
| bp | Base pairs |
| cpd | Cigarettes per day |
| HS | Heavy smoker, non-COPD |
| LS | Light smoker, non-COPD |
| S | Short allele |
| cAMP | Cyclic adenosine monophosphate |
| SS | Short allele homozygous |
| FEV1 | Forced expiratory volume in the first second |
| FVC | Forced vital capacity |
| LL | Large homozygous genotype |
| SL | Short–large heterozygous genotype |
| AF | Allele frequencies |
| GF | Genotype frequencies |
| OR | Odds ratio |
| CI 95% | Confidence interval at 95% |
| SNP | Single nucleotide polymorphism |
| INER | Instituto Nacional de Enfermedades Respiratorias Ismael Cosío Villegas |
| EDAS | Electrophoresis Documentation and Analysis System |
| HWE | Hardy–Weinberg equilibrium |
References
- Instituto Nacional de Psiquiatría Ramón de la Fuente Muñiz; Instituto Nacional de Salud Pública, Comisión Nacional Contra las Adicciones, Secretaría de Salud. Encuesta Nacional de Consumo de Drogas, Alcohol y Tabaco 2016–2017: Reporte de Tabaco; Reynales-Shigematsu, L.M., Zavala Arciniega, L., Paz-Ballesteros, W.C., Gutiérrez-Torres, D.S., García-Buendía, J.C., Rodriguez-Andrade, M.A., Gutiérrez-Reyes, J., Franco-Núñez, A., Romero-Martínez, M., y Mendoza-Alvarado, L., Eds.; INPRFM: Ciudad de México, Mexico, 2017.
- Perez-Rubio, G.; Cordoba-Lanus, E.; Cupertino, P.; Cartujano-Barrera, F.; Campos, A.; Falfan-Valencia, R. Role of genetic susceptibility in nicotine addiction and chronic obstructive pulmonary disease. Rev. Investig. Clin. 2019, 71, 36–54. [Google Scholar] [CrossRef] [PubMed]
- Salamone, J.D.; Correa, M.; Mingote, S.; Weber, S.M. Nucleus accumbens dopamine and the regulation of effort in food-seeking behavior: Implications for studies of natural motivation, psychiatry, and drug abuse. J. Pharmacol. Exp. Ther. 2003, 305, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Van Tol, H.H.; Bunzow, J.R.; Guan, H.C.; Sunahara, R.K.; Seeman, P.; Niznik, H.B.; Civelli, O. Cloning of a human dopamine D4 receptor gene with high affinity for the antipsychotic clozapine. Nature 1991, 350, 610–614. [Google Scholar] [CrossRef] [PubMed]
- Munafó, M.; Clark, T.; Moore, L.; Payne, E.; Walton, R.; Flint, J. Genetic polymorphisms and personality in healthy adults: A systematic review and metaanalysis. Mol. Psychiatry 2003, 8, 471–484. [Google Scholar] [CrossRef] [PubMed]
- Asghari, V.; Sanyal, S.; Buchwaldt, S.; Peterson, A.; Jovanovic, V. Modulation of intracellular cyclic AMP levels by different human dopamine D4 receptor variants. J. Neurochem. 1995, 65, 1157–1165. [Google Scholar] [CrossRef]
- Bergen, A.W.; Javitz, H.S.; Su, L.; He, Y.; Conti, D.V.; Benowitz, N.L.; Tyndale, R.F.; Lerman, C.; Swan, G.E. The DRD4 exon III VNTR, bupropion, and associations with prospective abstinence. Nicotine Tob. Res. 2013, 15, 1190–1200. [Google Scholar] [CrossRef]
- David, S.P.; Munafò, M.R.; Johansen-Berg, H.; MacKillop, J.; Sweet, L.H.; Cohen, R.A.; Niaura, R.; Rogers, R.D.; Matthews, P.M.; Walton, R.T. Effects of Acute Nicotine Abstinence on Cue-elicited Ventral Striatum/Nucleus Accumbens Activation in Female Cigarette Smokers: A Functional Magnetic Resonance Imaging Study. Brain Imaging Behav. 2007, 1, 43–57. [Google Scholar] [CrossRef]
- Pérez-Padilla, J.; Regalado-Pineda, J.; Vázquez-García, J. Reproducibility of spirometry in Mexican workers and international reference values. Salud Publica Mex. 2001, 43, 113–121. [Google Scholar] [CrossRef]
- Camarena, B.; Loyzaga, C.; Aguilar, A.; Weissbecker, K.; Nicolini, H. Association study between the dopamine receptor D 4 gene and obsessive-compulsive disorder. Eur. Neuropsychopharmacol. 2007, 17, 406–409. [Google Scholar] [CrossRef]
- Dean, A.G.; Arner, T.G.; Sunki, G.G.; Friedman, R.; Lantinga, M.; Sangam, S.; Zubieta, J.C.; Sullivan, K.M.; Brendel, K.A.; Gao, Z.; et al. Epi Info™, a Database and Statistics Program for Public Health Professionals; CDC: Atlanta, GA, USA, 2011. [Google Scholar]
- Ye, J.; Coulouris, G.; Zaretskaya, I.; Cutcutache, I.; Rozen, S.; Madden, T.L. Primer-BLAST: A tool to design target-specific primers for polymerase chain reaction. BMC Bioinform. 2012, 13, 134. [Google Scholar] [CrossRef]
- Mukaka, M.M. Statistics corner: A guide to appropriate use of correlation coefficient in medical research. Malawi Med. J. 2012, 24, 69–71. [Google Scholar] [PubMed]
- Wickham, H. Ggplot2:Elegant Graphics for Data Analysis; Springer: Berlin/Heidelberg, Germany, 2016. [Google Scholar]
- RStudio Team. RStudio: Integrated Development for, R. RStudio; RStudio, Inc.: Boston, MA, USA, 2015. [Google Scholar]
- Epidat: Programa para análisis epidemiológico de datos tabulados, versión 3.1; Dirección Xeral de Saúde Pública, Consellería de Sanidade-Xunta de Galicia: Santiago, Spain; Área de Análisis de Salud y Sistemas de Información Sanitaria, Organización Panamericana de la Salud (OPS/OMS): Washington, DC, USA, January 2006.
- Purcell, S.; Neale, B.; Todd-Brown, K.; Thomas, L.; Ferreira, M.A.; Bender, D.; Maller, J.; Sklar, P.; De Bakker, P.I.; Daly, M.J.; et al. PLINK: A Tool Set for Whole-Genome Association and Population-Based Linkage Analyses. Am. J. Hum. Genet. 2007, 81, 559–575. [Google Scholar] [CrossRef] [PubMed]
- Hardy-Weinberg Equilibrium Learn Science at Scitable. Available online: https://www.nature.com/scitable/definition/hardy-weinberg-equilibrium-122/ (accessed on 23 December 2019).
- Harrell, P.T.; Lin, H.-Y.; Park, J.Y.; Blank, M.D.; Drobes, D.J. Dopaminergic Genetic Variation Moderates the Effect of Nicotine on Cigarette Reward. Psychopharmacology 2016, 233, 351–360. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hutchison, K.; LaChance, H.; Niaura, R.; Bryan, A.; Smolen, A. The DRD4 VNTR polymorphism influences reactivity to smoking cues. J. Abnorm. Psychol. 2002, 111, 134–143. [Google Scholar] [CrossRef]
- Ellis, J.A.; Olsson, C.A.; Moore, E.; Greenwood, P.; van de Ven, M.O.M.; Patton, G.C. A role for the DRD4 exon III VNTR in modifying the association between nicotine dependence and neuroticism. Nicotine Tob. Res. 2011, 13, 64–69. [Google Scholar] [CrossRef]
- Eisenberg, D.T.A.; Apicella, C.L.; Campbell, B.C.; Dreber, A.; Garcia, J.R.; Lum, J.K. Assortative human pair-bonding for partner ancestry and allelic variation of the dopamine receptor D4 (DRD4) gene. Soc. Cognit. Affect. Neurosci. 2009, 5, 194–202. [Google Scholar] [CrossRef]
- Minisini, R.; Boccato, E.; Favretto, S.; Alaimo, E.; Smirne, C.; Burlone, M.E.; Bocchetta, S.; Vandelli, C.; Colletta, C.; Colletta, A.; et al. Fibrosis Progression in HCV Carriers with Mild Hepatitis Who Possess the High-Repetition Variant of the DRD4 Gene, a Genetic Marker for Binge-Drinking and Risk-Seeking Behavior: A Longitudinal Study. Alcohol. Clin. Exp. Res. 2013, 37, 891–895. [Google Scholar] [CrossRef]
- Haberstick, B.C.; Smolen, A.; Stetler, G.L.; Tabor, J.W.; Roy, T.; Casey, H.R.; Pardo, A.; Roy, F.; Ryals, L.A.; Hewitt, C.; et al. Simple sequence repeats in the national longitudinal study of adolescent health: An ethnically diverse resource for genetic analysis of health and behavior. Behav. Genet. 2014, 44, 487–497. [Google Scholar] [CrossRef][Green Version]
- Szekely, A.; Kotyuk, E.; Bircher, J.; Vereczkei, A.; Balota, D.A.; Sasvari-Szekely, M.; Ronai, Z. Association between age and the 7 repeat allele of the dopamine D4 receptor gene. PLoS ONE 2016, 11, e0167753. [Google Scholar] [CrossRef]
- Oldenhof, J.; Vickery, R.; Anafi, M.; Oak, J.; Ray, A.; Schoots, O.; Pawson, T.; von Zastrow, M.; Van Tol, H.H. SH3-binding domains in the dopamine D4 receptor. Biochemistry 1998, 37, 15726–15736. [Google Scholar] [CrossRef][Green Version]
- Hamarman, S.; Fossella, J.; Ulger, C.; Brimacombe, M.; Dermody, J. Dopamine receptor 4 (DRD4) 7-repeat allele predicts methylphenidate dose response in children with attention deficit hyperactivity disorder: A pharmacogenetic study. J. Child Adolesc. Psychopharmacol. 2004, 14, 564–574. [Google Scholar] [CrossRef] [PubMed]
- Brody, A.L.; Mandelkern, M.A.; Olmstead, R.E.; Scheibal, D.; Hahn, E.; Shiraga, S.; Zamora-Paja, E.; Farahi, J.; Saxena, S.; London, E.D.; et al. Gene variants of brain dopamine pathways and smoking-induced dopamine release in the ventral caudate/nucleus accumbens. Arch. Gen. Psychiatry 2006, 63, 808–816. [Google Scholar] [CrossRef] [PubMed]
- Brown, A.K.; Mandelkern, M.A.; Farahi, J.; Robertson, C.; Ghahremani, D.G.; Sumerel, B.; Moallem, N.; London, E.D. Sex Differences in Striatal Dopamine D2/D3 Receptor Availability in Smokers and Nonsmokers. Int. J. Neuropsychopharmacol. 2012, 15, 989–994. [Google Scholar] [CrossRef] [PubMed]
- Cosgrove, K.P.; Wang, S.; Kim, S.J.; McGovern, E.; Nabulsi, N.; Gao, H.; Labaree, D.; Tagare, H.D.; Sullivan, J.M.; Morris, E.D. Sex Differences in the Brain’s Dopamine Signature of Cigarette Smoking. J. Neurosci. 2014, 34, 16851–16855. [Google Scholar] [CrossRef]
- David, S.; Munafó, M.; Murphy, M.; Proctor, M.; Walton, R.; Johnstone, E. Genetic variation in the dopamine D4 receptor (DRD4) gene and smoking cessation: Follow-up of a randomised clinical trial of transdermal nicotine patch. Pharm. J. 2008, 8, 122–128. [Google Scholar] [CrossRef][Green Version]
- Leventhal, A.M.; David, S.P.; Brightman, M.; Strong, D.; McGeary, J.E.; Brown, R.A.; Lloyd-Richardson, E.E.; Munafò, M.; Uhl, G.R.; Niaura, R. Dopamine D4 Receptor Gene Variation Moderates the Efficacy of Bupropion for Smoking Cessation. Pharm. J. 2012, 12, 86–92. [Google Scholar] [CrossRef]
- Babic, M.; Nedic, G.; Muck-Seler, D.; Borovecki, F.; Pivac, N. Lack of association between dopamine receptor D4 variable numbers of tandem repeats gene polymorphism and smoking. Neurosci. Lett. 2012, 520, 67–70. [Google Scholar] [CrossRef][Green Version]
- Vereczkei, A.; Demetrovics, Z.; Szekely, A.; Sarkozy, P.; Antal, P.; Szilagyi, A.; Sasvari-Szekely, M.; Barta, C. Multivariate Analysis of Dopaminergic Gene Variants as Risk Factors of Heroin Dependence. PLoS ONE 2013, 8, e66592. [Google Scholar] [CrossRef]
- Pérez-Rubio, G.; Ramírez-Venegas, A.; Díaz, V.N.; Gómez, L.G.; Fabián, K.E.; Carmona, S.G.; López-Flores, L.A.; Ambrocio-Ortiz, E.; Romero, R.C.; Alcantar-Ayala, N.; et al. Polymorphisms in HTR2A and DRD4 Predispose to Smoking and Smoking Quantity. PLoS ONE 2017, 12, e0170019. [Google Scholar] [CrossRef]
- David, S.P.; Strong, D.R.; Leventhal, A.M.; Lancaster, M.A.; McGeary, J.E.; Munafò, M.R.; Bergen, A.W.; Swan, G.E.; Benowitz, N.L.; Tyndale, R.F.; et al. Influence of a dopamine pathway additive genetic efficacy score on smoking cessation: Results from two randomized clinical trials of bupropion. Addiction 2013, 108, 2202–2211. [Google Scholar] [CrossRef]
- Juárez-Cedillo, T.; Zuñiga, J.; Acuña-Alonzo, V.; Pérez-Hernández, N.; Rodríguez-Pérez, J.M.; Barquera, R.; Gallardo, G.J.; Sánchez-Arenas, R.; del Carmen García-Peña, M.; Granados, J.; et al. Genetic admixture and diversity estimations in the Mexican Mestizo population from Mexico City using 15 STR polymorphic markers. Forensic Sci. Int. Genet. 2008, 2, e37–e39. [Google Scholar] [CrossRef] [PubMed]
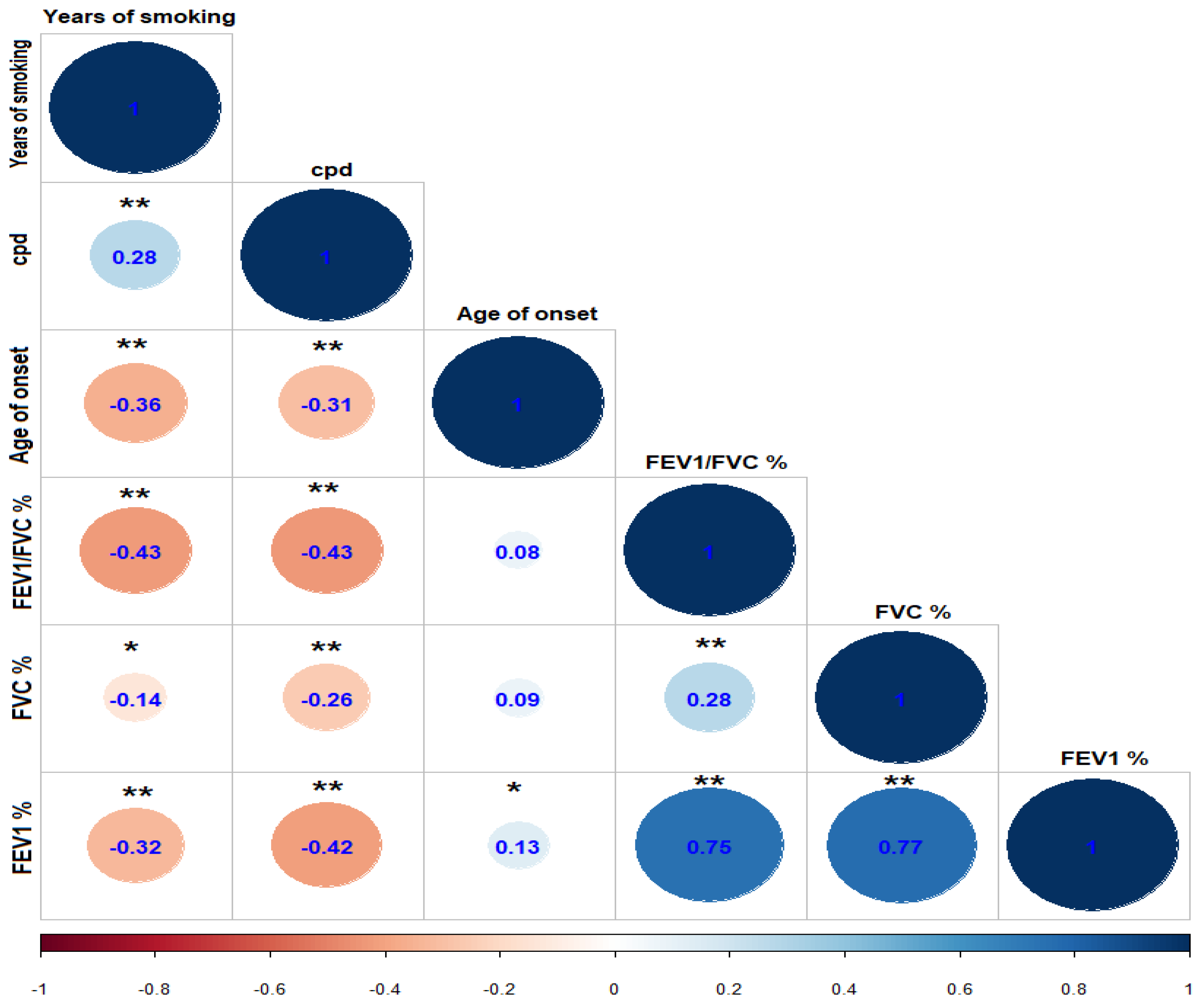
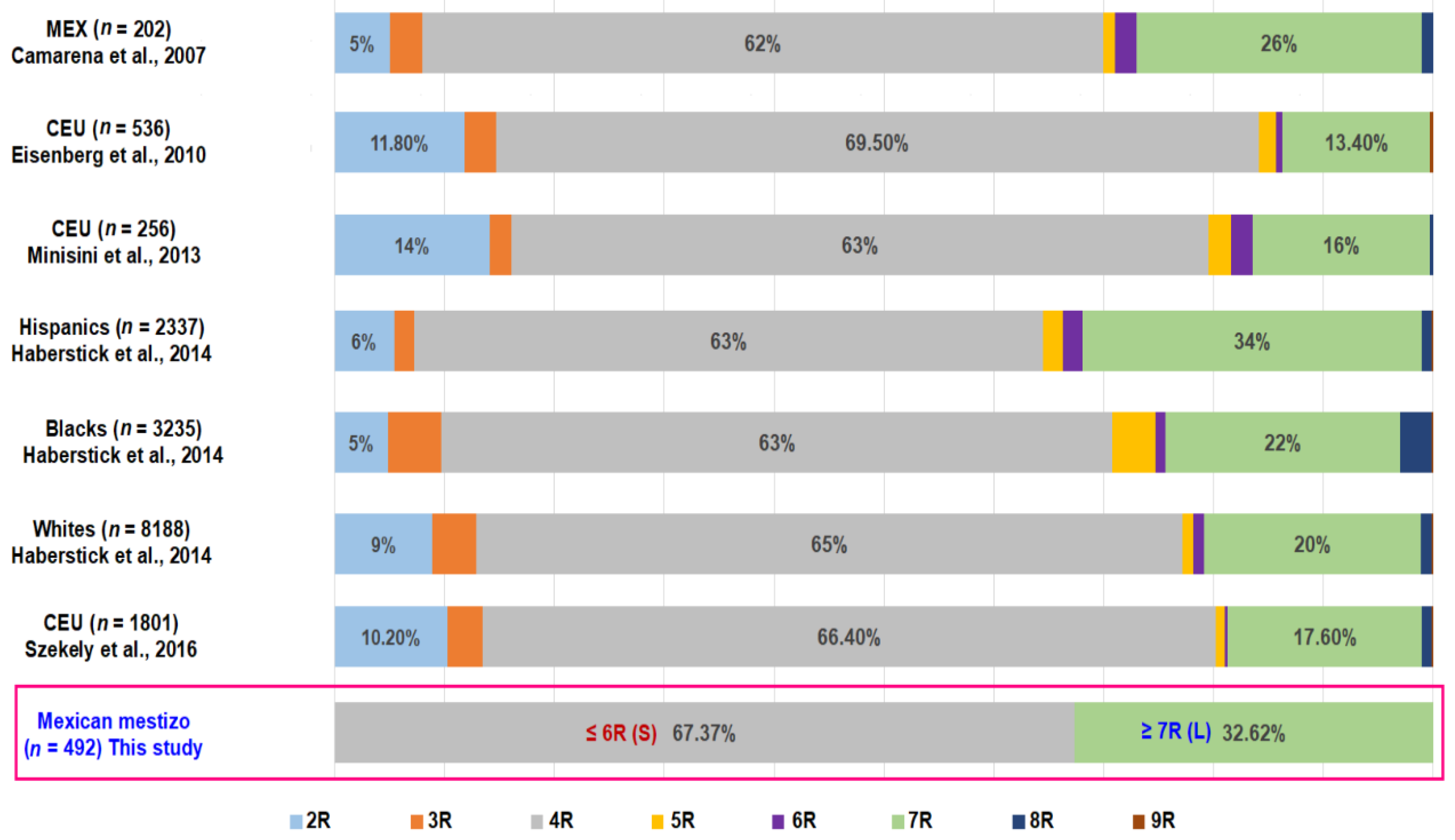
| Variable | COPD n = 164 | HS n = 164 | LS n = 164 | p-Value * |
|---|---|---|---|---|
| Age (years) | 59 (45–73) | 53 (30–75) | 50 (35–75) | <0.001 |
| Weight (kg) | 69 (46–91) | 70 (45–118) | 70 (50–109) | 0.003 |
| Height (m) | 1.63 (1.40–1.88) | 1.65 (1.43–1.86) | 1.60 (1.40–1.88) | 0.002 |
| BMI | 25.7 (16.3–41.3) | 26.9 (15.9–46.6) | 27.1 (17.2–53.6) | 0.001 |
| Years of smoking | 43 (28–60) | 36 (5–62) | 29 (3–52) | <0.001 |
| cpd | 20 (20–80) | 20 (20–40) | 8 (1–10) | <0.001 |
| TI | 43 (28–239) | 36 (5–123) | 12 (0.1–26) | <0.001 |
| Age of smoking onset | 17 (7–30) | 15 (9–35) | 18 (12–45) | <0.001 |
| Fagerström test | 7 (0–9) | 6 (1–10) | 3 (0–8) | <0.001 |
| FVC (%) | 82 (55–135) | 93 (54–133) | 100 (71–128) | <0.001 |
| FEV1 (%) | 68 (31–112) | 92 (50–128) | 101 (67–131) | <0.001 |
| FEV1/FVC (%) | 60 (44–81) | 80 (58–101) | 83.7 (64–97) | <0.001 |
| DRD4 | COPD (n = 164) | HS (n = 164) | LS (n = 164) | p-Value | |||||
|---|---|---|---|---|---|---|---|---|---|
| n | AF/GF% | n | AF/GF% | n | AF/GF% | COPD vs. HS | COPD vs. LS | HS vs. LS | |
| S | 213 | 64.93 | 222 | 67.68 | 228 | 69.51 | 0.508 | 0.244 | 0.674 |
| L | 115 | 35.06 | 106 | 32.31 | 100 | 30.48 | |||
| SS | 71 | 43.30 | 75 | 45.73 | 84 | 51.21 | |||
| SL | 71 | 43.30 | 72 | 43.90 | 60 | 36.58 | 0.463 | 0.233 | 0.625 |
| LL | 22 | 13.40 | 17 | 10.36 | 20 | 12.19 | |||
| Men | COPD (n = 130) | LS (n = 80) | p-Value | OR (CI 95%) | ||
|---|---|---|---|---|---|---|
| n | F% | n | F% | |||
| SS | 53 | 40.7 | 47 | 58.8 | 0.0168 | |
| SL + LL | 77 | 59.2 | 33 | 41.2 | 2.06 (1.17–3.64) | |
| HS (n = 93) | LS (n = 80) | |||||
| n | F% | n | F% | |||
| SS | 40 | 43.0 | 47 | 58.8 | 0.0559 | |
| SL + LL | 53 | 57.0 | 33 | 41.2 | 1.88 (1.03–3.45) | |
| COPD + HS (n = 223) | LS (n = 80) | |||||
| n | F% | n | F% | |||
| SS | 93 | 41.7 | 47 | 58.8 | 0.0127 | |
| SL + LL | 130 | 58.3 | 33 | 41.2 | 1.99 (1.18–3.34) | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pérez-Rubio, G.; García-Carmona, S.; García-Gómez, L.; Hernández-Pérez, A.; Ramírez-Venegas, A.; López-Flores, L.A.; Sansores, R.; Falfán-Valencia, R. The VNTR 48 bp Polymorphism in the DRD4 Gene Is Associated with Higher Tobacco Smoking in Male Mexican Mestizo Smokers with and without COPD. Diagnostics 2020, 10, 16. https://doi.org/10.3390/diagnostics10010016
Pérez-Rubio G, García-Carmona S, García-Gómez L, Hernández-Pérez A, Ramírez-Venegas A, López-Flores LA, Sansores R, Falfán-Valencia R. The VNTR 48 bp Polymorphism in the DRD4 Gene Is Associated with Higher Tobacco Smoking in Male Mexican Mestizo Smokers with and without COPD. Diagnostics. 2020; 10(1):16. https://doi.org/10.3390/diagnostics10010016
Chicago/Turabian StylePérez-Rubio, Gloria, Salvador García-Carmona, Leonor García-Gómez, Andrea Hernández-Pérez, Alejandra Ramírez-Venegas, Luis Alberto López-Flores, Raúl Sansores, and Ramcés Falfán-Valencia. 2020. "The VNTR 48 bp Polymorphism in the DRD4 Gene Is Associated with Higher Tobacco Smoking in Male Mexican Mestizo Smokers with and without COPD" Diagnostics 10, no. 1: 16. https://doi.org/10.3390/diagnostics10010016
APA StylePérez-Rubio, G., García-Carmona, S., García-Gómez, L., Hernández-Pérez, A., Ramírez-Venegas, A., López-Flores, L. A., Sansores, R., & Falfán-Valencia, R. (2020). The VNTR 48 bp Polymorphism in the DRD4 Gene Is Associated with Higher Tobacco Smoking in Male Mexican Mestizo Smokers with and without COPD. Diagnostics, 10(1), 16. https://doi.org/10.3390/diagnostics10010016







