Down-Grading of Ipsilateral Hydronephrosis by Neoadjuvant Chemotherapy Correlates with Favorable Oncological Outcomes in Patients Undergoing Radical Nephroureterectomy for Ureteral Carcinoma
Abstract
:1. Introduction
2. Materials and Methods
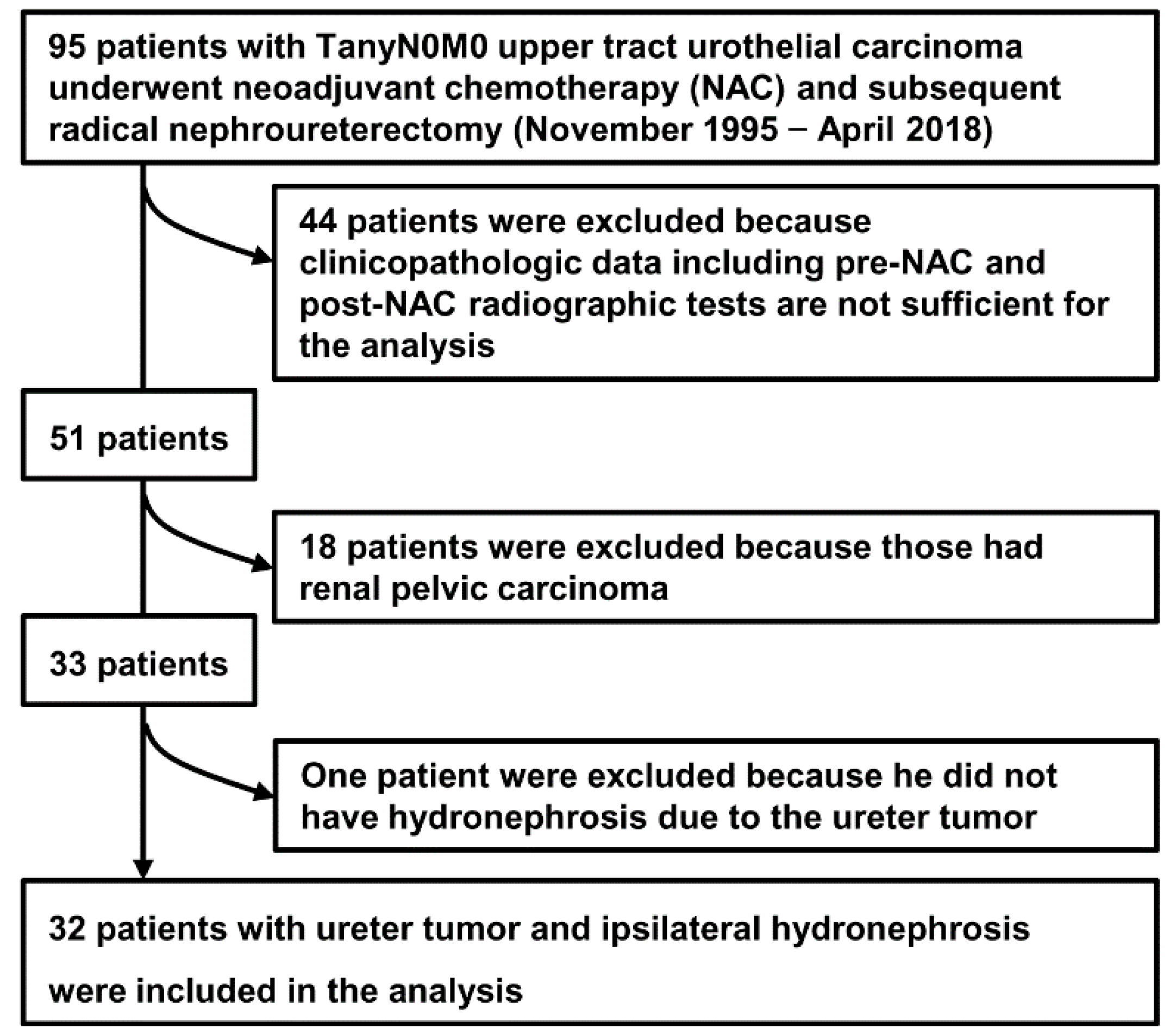
2.1. Study Cohort and Data Collection
2.2. Preoperative Systemic Chemotherapy
2.3. Image Interpretation for Ureteral Tumor and Hydronephrosis
2.4. Statistical Analysis
3. Results
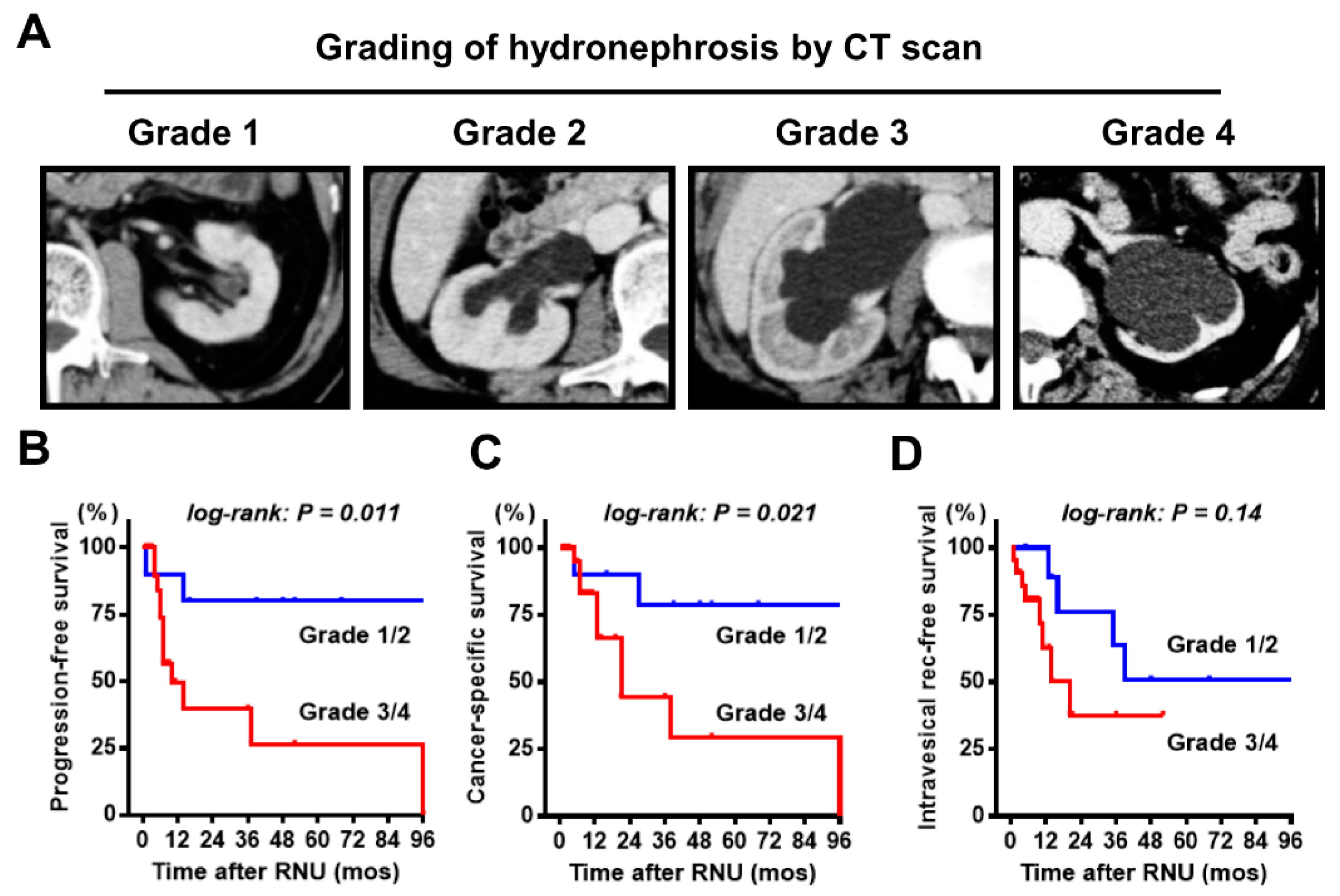
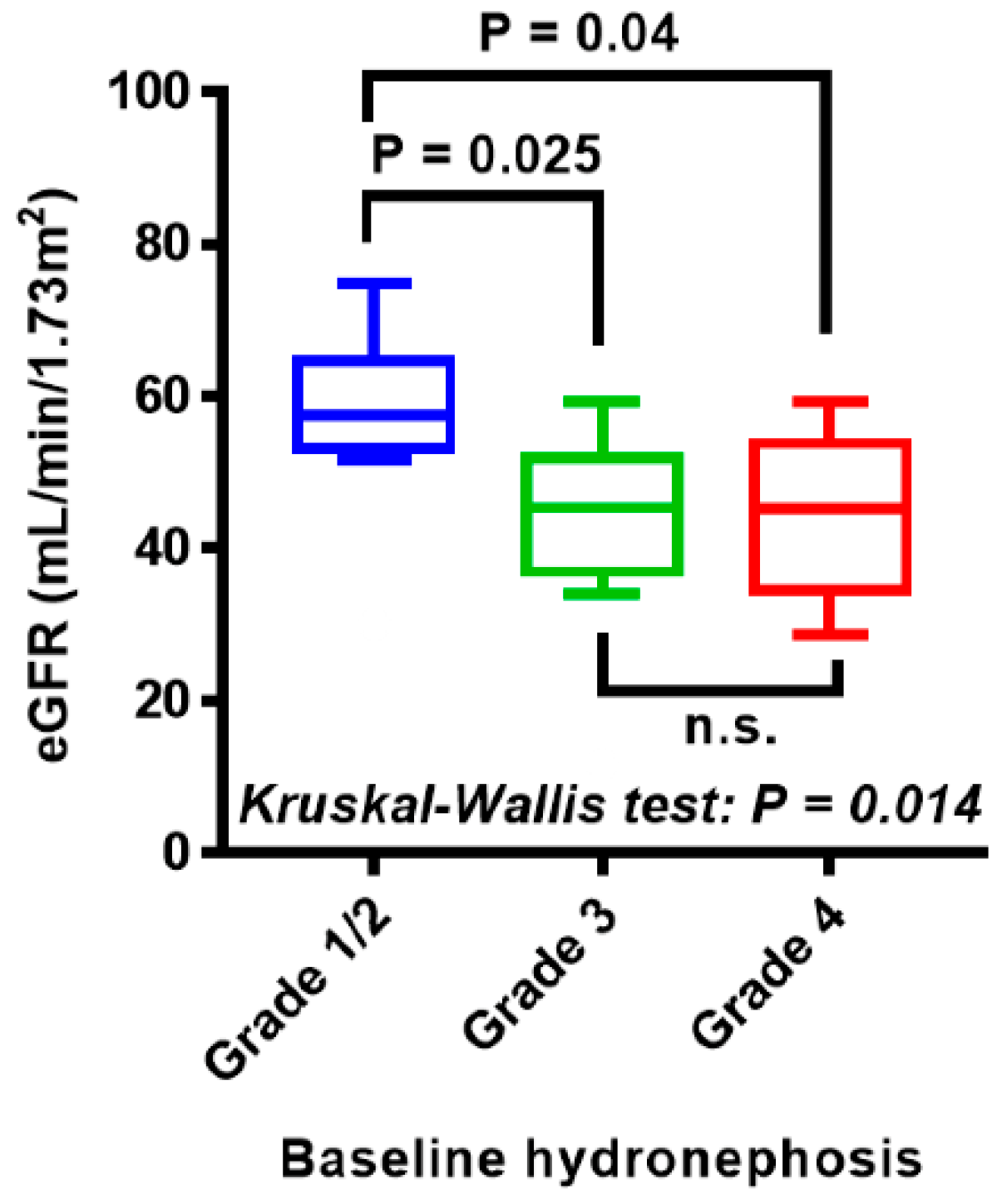
3.1. Baseline Characteristics Including Ipsilateral Hydronephrosis Grade
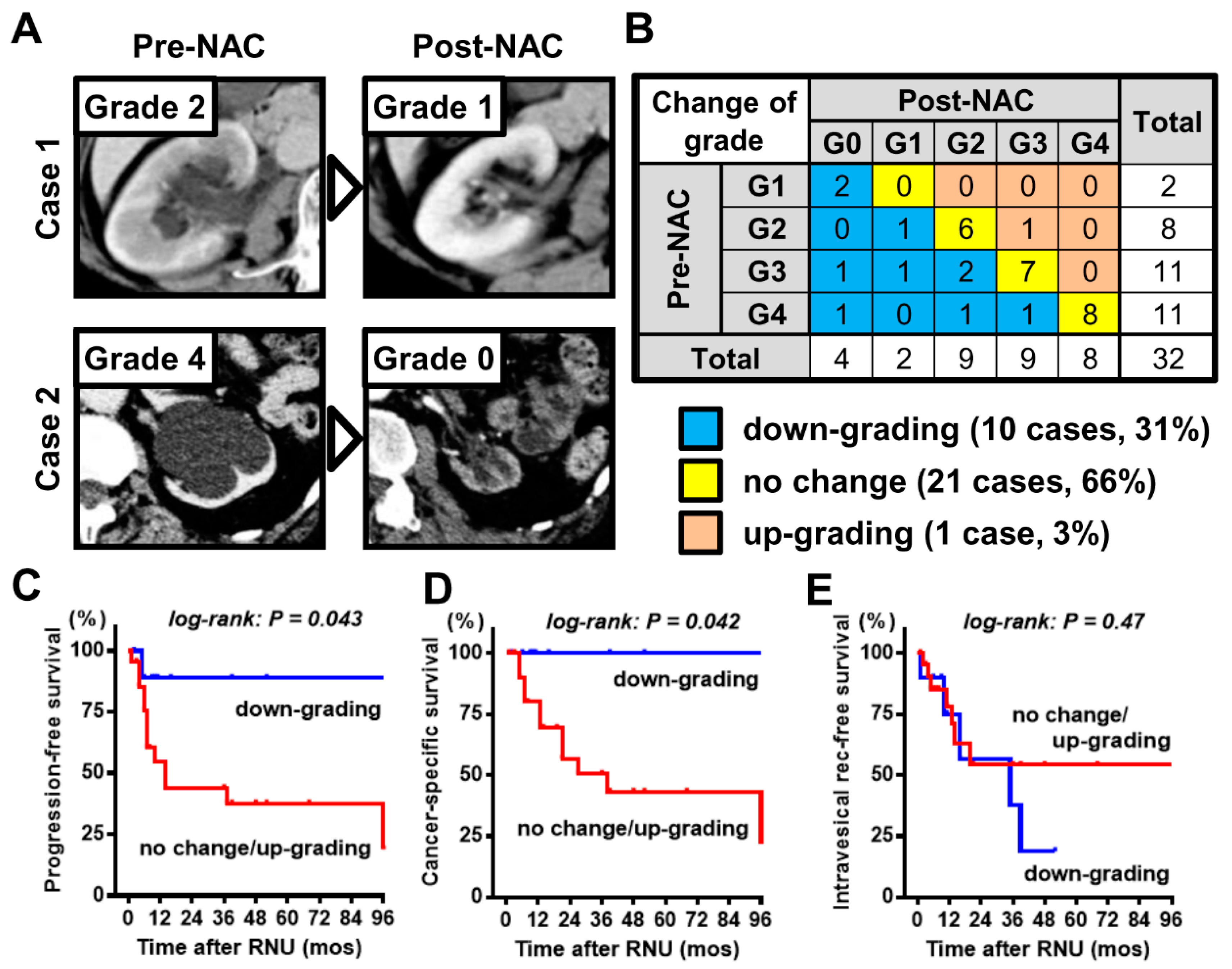
3.2. Clinical Impact of Change in Hydronephrosis Grade Induced by NAC
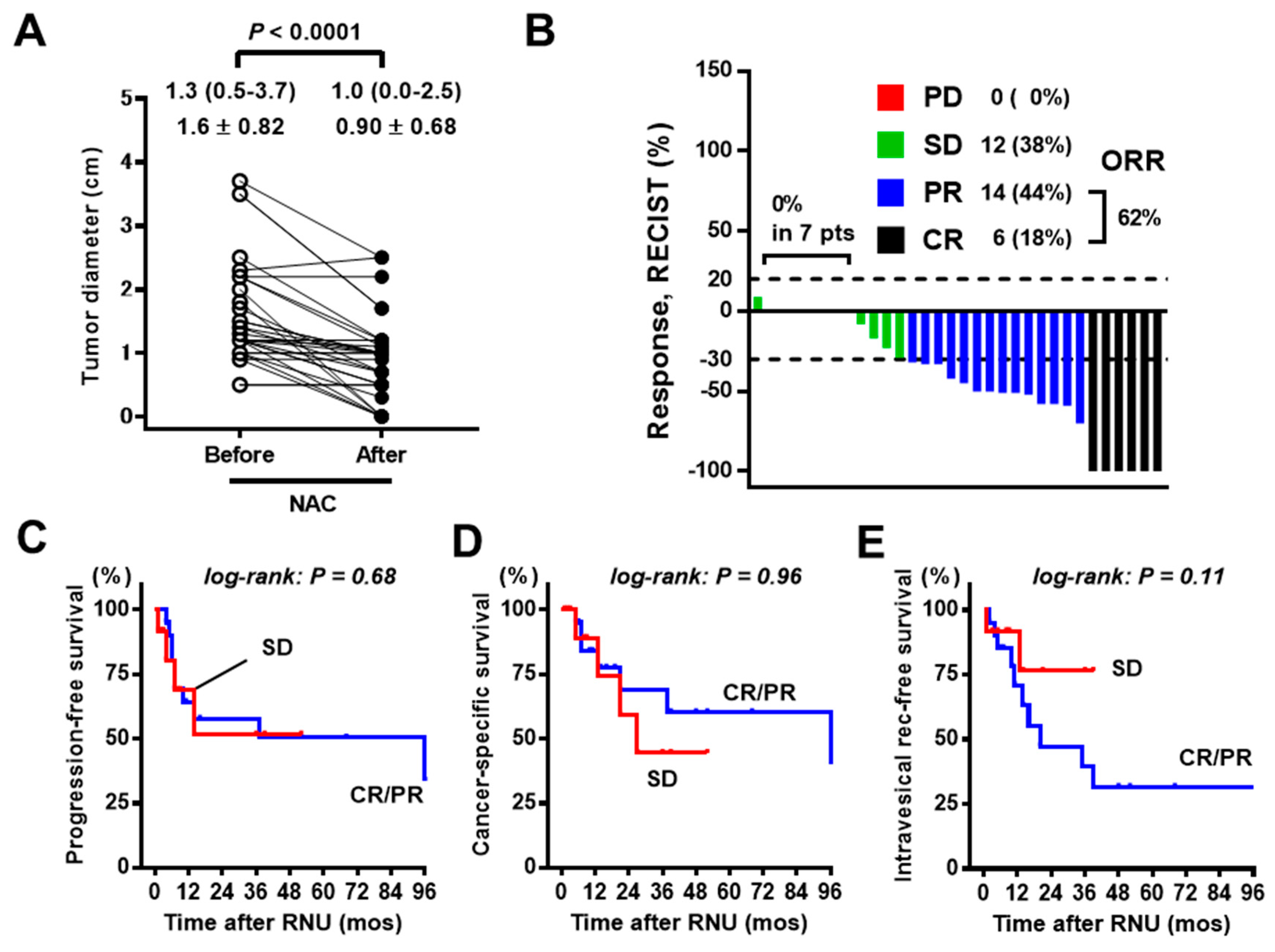
3.3. Analysis of Tumor Response to NAC
3.4. Prognostic Value of NAC-Induced Changes and Other Potential Factors
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Miyake, M.; Tatsumi, Y.; Fujimoto, K.; Nagao, K.; Sakano, S.; Matsuyama, H.; Inamoto, T.; Azuma, H.; Yasumoto, H.; Shiina, H.; et al. Changes in oncological outcomes after radical nephroureterectomy in patients with upper urinary tract urothelial carcinoma treated in the last two decades: A retrospective analysis based on a multicenter collaborative study. Jpn. J. Clin. Oncol. 2016, 46, 1148–1155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raman, J.D.; Messer, J.; Sielatycki, J.A.; Hollenbeak, C.S. Incidence and survival of patients with carcinoma of the ureter and renal pelvis in the USA, 1973–2005. BJU Int. 2011, 107, 1059–1064. [Google Scholar] [CrossRef] [PubMed]
- Munoz, J.J.; Ellison, L.M. Upper tract urothelial neoplasms: Incidence and survival during the last 2 decades. J. Urol. 2000, 164, 1523–1525. [Google Scholar] [CrossRef]
- Adibi, M.; Youssef, R.; Shariat, S.F.; Lotan, Y.; Wood, C.G.; Sagalowsky, A.I.; Zigeuner, R.; Montorsi, F.; Bolenz, C.; Margulis, V. Oncological outcomes after radical nephroureterectomy for upper tract urothelial carcinoma: Comparison over the three decades. Int. J. Urol. 2012, 19, 1060–1066. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Birtle, A.J.; Chester, J.D.; Jones, R.J.; Johnson, M.; Hill, M.; Bryan, R.T.; Catto, J.; Donovan, J.; French, A.; Harris, C.; et al. Results of POUT: A phase III randomised trial of perioperative chemotherapy versus surveillance in upper tract urothelial cancer (UTUC). J. Clin. Oncol. 2018, 36 (Suppl. 6), 407. [Google Scholar] [CrossRef]
- Gregg, R.W.; Vera-Badillo, F.E.; Booth, C.M.; Mahmud, A.; Brundage, M.; Leveridge, M.J.; Hanna, T.P. Perioperative chemotherapy for urothelial carcinoma of the upper urinary tract: A systematic review and meta-analysis. Crit. Rev. Oncol. Hematol. 2018, 128, 58–64. [Google Scholar] [CrossRef]
- Porten, S.; Siefker-Radtke, A.O.; Xiao, L.; Margulis, V.; Kamat, A.M.; Wood, C.G.; Jonasch, E.; Dinney, C.P.; Matin, S.F. Neoadjuvant chemotherapy improves survival of patients with upper tract urothelial carcinoma. Cancer 2014, 120, 1794–1799. [Google Scholar] [CrossRef]
- Mbeutcha, A.; Rouprêt, M.; Kamat, A.M.; Karakiewicz, P.I.; Lawrentschuk, N.; Novara, G.; Raman, J.D.; Seitz, C.; Xylinas, E.; Shariat, S.F. Prognostic factors and predictive tools for upper tract urothelial carcinoma: A systematic review. World J. Urol. 2017, 35, 337–353. [Google Scholar] [CrossRef]
- Itami, Y.; Miyake, M.; Tatsumi, Y.; Gotoh, D.; Hori, S.; Morizawa, Y.; Iida, K.; Ohnishi, K.; Nakai, Y.; Inoue, T.; et al. Preoperative predictive factors focused on inflammation-, nutrition-, and muscle-status in patients with upper urinary tract urothelial carcinoma undergoing nephroureterectomy. Int. J. Clin. Oncol. 2019, 24, 533–545. [Google Scholar] [CrossRef]
- Ito, Y.; Kikuchi, E.; Tanaka, N.; Miyajima, A.; Mikami, S.; Jinzaki, M.; Oya, M. Preoperative hydronephrosis grade independently predicts worse pathological outcomes in patients undergoing nephroureterectomy for upper tract urothelial carcinoma. J. Urol. 2011, 185, 1621–1626. [Google Scholar] [CrossRef]
- Kohada, Y.; Hayashi, T.; Goto, K.; Kobatake, K.; Abdi, H.; Honda, Y.; Sentani, K.; Inoue, S.; Teishima, J.; Awai, K.; et al. Preoperative risk classification using neutrophil-lymphocyte ratio and hydronephrosis for upper tract urothelial carcinoma. Jpn. J. Clin. Oncol. 2018, 48, 841–850. [Google Scholar] [CrossRef] [PubMed]
- Bozzini, G.; Nison, L.; Colin, P.; Ouzzane, A.; Yates, D.R.; Audenet, F.; Pignot, G.; Arvin-Berod, A.; Merigot, O.; Guy, L.; et al. Influence of preoperative hydronephrosis on the outcome of urothelial carcinoma of the upper urinary tract after nephroureterectomy: The results from a multi-institutional French cohort. World J. Urol. 2013, 31, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Cho, K.S.; Hong, S.J.; Cho, N.H.; Choi, Y.D. Grade of hydronephrosis and tumor diameter as preoperative prognostic factors in ureteral transitional cell carcinoma. Urology 2007, 70, 662–666. [Google Scholar] [CrossRef] [PubMed]
- Thiel, D.D.; Jorns, J.; Lohse, C.M.; Cheville, J.C.; Thompson, R.H.; Parker, A.S. Maximum tumor diameter is not an accurate predictor of renal cell carcinoma tumor volume. Scand. J. Urol. 2013, 47, 472–475. [Google Scholar] [CrossRef] [PubMed]
- Asai, S.; Fukumoto, T.; Watanabe, R.; Koyama, K.; Sawada, Y.; Noda, T.; Miura, N.; Yanagihara, Y.; Miyauchi, Y.; Miyagawa, M.; et al. New classification of hydronephrosis on 18F-FDG-PET/CT predicts post-operative renal function and muscle-invasive disease in patients with upper urinary tract urothelial carcinoma. Jpn. J. Clin. Oncol. 2018, 48, 1022–1027. [Google Scholar] [CrossRef]
- Grossman, H.B.; Natale, R.B.; Tangen, C.M.; Speights, V.O.; Vogelzang, N.J.; Trump, D.L.; deVere White, R.W.; Sarosdy, M.F.; Wood, D.P., Jr.; Raghavan, D.; et al. Neoadjuvant chemotherapy plus cystectomy compared with cystectomy alone for locally advanced bladder cancer. N. Engl. J. Med. 2003, 349, 859–866. [Google Scholar] [CrossRef]
- International Collaboration of Trialists; Medical Research Council Advanced Bladder Cancer Working Party (Now the National Cancer Research Institute Bladder Cancer Clinical Studies Group); European Organisation for Research and Treatment of Cancer Genito-Urinary Tract Cancer Group; Australian Bladder Cancer Study Group; National Cancer Institute of Canada Clinical Trials Group; Finnbladder; Norwegian Bladder Cancer Study Group; Club Urologico Espanol de Tratamiento Oncologico Group; Griffiths, G.; Hall, R.; et al. International phase III trial assessing neoadjuvant cisplatin, methotrexate, and vinblastine chemotherapy for muscle-invasive bladder cancer: Long-term results of the BA06 30894 trial. J. Clin. Oncol. 2011, 29, 2171–2177. [Google Scholar] [CrossRef] [Green Version]
- Millikan, R.; Dinney, C.; Swanson, D.; Sweeney, P.; Ro, J.Y.; Smith, T.L.; Williams, D.; Logothetis, C. Integrated therapy for locally advanced bladder cancer: Final report of a randomized trial of cystectomy plus adjuvant M-VAC versus cystectomy with both preoperative and postoperative M-VAC. J. Clin. Oncol. 2001, 19, 4005–4013. [Google Scholar] [CrossRef]
- Aziz, A.; Dobruch, J.; Hendricksen, K.; Kluth, L.A.; Necchi, A.; Noon, A.; Rink, M.; Roghmann, F.; Seiler, R.; Gontero, P.; et al. Perioperative chemotherapy in upper tract urothelial carcinoma: A comprehensive review. World J. Urol. 2017, 35, 1401–1407. [Google Scholar] [CrossRef]
- Igawa, M.; Urakami, S.; Shiina, H.; Kishi, H.; Himeno, Y.; Ishibe, T.; Kadena, H.; Usui, T. Neoadjuvant chemotherapy for locally advanced urothelial cancer of the upper urinary tract. Urol. Int. 1995, 55, 74–77. [Google Scholar] [CrossRef]
- Matin, S.F.; Margulis, V.; Kamat, A.; Wood, C.G.; Grossman, H.B.; Brown, G.A.; Dinney, C.P.; Millikan, R.; Siefker-Radtke, A.O. Incidence of downstaging and complete remission after neoadjuvant chemotherapy for high-risk upper tract transitional cell carcinoma. Cancer 2010, 116, 3127–3134. [Google Scholar] [CrossRef]
- Almassi, N.; Gao, T.; Lee, B.; Stein, R.J.; Haber, G.P.; Ornstein, M.C.; Rini, B.I.; Gilligan, T.D.; Garcia, J.A.; Stephenson, A.J.; et al. Impact of neoadjuvant chemotherapy on pathologic response in patients with upper tract urothelial carcinoma undergoing extirpative surgery. Clin. Genitourin. Cancer 2018, 16, e1237–e1242. [Google Scholar] [CrossRef]
| Variables | n (%) | |
|---|---|---|
| Total | 32 (100%) | |
| Age at diagnosis | Mean ± SD | 69 ± 8.9 |
| Sex | Male | 22 (69%) |
| Female | 10 (31%) | |
| Location of main tumor | Upper | 6 (19%) |
| Middle | 10 (31%) | |
| Lower | 16 (50%) | |
| Baseline clinical T category † | cT1/2 | 13 (40%) |
| cT3 | 19 (60%) | |
| Long diameter of index tumor (cm) | Median (IQR) | 1.3 (1.0–2.2) |
| Mean ± SD | 1.6 ± 0.82 | |
| Estimated volume of index tumor (cm3) | Median (IQR) | 0.79 (0.49–2.6) |
| Mean ± SD | 2.5 ± 4.0 | |
| Baseline CRP level | Mean ± SD | 0.45 ± 1.26 |
| Baseline NLR | Mean ± SD | 3.26 ± 2.40 |
| Hydronephrosis due to ureteral tumor | Grade 1 | 2 (6%) |
| Grade 2 | 8 (25%) | |
| Grade 3 | 11 (34%) | |
| Grade 4 | 11 (34%) | |
| Neoadjuvant chemotherapy regimen | GC | 24 (75%) |
| M-VAC | 3 (9%) | |
| Other | 5 (16%) | |
| Pathological T category at RNU † | pT0 | 4 (13%) |
| pTa | 2 (6%) | |
| pT1 | 6 (19%) | |
| pT2 | 8 (25%) | |
| pT3 | 11 (34%) | |
| pT4 | 1 (3%) | |
| Pathological N category at RNU † | N0 | 28 (89%) |
| N1–2 | 4 (11%) | |
| Adjuvant chemotherapy | No | 28 (89%) |
| GC | 3 (9%) | |
| M-VAC | 1 (3%) | |
| Variables | Change in Hydronephrosis Grade | p Value | ||
|---|---|---|---|---|
| Down-Grading | No Change/Up-Grading | |||
| Total | 10 (31%) | 22 (69%) | - | |
| Age at diagnosis | Mean ± SD | 65.1 ± 10.7 | 71.1 ± 7.5 | 0.09 |
| Sex | Male | 8 (80%) | 14 (64%) | 0.44 |
| Female | 2 (20%) | 8 (36%) | ||
| Location of main tumor | Upper | 2 (20%) | 4 (18%) | 0.64 |
| Middle | 2 (20%) | 8 (36%) | ||
| Lower | 6 (60%) | 10 (46%) | ||
| Baseline clinical T category † | cT1/2 | 3 (30%) | 7 (32%) | 0.92 |
| cT3 | 7 (70%) | 15 (68%) | ||
| Long tumor diameter (cm) | Mean ± SD | 1.9 ± 1.1 | 1.5 ± 0.68 | 0.60 |
| Estimated tumor volume (cm3) | Mean ± SD | 3.7 ± 5.4 | 2.0 ± 3.4 | 0.59 |
| Baseline CRP level | Mean ± SD | 0.79 ± 2.11 | 0.30 ± 0.61 | 0.96 |
| Baseline NLR | Mean ± SD | 3.12 ± 1.39 | 3.33 ± 2.92 | 0.57 |
| Baseline hydronephrosis | Grade 1 | 2 (20%) | 0 (0%) | 0.12 |
| Grade 2 | 1 (10%) | 7 (32%) | ||
| Grade 3 | 4 (40%) | 7 (32%) | ||
| Grade 4 | 3 (30%) | 8 (36%) | ||
| NAC regimen | GC | 6 (60%) | 18 (82%) | 0.33 ‡ |
| M-VAC | 0 (0%) | 3 (14%) | ||
| Other | 4 (40%) | 1 (4%) | ||
| Number of Cycles | Change in Hydronephrosis | Total | ||
|---|---|---|---|---|
| Down-Grading | No Change | Up-Grading | ||
| 1 | 4 (44%) | 5 (56%) | 0 (0%) | 9 (100%) |
| 2 | 5 (26%) | 13 (68%) | 1 (5%) | 19 (100%) |
| 3 | 1 (25%) | 3 (75%) | 0 (0%) | 4 (100%) |
| Total | 10 (31%) | 21 (66%) | 1 (3%) | 32 (100%) |
| Variables | Progression-Free Survival | Cancer-Specific Survival | IVR-Free Survival | ||||||
|---|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | p | HR | 95% CI | p | HR | 95% CI | p | |
| Age | |||||||||
| 70 or less | 1 | 1 | 1 | ||||||
| more than 70 | 1.52 | 0.51–4.5 | 0.45 | 1.47 | 0.43–5.0 | 0.54 | 1.07 | 0.34–3.37 | 0.91 |
| Sex | |||||||||
| Male | 1 | 1 | 1 | ||||||
| Female | 0.68 | 0.24–2.0 | 0.45 | 0.53 | 0.16–1.7 | 0.26 | 0.65 | 0.21–2.02 | 0.46 |
| Baseline clinical T | |||||||||
| cT1/2 | 1 | 1 | 1 | ||||||
| T3 | 3.53 | 1.23–10.1 | 0.03 | 2.67 | 0.82–8.70 | 0.12 | 1.27 | 0.41–4.00 | 0.68 |
| Baseline hydronephrosis | |||||||||
| Grade 1/2 | 1 | 1 | 1 | ||||||
| Grade 3/4 | 5.10 | 1.78–14.6 | 0.01 | 4.56 | 1.40–14.9 | 0.024 | 2.32 | 0.75–8.25 | 0.14 |
| Baseline blood CRP level (mg/dL) | |||||||||
| less than 0.1 | 1 | 1 | 1 | ||||||
| 0.1 or more | 1.74 | 0.58–5.18 | 0.29 | 1.79 | 0.52–6.13 | 0.35 | 1.33 | 0.41–4.22 | 0.13 |
| Baseline NLR | |||||||||
| less than 2.6 | 1 | 1 | 1 | ||||||
| 2.6 or more | 0.61 | 0.20–1.90 | 0.32 | 0.97 | 0.27–3.46 | 0.96 | 1.31 | 0.37–4.59 | 0.59 |
| Change in hydronephrosis | |||||||||
| Down-grading | 1 | 1 | 1 | ||||||
| No change/up-grading | 6.07 | 1.97–18.7 | 0.04 | 4.16 | 1.07–16.4 | 0.042 | 0.66 | 0.19–2.16 | 0.47 |
| Response, RECIST criteria | |||||||||
| CR/PR | 1 | 1 | 1 | ||||||
| SD | 1.11 | 0.32–3.8 | 0.87 | 1.5 | 0.39–6.0 | 0.69 | 0.50 | 0.15–1.7 | 0.27 |
| Response in tumor volume | |||||||||
| CR/PR | 1 | 1 | 1 | ||||||
| SD/PD | 0.78 | 0.23–2.6 | 0.68 | 0.97 | 0.26–3.6 | 0.96 | 0.22 | 0.06–1.24 | 0.11 |
| Response in CRP level | |||||||||
| Decreased | 1 | 1 | 1 | ||||||
| No change/increased | 0.46 | 0.15–1.70 | 0.15 | 1.01 | 0.22–4.75 | 0.99 | 0.24 | 0.05–1.17 | 0.09 |
| Response in NLR | |||||||||
| Decreased | 1 | 1 | 1 | ||||||
| No change/increased | 1.06 | 0.34–3.30 | 0.91 | 0.88 | 0.25–3.10 | 0.84 | 0.81 | 0.23–2.80 | 0.73 |
| Pathological T at RNU specimen | |||||||||
| pT0 | 1 | 1 | 1 | ||||||
| pTa/1 | NA | NA | 0.05 | NA | NA | 0.056 | 2.32 | 0.38–14.3 | 0.44 |
| pT2–4 | 1.47 | 0.40–5.52 | 0.58 | 1.36 | 0.34–5.52 | 0.67 | 2.98 | 0.65–13.5 | 0.27 |
| LVI at RNU specimen | |||||||||
| No | 1 | 1 | 1 | ||||||
| Yes | 4.14 | 1.10–15.6 | 0.03 | 4.10 | 1.76–47.1 | 0.0084 | 2.21 | 0.41–11.8 | 0.19 |
| Variables | Progression-Free Survival | ||
|---|---|---|---|
| HR | 95% CI | p Value | |
| Baseline clinical T | |||
| cT1/2 | 1 | ||
| T3 | 6.12 | 1.45–25.8 | 0.014 |
| Change in hydronephrosis | |||
| Down-grading | 1 | ||
| No change/up-grading | 10.4 | 1.25–86.4 | 0.030 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miyake, M.; Marugami, N.; Fujiwara, Y.; Komura, K.; Inamoto, T.; Azuma, H.; Matsumoto, H.; Matsuyama, H.; Fujimoto, K. Down-Grading of Ipsilateral Hydronephrosis by Neoadjuvant Chemotherapy Correlates with Favorable Oncological Outcomes in Patients Undergoing Radical Nephroureterectomy for Ureteral Carcinoma. Diagnostics 2020, 10, 10. https://doi.org/10.3390/diagnostics10010010
Miyake M, Marugami N, Fujiwara Y, Komura K, Inamoto T, Azuma H, Matsumoto H, Matsuyama H, Fujimoto K. Down-Grading of Ipsilateral Hydronephrosis by Neoadjuvant Chemotherapy Correlates with Favorable Oncological Outcomes in Patients Undergoing Radical Nephroureterectomy for Ureteral Carcinoma. Diagnostics. 2020; 10(1):10. https://doi.org/10.3390/diagnostics10010010
Chicago/Turabian StyleMiyake, Makito, Nagaaki Marugami, Yuya Fujiwara, Kazumasa Komura, Teruo Inamoto, Haruhito Azuma, Hiroaki Matsumoto, Hideyasu Matsuyama, and Kiyohide Fujimoto. 2020. "Down-Grading of Ipsilateral Hydronephrosis by Neoadjuvant Chemotherapy Correlates with Favorable Oncological Outcomes in Patients Undergoing Radical Nephroureterectomy for Ureteral Carcinoma" Diagnostics 10, no. 1: 10. https://doi.org/10.3390/diagnostics10010010
APA StyleMiyake, M., Marugami, N., Fujiwara, Y., Komura, K., Inamoto, T., Azuma, H., Matsumoto, H., Matsuyama, H., & Fujimoto, K. (2020). Down-Grading of Ipsilateral Hydronephrosis by Neoadjuvant Chemotherapy Correlates with Favorable Oncological Outcomes in Patients Undergoing Radical Nephroureterectomy for Ureteral Carcinoma. Diagnostics, 10(1), 10. https://doi.org/10.3390/diagnostics10010010






