How Prebiotic Chemistry and Early Life Chose Phosphate
Abstract
1. Introduction
2. Phosphoryl Transfer Pathways
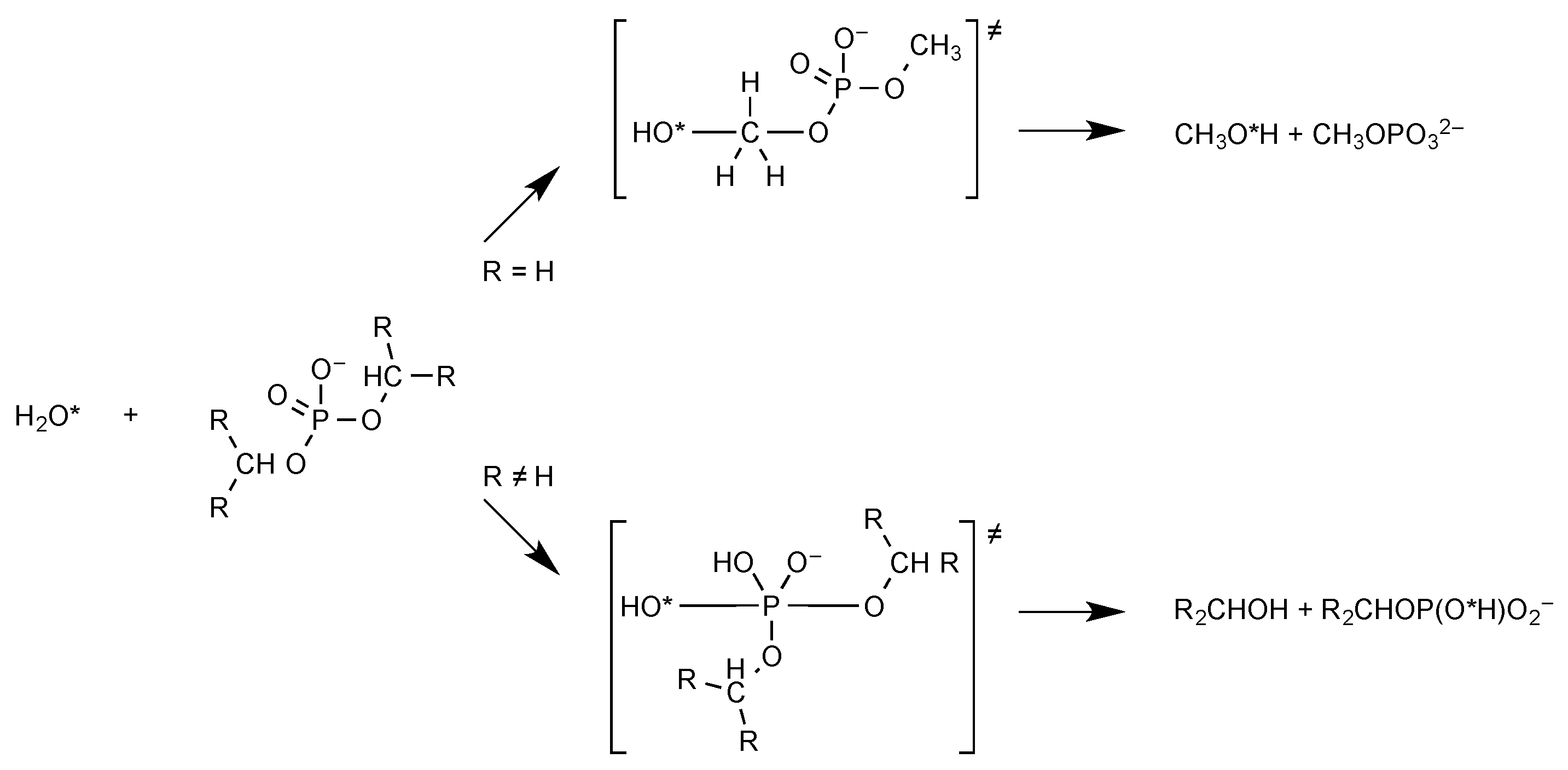
2.1. Phosphate Esters
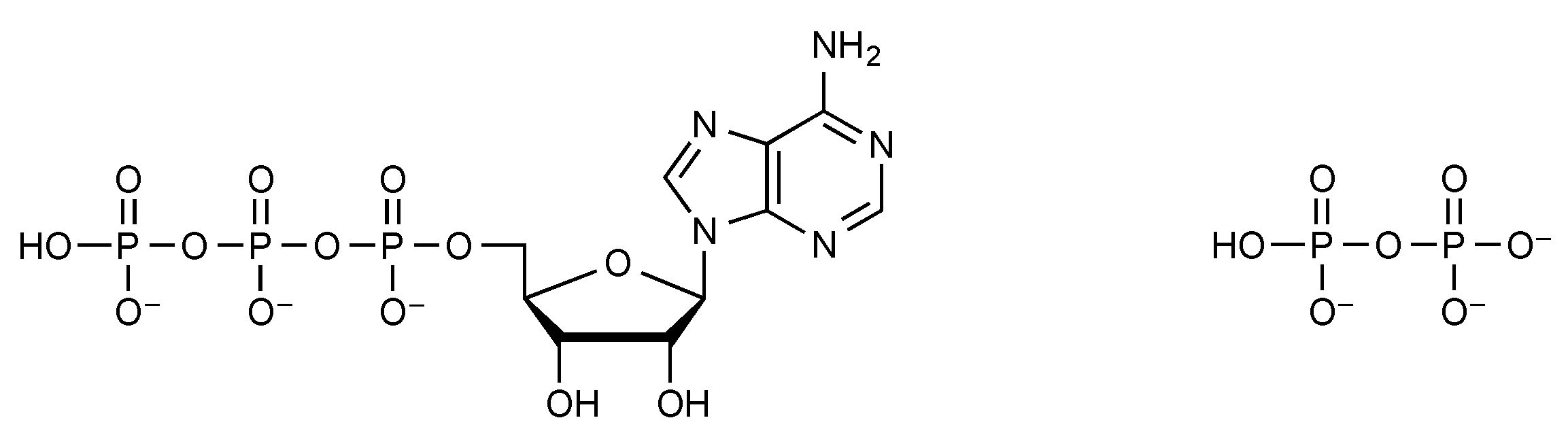
2.2. Phosphate Anhydrides
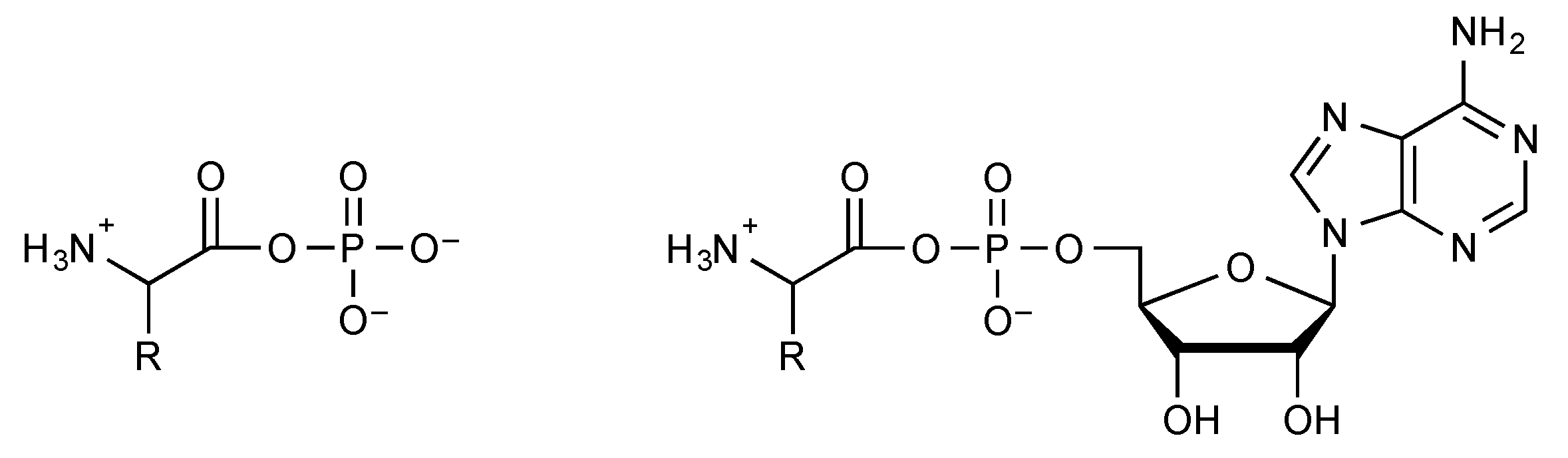
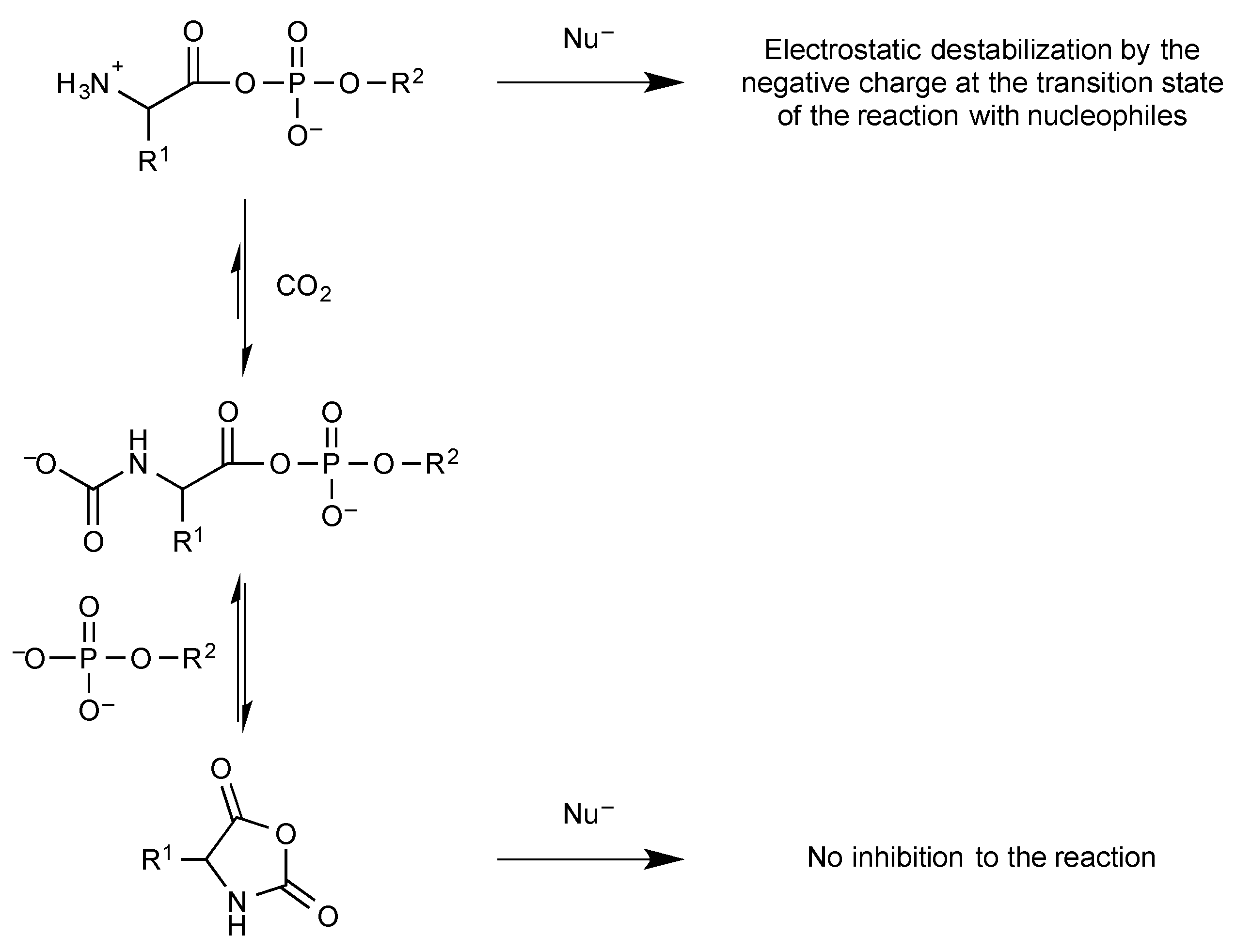
2.3. Phosphate Mixed Anhydrides
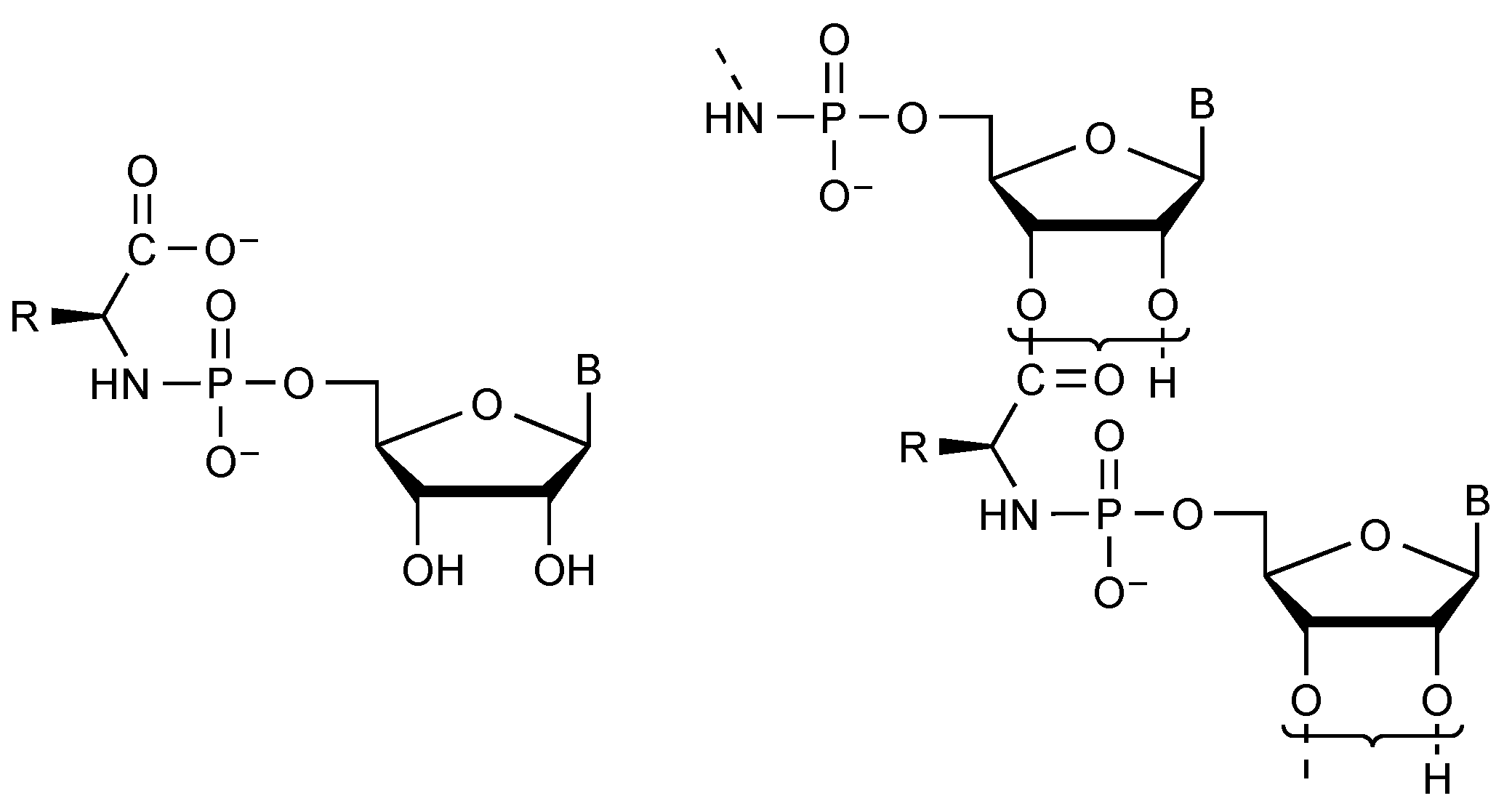
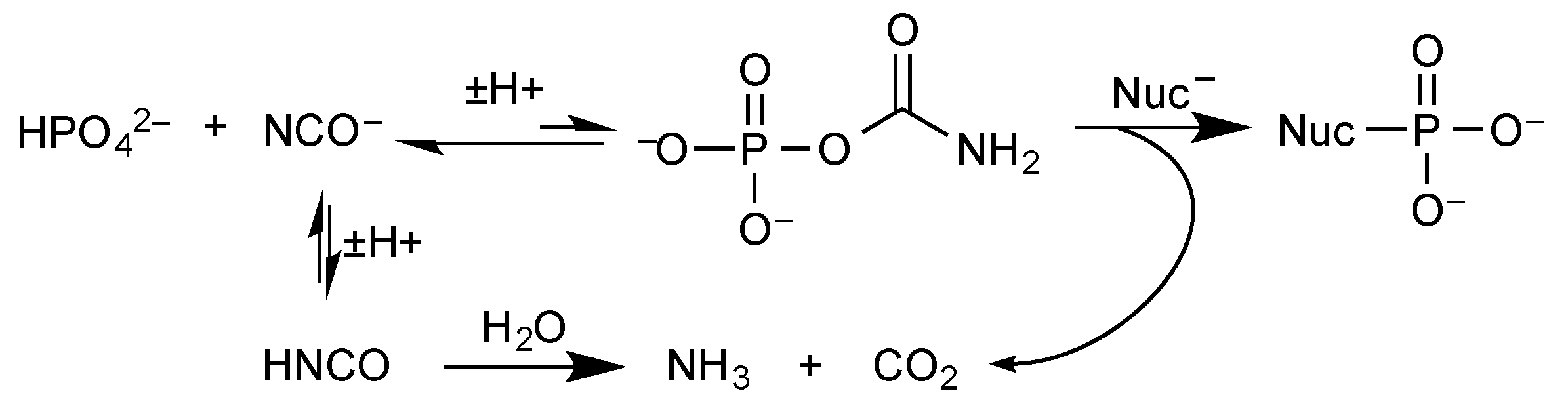
2.4. Phosphoramidates
3. Which Phosphate Derivatives Could Play A Role as Early Energy Currencies?
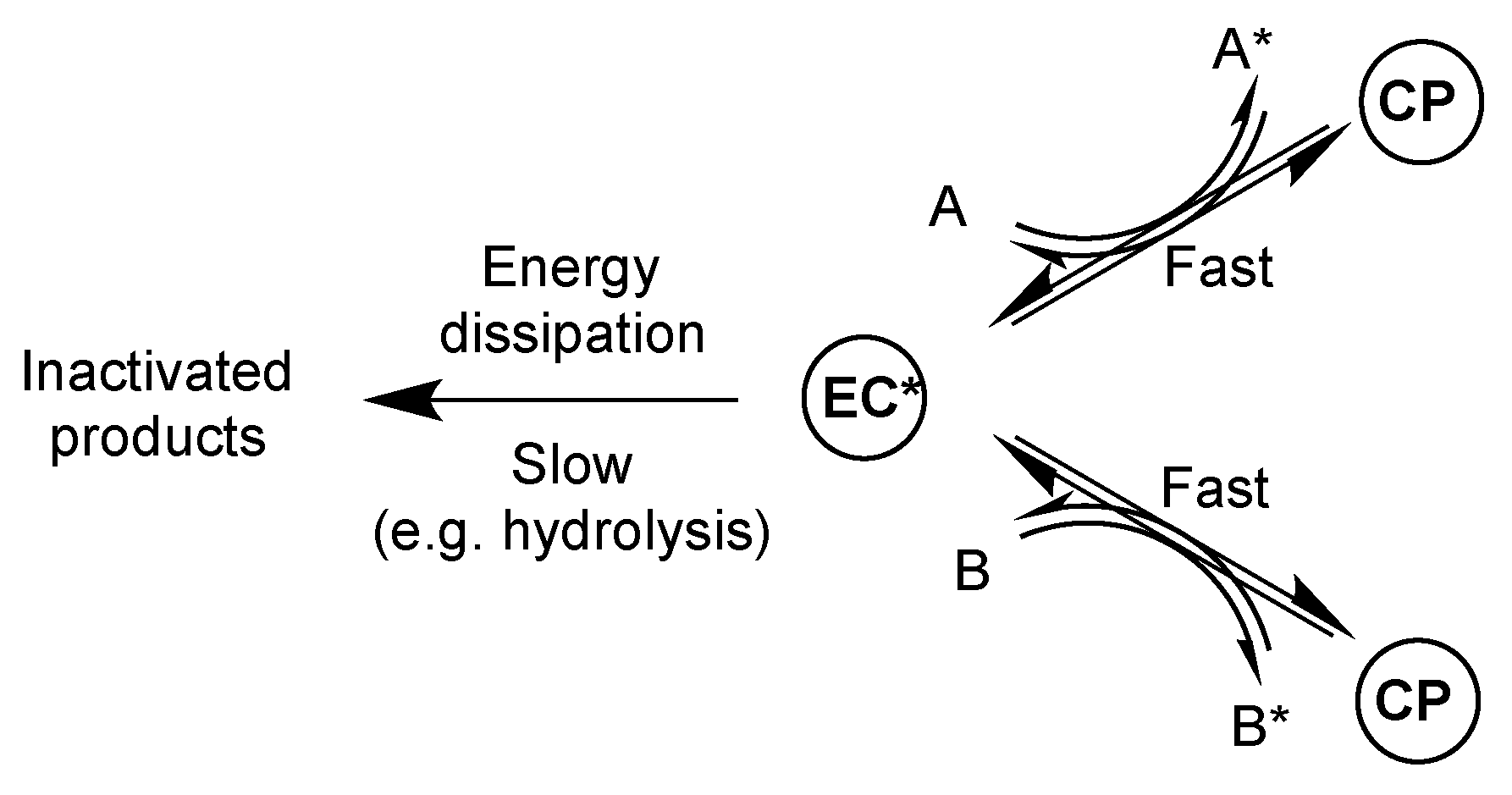
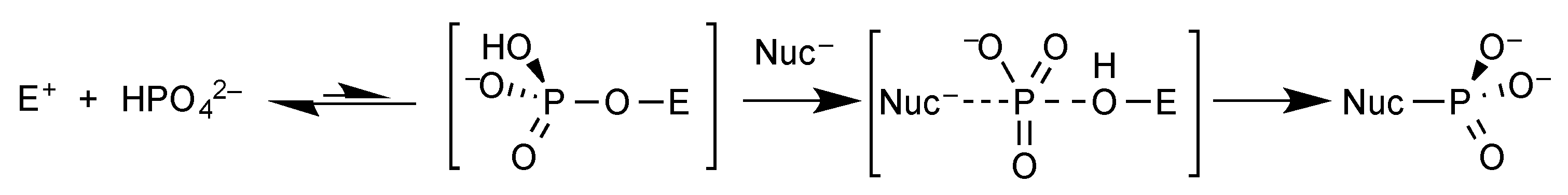
4. The Question of Prebiotic Phosphorylation
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Lipmann, F. Metabolic generation and utilization of phosphate bond energy. Adv. Enzymol. Relat. Areas Mol. Biol. 1941, 1, 99–162. [Google Scholar]
- Lipmann, F. Phosphorus Metabolism; McElroy, W.D., Glass, H.B., Eds.; Johns Hopkins Press: Baltimore, MD, USA, 1951; Volume 1, p. 521. [Google Scholar]
- Westheimer, F.H. Why nature chose phosphate. Science 1987, 235, 1173–1178. [Google Scholar] [CrossRef] [PubMed]
- Kumler, W.D.; Eiler, J.J. The Acid Strength of Mono and Diesters of Phosphoric Acid. The n-Alkyl Esters from Methyl to Butyl, the Esters of Biological Importance, and the Natural Guanidine Phosphoric Acids. J. Am. Chem. Soc. 1943, 65, 2355–2361. [Google Scholar] [CrossRef]
- Wolfenden, R.; Ridgway, C.; Young, G. Spontaneous hydrolysis of ionized phosphate monoesters and diesters and the proficiencies of phosphatases and phosphodiesterases as catalysts. J. Am. Chem. Soc. 1998, 120, 833–834. [Google Scholar] [CrossRef]
- Bowler, M.W.; Cliff, M.J.; Waltho, J.P.; Blackburn, G.M. Why did Nature select phosphate for its dominant roles in biology? New J. Chem. 2010, 34, 784–794. [Google Scholar] [CrossRef]
- Goldford, J.E.; Hartman, H.; Smith, T.F.; Segrè, D. Remnants of an Ancient Metabolism without Phosphate. Cell 2017, 168, 1126–1134. [Google Scholar] [CrossRef] [PubMed]
- Martin, W.F.; Thauer, R.K. Energy in Ancient Metabolism. Cell 2017, 168, 953–955. [Google Scholar] [CrossRef] [PubMed]
- Pasek, M.A.; Kee, T.P.; Bryant, D.E.; Pavlov, A.A.; Lunine, J.I. Production of Potentially Prebiotic Condensed Phosphates by Phosphorus Redox Chemistry. Angew. Chem. Int. Ed. 2008, 47, 7918–7920. [Google Scholar] [CrossRef] [PubMed]
- Bryant, D.E.; Greenfield, D.; Walshaw, R.D.; Evans, S.M.; Nimmo, A.E.; Smith, C.L.; Wang, L.; Pasek, M.A.; Kee, T.P. Electrochemical studies of iron meteorites: Phosphorus redox chemistry on the early Earth. Int. J. Astrobiol. 2009, 8, 27–36. [Google Scholar] [CrossRef]
- Bryant, D.E.; Marriott, K.E.R.; Macgregor, S.A.; Kilner, C.; Pasek, M.A.; Kee, T.P. On the prebiotic potential of reduced oxidation state phosphorus: The H-phosphinate–pyruvate system. Chem. Commun. 2010, 46, 3726–3728. [Google Scholar] [CrossRef] [PubMed]
- Kaye, K.; Bryant, D.; Marriott, K.; Ohara, S.; Fishwick, C.; Kee, T. Selective Phosphonylation of 5′-Adenosine Monophosphate (5′-AMP) via Pyrophosphite [PPi(III)]. Orig. Life Evol. Biosph. 2016, 46, 425–434. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, G.K.; Lad, C.; Wyman, P.; Williams, N.H.; Wolfenden, R. The time required for water attack at the phosphorus atom of simple phosphodiesters and of DNA. Proc. Natl. Acad. Sci. USA 2006, 103, 4052–4055. [Google Scholar] [CrossRef] [PubMed]
- Wolfenden, R. Benchmark reaction rates, the stability of biological molecules in water, and the evolution of catalytic power in enzymes. Annu. Rev. Biochem. 2011, 80, 645–667. [Google Scholar] [CrossRef] [PubMed]
- Kirby, A.J. Effective molarities for intramolecular reactions. Adv. Phys. Org. Chem. 1980, 17, 183–278. [Google Scholar]
- Pascal, R. Catalysis through Induced Intramolecularity: What Can Be Learned by Mimicking Enzymes with Carbonyl Compounds that Covalently Bind Substrates? Eur. J. Org. Chem. 2003, 2003, 1813–1824. [Google Scholar] [CrossRef]
- Pascal, R. Kinetic Barriers and the Self-organization of Life. Isr. J. Chem. 2015, 55, 865–874. [Google Scholar] [CrossRef]
- Li, B.-J.; El-Nachef, C.; Beauchemin, A. Organocatalysis Using Aldehydes: The Development and Improvement of Catalytic Hydroaminations, Hydrations and Hydrolyses. Chem. Commun. 2017, 53, 13192–13204. [Google Scholar] [CrossRef] [PubMed]
- Kirby, A.J.; Varvoglis, A.G. The Reactivity of Phosphate Esters. Monoester Hydrolysis. J. Am. Chem. Soc. 1967, 89, 415–423. [Google Scholar] [CrossRef]
- Westheimer, F.H. Monomeric metaphosphate ion. Chem. Rev. 1981, 81, 313–326. [Google Scholar] [CrossRef]
- Kamerlin, S.C.; Sharma, P.K.; Prasad, R.B.; Warshel, A. Why nature really chose phosphate. Q. Rev. Biophys. 2013, 46, 1–132. [Google Scholar] [CrossRef] [PubMed]
- Lipmann, F. Projecting backward from the present stage of evolution of biosynthesis. In The Origins of Prebiological Systems and of Their Molecular Matrices; Fox, S.W., Ed.; Academic Press: New York, NY, USA, 1965; pp. 259–280. [Google Scholar]
- Miller, S.L.; Parris, M. Synthesis of pyrophosphate under primitive Earth conditions. Nature 1964, 204, 1248–1250. [Google Scholar] [CrossRef]
- Vieyra, A.; Meyer-Fernandes, J.R.; Gama, O.B. Phosphorolysis of acetyl phosphate by orthophosphate with energy conservation in the phosphoanhydride linkage of pyrophosphate. Arch. Biochem. Biophys. 1985, 238, 574–583. [Google Scholar] [CrossRef]
- Vieyra, A.; Gueiros-Filho, F.; Meyer-Fernandes, J.R.; Costa-Sarmento, G.; De Souza-Barros, F. Reactions involving carbamyl phosphate in the presence of precipitated calcium phosphate with formation of pyrophosphate: A model for primitive energy-conservation pathways. Orig. Life Evol. Biosph. 1995, 25, 335–350. [Google Scholar] [CrossRef] [PubMed]
- Keefe, A.D.; Miller, S.L. Are polyphosphate or phosphate esters prebiotic reagents? J. Mol. Evol. 1995, 41, 693–702. [Google Scholar] [CrossRef] [PubMed]
- Keefe, A.D.; Miller, S.L. Potentially prebiotic synthesis of condensed phosphates. Orig. Life Evol. Biosph. 1996, 26, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Deamer, D.W. The First Living Systems: A Bioenergetic Perspective. Microbiol. Mol. Biol. Rev. 1997, 61, 239–261. [Google Scholar] [PubMed]
- Russell, M.J. The Alkaline Solution to the Emergence of Life: Energy, Entropy and Early Evolution. Acta Biotheor. 2007, 55, 133–179. [Google Scholar] [CrossRef] [PubMed]
- Hagan, W.J., Jr.; Parker, A.; Steuerwald, A.; Hathaway, M. Phosphate Solubility and the Cyanate-Mediated Synthesis of Pyrophosphate. Orig. Life Evol. Biosph. 2007, 37, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Szostak, J. The Origin of Life on Earth and the Design of Alternative Life Forms. Mol. Front. J. 2017, 1, 121–131. [Google Scholar] [CrossRef]
- Lipmann, F. Acetyl Phosphate. Adv. Enzymol. Relat. Areas Mol. Biol. 1946, 6, 231–267. [Google Scholar]
- Jencks, W.P. Free energies of hydrolysis and decarboxylation. In Handbook of Biochemistry and Molecular Biology, 3rd ed.; Fasman, G.D., Ed.; CRC Press: Cleveland, OH, USA, 1976; Volume 1, pp. 296–304. [Google Scholar]
- Di Sabato, G.; Jencks, W.P. Mechanism and catalysis of reactions of acyl phosphates II. Hydrolysis. J. Am. Chem. Soc. 1961, 83, 4400–4405. [Google Scholar] [CrossRef]
- Biron, J.-P.; Pascal, R. Amino acid N-carboxyanhydrides: Activated peptide monomers behaving as phosphate-activating agents in aqueous solution. J. Am. Chem. Soc. 2004, 126, 9198–9199. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, C.J.; Wallace, R.G. A phosphate-catalysed acyl transfer reaction. Hydrolysis of 4-nitrophenyl acetate in phosphate buffers. Aust. J. Chem. 1984, 37, 2559–2569. [Google Scholar] [CrossRef]
- Andrés, G.O.; Granados, A.M.; de Rossi, R.H. Kinetic Study of the Hydrolysis of Phthalic Anhydride and Aryl Hydrogen Phthalates. J. Org. Chem. 2001, 66, 7653–7657. [Google Scholar] [CrossRef] [PubMed]
- El Seoud, O.A.; Ruasse, M.-F.; Rodrigues, W.A. Kinetics and mechanism of phosphate-catalyzed hydrolysis of benzoate esters: Comparison with nucleophilic catalysis by imidazole and o-iodosobenzoate. J. Chem. Soc. Perkin Trans. 2 2002, 1053–1058. [Google Scholar] [CrossRef]
- Gill, M.S.; Neverov, A.A.; Brown, R.S. Dissection of nucleophilic and general base roles for the reaction of phosphate with p-nitrophenylthiolacetate, p-nitrophenylthiolformate, phenylthiolacetate. J. Org. Chem. 1997, 62, 7351–7357. [Google Scholar] [CrossRef] [PubMed]
- Thomas, G.L.; Payne, R.J. Phosphate-assisted peptide ligation. Chem. Commun. 2009, 4260–4262. [Google Scholar] [CrossRef] [PubMed]
- Higuchi, T.; Flynn, G.L.; Shah, A.C. Reversible Formation and Hydrolysis of Phthaloyl and Succinyl Monophosphates in Aqueous Solution. J. Am. Chem. Soc. 1967, 89, 616–622. [Google Scholar] [CrossRef]
- Hagan, W.J., Jr. Uracil-Catalyzed Synthesis of Acetyl Phosphate: A Photochemical Driver for Protometabolism. ChemBioChem 2010, 11, 383–387. [Google Scholar] [CrossRef] [PubMed]
- Whicher, A.; Camprubi, E.; Pinna, S.; Herschy, B.; Lane, N. Acetyl Phosphate as a Primordial Energy Currency at the Origin of Life. Orig. Life Evol. Biosph. 2018, 48, 159–179. [Google Scholar] [CrossRef] [PubMed]
- Wells, T.N.C.; Ho, C.K.; Fersht, A.R. Free Energy of Hydrolysis of Tyrosyl Adenylate and Its Binding to Wild-Type and Engineered Mutant Tyrosyl-tRNA Synthetases. Biochemistry 1986, 25, 6603–6608. [Google Scholar] [CrossRef] [PubMed]
- Ribas de Pouplana, L.; Schimmel, P. Aminoacyl-tRNA synthetases: Potential markers of genetic code development. Trends Biochem. Sci. 2001, 26, 591–596. [Google Scholar] [CrossRef]
- Pascal, R.; Boiteau, L.; Commeyras, A. From the Prebiotic Synthesis of α-Amino Acids Towards a Primitive Translation Apparatus for the Synthesis of Peptides. Top. Curr. Chem. 2005, 259, 69–122. [Google Scholar]
- Pascal, R.; Boiteau, L. Energetic constraints on prebiotic pathways: Application to the emergence of translation. In Origin and Evolution of Life: An Astrobiology Perspective; Gargaud, M., Lopez-Garcia, P., Martin, H., Eds.; Cambridge University Press: Cambridge, UK, 2011; pp. 247–258. [Google Scholar]
- Liu, Z.; Beaufils, D.; Rossi, J.-C.; Pascal, R. Evolutionary importance of the intramolecular pathways of hydrolysis of phosphate ester mixed anhydrides with amino acids and peptides. Sci. Rep. 2014, 4, 7440. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Rigger, L.; Rossi, J.-C.; Sutherland, J.D.; Pascal, R. Mixed Anhydride Intermediates in the Reaction of 5(4H)-Oxazolones with Phosphate Esters and Nucleotides. Chem. Eur. J. 2016, 22, 14940–14949. [Google Scholar] [CrossRef] [PubMed]
- Biron, J.-P.; Parkes, A.L.; Pascal, R.; Sutherland, J.D. Expeditious, potentially primordial, aminoacylation of nucleotides. Angew. Chem. Int. Ed. Engl. 2005, 44, 6731–6734. [Google Scholar] [CrossRef] [PubMed]
- Leman, L.; Orgel, L.; Ghadiri, M.R. Amino Acid Dependent Formation of Phosphate Anhydrides in Water Mediated by Carbonyl Sulfide. J. Am. Chem. Soc. 2006, 128, 20–21. [Google Scholar] [CrossRef] [PubMed]
- Brack, A. Selective emergence and survival of early polypeptides in water. Orig. Life 1987, 17, 367–379. [Google Scholar] [CrossRef]
- Commeyras, A.; Taillades, J.; Collet, H.; Boiteau, L.; Vandenabeele-Trambouze, O.; Pascal, R.; Rousset, A.; Garrel, L.; Rossi, J.-C.; Biron, J.-P.; et al. Dynamic co-evolution of peptides and chemical energetics, a gateway to the emergence of homochirality and the catalytic activity of peptides. Orig. Life Evol. Biosph. 2004, 34, 35–55. [Google Scholar] [CrossRef] [PubMed]
- Leman, L.; Orgel, L.; Ghadiri, M.R. Carbonyl Sulfide–Mediated Prebiotic Formation of Peptides. Science 2004, 306, 283–286. [Google Scholar] [CrossRef] [PubMed]
- Danger, G.; Boiteau, L.; Cottet, H.; Pascal, R. The peptide formation mediated by cyanate revisited. N-carboxyanhydrides as accessible intermediates in the decomposition of N-carbamoylamino acids. J. Am. Chem. Soc. 2006, 128, 7412–7413. [Google Scholar] [PubMed]
- Wickramasinghe, N.S.M.D.; Staves, M.P.; Lacey, J.C. Stereoselective, nonenzymatic, intramolecular transfer of amino acids. Biochemistry 1991, 30, 2768–2772. [Google Scholar] [CrossRef] [PubMed]
- Paecht-Horowitz, M.; Berger, J.; Katchalsky, A. Prebiotic synthesis of polypeptides by heterogeneous polycondensation of aminoacid adenylates. Nature 1970, 228, 636–639. [Google Scholar] [CrossRef]
- Liu, Z.; Hanson, C.; Ajram, G.; Boiteau, L.; Rossi, J.-C.; Danger, G.; Pascal, R. 5(4H)-Oxazolones as Effective Aminoacylation Reagents for the 3′-Terminus of RNA. Synlett 2017, 28, 73–77. [Google Scholar]
- Bowler, F.R.; Chan, C.K.; Duffy, C.D.; Gerland, B.; Islam, S.; Powner, M.W.; Sutherland, J.D.; Xu, J. Prebiotically plausible oligoribonucleotide ligation facilitated by chemoselective acetylation. Nat. Chem. 2013, 5, 383–389. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Ajram, G.; Rossi, J.-C.; Pascal, R. The chemical likelihood of ribonucleotide-α-amino acid copolymers as players for early stages of evolution. J. Mol. Evol. 2019, in press. [Google Scholar] [CrossRef]
- Page, M.I.; Jencks, W.P. Entropic contribution to rate accelerations in enzymic and intramolecular reactions and the chelate effect. Proc. Natl. Acad. Sci. USA 1971, 68, 1678–1683. [Google Scholar] [CrossRef] [PubMed]
- De Duve, C. A research proposal on the origin of life. Orig. Life Evol. Biosph. 2003, 33, 559–574. [Google Scholar] [CrossRef] [PubMed]
- Adelfinskaya, O.; Herdewijn, P. Amino Acid Phosphoramidate Nucleotides as Alternative Substrates for HIV-1 Reverse Transcriptase. Angew. Chem. Int. Ed. 2007, 46, 4356–4358. [Google Scholar] [CrossRef] [PubMed]
- Adelfinskaya, O.; Terrazas, M.; Froeyen, M.; Marlière, P.; Nauwelaerts, K.; Herdewijn, P. Polymerase-catalyzed synthesis of DNA from phosphoramidate conjugates of deoxynucleotides and amino acids. Nucleic Acids Res. 2007, 35, 5060–5072. [Google Scholar] [CrossRef] [PubMed]
- Giraut, A.; Herdewijn, P. Influence of the Linkage between Leaving Group and Nucleoside on Substrate Efficiency for Incorporation in DNA Catalyzed by Reverse Transcriptase. ChemBioChem 2010, 11, 1399–1403. [Google Scholar] [CrossRef] [PubMed]
- Song, X.-P.; Bouillon, C.; Lescrinier, E.; Herdewijn, P. Iminodipropionic acid as the leaving group for DNA polymerization by HIV-1 reverse transcriptase. ChemBioChem 2011, 12, 1868–1880. [Google Scholar] [CrossRef] [PubMed]
- Song, X.-P.; Bouillon, C.; Lescrinier, E.; Herdewijn, P. Dipeptides as Leaving Group in the Enzyme-Catalyzed DNA Synthesis. Chem. Biodivers. 2012, 9, 2685–2700. [Google Scholar] [CrossRef] [PubMed]
- Giraut, A.; Abu El-Asrar, R.; Marlière, P.; Delarue, M.; Herdewijn, P. 2′-Deoxyribonucleoside phosphoramidate triphosphate analogues as alternative substrates for E. coli polymerase III. ChemBioChem 2012, 13, 2439–2444. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.M.; Liu, X.H.; Li, Y.M.; Ma, Y.; Tan, B.; Wan, R.; Zhao, Y.F. N-Phosphoryl amino acids and biomolecular origins. Orig. Life Evol. Biosph. 2004, 34, 455–464. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Deng, H.; Tang, G.; Liu, Y.; Xu, P.; Zhao, Y. Intermolecular Phosphoryl Transfer of N-Phosphoryl Amino Acids. Eur. J. Org. Chem. 2011, 2011, 3220–3228. [Google Scholar] [CrossRef]
- Ying, J.; Fu, S.; Li, X.; Fen, L.; Pengxiang, X.; Liu, Y.; Gao, X.; Zhao, Y. A plausible model correlates prebiotic peptide synthesis with the primordial genetic code. Chem. Commun. 2018, 54, 8598–8601. [Google Scholar] [CrossRef] [PubMed]
- Griesser, H.; Tremmel, P.; Kervio, E.; Pfeffer, C.; Steiner, U.E.; Richert, C. Ribonucleotides and RNA Promote Peptide Chain Growth. Angew. Chem. Int. Ed. 2017, 56, 1219–1223. [Google Scholar] [CrossRef] [PubMed]
- Jauker, M.; Griesser, H.; Richert, C. Spontaneous Formation of RNA Strands, Peptidyl RNA, and Cofactors. Angew. Chem. Int. Ed. 2015, 54, 14564–14569. [Google Scholar] [CrossRef] [PubMed]
- Krishnamurthy, R.; Arrhenius, G.; Eschenmoser, A. Formation of glycolaldehyde phosphate from glycolaldehyde in aqueous solution. Orig. Life Evol. Biosph. 1999, 29, 333–354. [Google Scholar] [CrossRef] [PubMed]
- Krishnamurthy, R.; Guntha, S.; Eschenmoser, A. Regioselective α-phosphorylation of aldoses in aqueous solution. Angew. Chem. Int. Ed. 2000, 39, 2281–2285. [Google Scholar] [CrossRef]
- Karki, M.; Gibard, C.; Bhowmik, S.; Krishnamurthy, R. Nitrogenous Derivatives of Phosphorus and the Origins of Life: Plausible Prebiotic Phosphorylating Agents in Water. Life 2017, 7, 32. [Google Scholar] [CrossRef] [PubMed]
- Gibard, C.; Bhowmik, S.; Karki, M.; Kim, E.-K.; Krishnamurthy, R. Phosphorylation, oligomerization and self-assembly in water under potential prebiotic conditions. Nat. Chem. 2017, 10, 212–217. [Google Scholar] [CrossRef] [PubMed]
- Lohrmann, R.; Orgel, L.E. Template-directed synthesis of high molecular weight polynucleotide analogues. Nature 1976, 261, 342–344. [Google Scholar] [CrossRef] [PubMed]
- Zielinski, W.S.; Orgel, L.E. Oligomerization of activated derivatives of 3′-amino-3′-deoxyguanosine on poly(C) and poly(dC) templates. Nucleic Acids Res. 1985, 13, 2469–2484. [Google Scholar] [CrossRef] [PubMed]
- Zielinski, W.S.; Orgel, L.E. Autocatalytic synthesis of a tetranucleotide analogue. Nature 1987, 327, 346–347. [Google Scholar] [CrossRef] [PubMed]
- Zielinski, W.S.; Orgel, L.E. Oligoaminonucleoside phosphoramidates. Oligomerization of dimers of 3′-amino-3′-deoxynudeotides (GC and CG) in aqueous solution. Nucleic Acids Res. 1987, 15, 1699–1715. [Google Scholar] [CrossRef] [PubMed]
- Zielinski, W.S.; Orgel, L.E. Polymerization of a Monomeric Guanosine Derivative in a Hydrogen-Bonded Aggregate. J. Mol. Evol. 1989, 29, 367–369. [Google Scholar] [CrossRef] [PubMed]
- Sievers, D.; von Kiedrowski, G. Self-replication of complementary nucleotide-based oligomers. Nature 1994, 369, 221–224. [Google Scholar] [CrossRef] [PubMed]
- Mansy, S.S.; Schrum, J.P.; Krishnamurthy, M.; Tobé, S.; Treco, D.A.; Szostak, J.W. Template-directed synthesis of a genetic polymer in a model protocell. Nature 2008, 454, 122–125. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Blain, C.; Zielinska, D.; Gryaznov, S.; Szostak, J. Fast and accurate nonenzymatic copying of an RNA-like synthetic genetic polymer. Proc. Natl. Acad. Sci. USA 2013, 110, 17732–17737. [Google Scholar] [CrossRef] [PubMed]
- Shim, J.L.; Lohrmann, R.; Orgel, L.E. Poly(U)-Directed Transamidation between Adenosine 5′-Phosphorimidazolide and 5′ Phosphoadenosine 2′(3′)-Glycine Ester. J. Am. Chem. Soc. 1974, 96, 5283–5284. [Google Scholar] [CrossRef] [PubMed]
- Nicolis, G.; Prigogine, I. Self-Organization in Nonequilibrium Systems: From Dissipative Structures to Order through Fluctuations; Wiley: New York, NY, USA, 1977. [Google Scholar]
- Eschenmoser, A. Chemistry of potentially prebiological natural products. Orig. Life Evol. Biosph. 1994, 24, 389–423. [Google Scholar] [CrossRef]
- Eschenmoser, A. Question 1: Commentary Referring to the Statement “The Origin of Life can be Traced Back to the Origin of Kinetic Control” and the Question “Do You Agree with this Statement; and How Would You Envisage the Prebiotic Evolutionary Bridge Between Thermodynamic and Kinetic Control?” Stated in Section 1.1. Orig. Life Evol. Biosph. 2007, 37, 309–314. [Google Scholar] [PubMed]
- Danger, G.; Plasson, R.; Pascal, R. Pathways for the formation and evolution of peptides in prebiotic environments. Chem. Soc. Rev. 2012, 41, 5416–5429. [Google Scholar] [CrossRef] [PubMed]
- Boiteau, L.; Pascal, R. Energy sources, self-organization, and the origin of life. Orig. Life Evol. Biosph. 2011, 41, 23–33. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, A.W. Phosphorus in prebiotic chemistry. Philos. Trans. R. Soc. Lond. Ser. B 2006, 361, 1743–1749. [Google Scholar] [CrossRef] [PubMed]
- Fernández-García, C.; Coggins, A.J.; Powner, M.W. A Chemist’s Perspective on the Role of Phosphorus at the Origins of Life. Life 2017, 7, 31. [Google Scholar] [CrossRef] [PubMed]
- Tordini, F.; Bencini, A.; Bruschi, M.; De Gioia, L.; Zampella, G.; Fantucci, P. Theoretical Study of Hydration of Cyanamide and Carbodiimide. J. Phys. Chem. A 2003, 107, 1188–1196. [Google Scholar] [CrossRef]
- Steinman, G.; Lemmon, R.M.; Calvin, M. Cyanamide: A possible key compound in chemical evolution. Proc. Natl. Acad. Sci. USA 1964, 52, 27–30. [Google Scholar] [CrossRef] [PubMed]
- Jones, M.E.; Spector, L.; Lipmann, F. Carbamyl phosphate, the carbamyl donor in enzymatic citrulline synthesis. J. Am. Chem. Soc. 1955, 77, 819–820. [Google Scholar] [CrossRef]
- Jones, M.E.; Lipmann, F. Chemical and enzymatic synthesis of carbamyl phosphate. Proc. Natl. Acad. Sci. USA 1960, 46, 1194–1205. [Google Scholar] [CrossRef] [PubMed]
- Halmann, M.; Lapidot, A.; Samuel, D. Kinetic and tracer studies of the reaction of carbamoyl phosphate in aqueous solution. J. Chem. Soc. 1962, 1944–1957. [Google Scholar] [CrossRef]
- Allen, C.M., Jr.; Jones, M.E. Decomposition of Carbamylphosphate in Aqueous Solutions. Biochemistry 1964, 3, 1238–1247. [Google Scholar] [CrossRef] [PubMed]
- Vogels, G.D.; Uffink, L.; Van der Drift, C. Cyanate decomposition catalyzed by certain divalent anions. Recl. Trav. Chim. Pays-Bas 1970, 89, 500–508. [Google Scholar] [CrossRef]
- Danger, G.; Charlot, S.; Boiteau, L.; Pascal, R. Activation of carboxyl group with cyanate: Peptide bond formation from dicarboxylic acids. Amino Acids 2012, 42, 2331–2341. [Google Scholar] [CrossRef] [PubMed]
- Saygin, Ö. Non-enzymatic photophosphorylation with visible-light—A possible mode of prebiotic ATP. Naturwissenschaften 1981, 68, 617–619. [Google Scholar] [CrossRef]
- Saygin, Ö. Non-enzymatic phosphorylation of acetate by carbamyl-phosphate—A model reaction for prebiotic activation of carboxyl groups. Orig. Life 1983, 13, 43–48. [Google Scholar] [CrossRef]
- Saygin, Ö. Photochemical carbamylphosphate formation and metal ion-catalysed transphosphorylations between carbamylphosphate and adenine nucleotides or carboxyl groups. Orig. Life 1984, 14, 131–137. [Google Scholar] [CrossRef]
- Hosseini, M.W.; Lehn, J.M. Supramolecular catalysis of phosphoryl transfer: Pyrophosphate synthesis from acetyl phosphate mediated by macrocyclic polyamines. J. Am. Chem. Soc. 1987, 109, 7047–7058. [Google Scholar] [CrossRef]
- Hosseini, M.W.; Lehn, J.M. Supramolecular catalysis of adenosine triphosphate synthesis in aqueous solution mediated by a macrocyclic polyamine and divalent metal cations. J. Chem. Soc. Chem. Commun. 1991, 451–453. [Google Scholar] [CrossRef]
- Costanzo, G.; Saladino, R.; Crestini, C.; Ciciriello, F.; Di Mauro, E. Nucleoside Phosphorylation by Phosphate Minerals. J. Biol. Chem. 2007, 282, 16729–16735. [Google Scholar] [CrossRef] [PubMed]
- Lohrmann, R.; Orgel, L.E. Urea-Inorganic Phosphate Mixtures as Prebiotic Phosphorylating Agents. Science 1971, 171, 490–494. [Google Scholar] [CrossRef] [PubMed]
- Reimann, R.; Zubay, G. Nucleoside phosphorylation: A feasible step in the prebiotic pathway to RNA. Orig. Life Evol. Biosph. 1999, 29, 229–247. [Google Scholar] [CrossRef] [PubMed]
- Albertsen, A.; Duffy, C.; Sutherland, J.; Monnard, P. Self-Assembly of Phosphate Amphiphiles in Mixtures of Prebiotically Plausible Surfactants. Astrobiology 2014, 14, 462–472. [Google Scholar] [CrossRef] [PubMed]
- Orgel, L.E.; Lohrmann, R. Prebiotic Chemistry and Nucleic Acid replication. Acc. Chem. Res. 1974, 7, 368–377. [Google Scholar] [CrossRef]
- Osterberg, R.; Orgel, L.E. Polyphosphate and Trimetaphosphate Formation under Potentially Prebiotic Conditions. J. Mol. Evol. 1972, 1, 241–248. [Google Scholar] [CrossRef] [PubMed]
- Fiore, M.; Madanamoothoo, W.; Berlioz-Barbier, A.; Maniti, O.; Girard-Egrot, A.; Buchet, R.; Strazewski, P. Giant vesicles from rehydrated crude mixtures containing unexpected mixtures of amphiphiles formed under plausibly prebiotic conditions. Org. Biomol. Chem. 2017, 15, 4231–4240. [Google Scholar] [CrossRef] [PubMed]
- Shaw, W.H.R.; Bordeaux, J.J. The Decomposition of Urea in Aqueous Media. J. Am. Chem. Soc. 1955, 77, 4729–4733. [Google Scholar] [CrossRef]
- Orgel, L.E. Prebiotic chemistry and the origin of the RNA world. Crit. Rev. Biochem. Mol. Biol. 2004, 39, 99–123. [Google Scholar] [PubMed]
- Fahrenbach, A.C.; Giurgiu, C.; Tam, C.P.; Li, L.; Hongo, Y.; Aono, M.; Szostak, J.W. Common and Potentially Prebiotic Origin for Precursors of Nucleotide Synthesis and Activation. J. Am. Chem. Soc. 2017, 139, 8780–8783. [Google Scholar] [CrossRef] [PubMed]
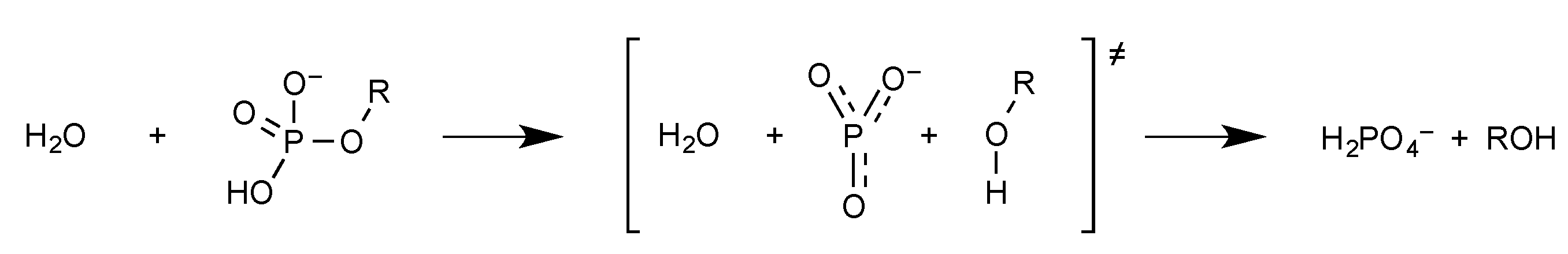
| Reagent | Product(s) | ΔG°’ kJ mol−1 | Reference |
|---|---|---|---|
| PPi | 2 Pi | −19 | [33] |
| ATP | AMP + PPi | −32.2 | [33] |
| ATP | ADP + Pi | −30.5 | [33] |
| Acetyl phosphate | AcOH + Pi | −43.1 | [33] |
| Carbamyl phosphate | CO2 + NH3 + Pi | ca. −51 1 | [33] |
| Aminoacyl phosphate | Amino acid + Pi | ca. −50 | [91] |
| Aminoacyl adenylate | Amino acid + AMP | −70 | [44] |
| Phosphoenol pyruvate | Pyruvate + Pi | −62 | [33] |
| Reagent | Product(s) | ΔG°’ kJ mol−1 | Reference |
|---|---|---|---|
| HNCO | CO2 + NH3 | −54 | [91] |
| Urea | CO2 + NH3 | −28 | [91] |
| Cyanamide | Isourea | −83 | [94] |
| Carbodiimide | Isourea | −97 | [94] |
| Acetic anhydride | Acetic acid | −91 | [33] |
| NCA | Amino acid + CO2 | −60 | [46] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Z.; Rossi, J.-C.; Pascal, R. How Prebiotic Chemistry and Early Life Chose Phosphate. Life 2019, 9, 26. https://doi.org/10.3390/life9010026
Liu Z, Rossi J-C, Pascal R. How Prebiotic Chemistry and Early Life Chose Phosphate. Life. 2019; 9(1):26. https://doi.org/10.3390/life9010026
Chicago/Turabian StyleLiu, Ziwei, Jean-Christophe Rossi, and Robert Pascal. 2019. "How Prebiotic Chemistry and Early Life Chose Phosphate" Life 9, no. 1: 26. https://doi.org/10.3390/life9010026
APA StyleLiu, Z., Rossi, J.-C., & Pascal, R. (2019). How Prebiotic Chemistry and Early Life Chose Phosphate. Life, 9(1), 26. https://doi.org/10.3390/life9010026





