The Nitrogen Heterocycle Content of Meteorites and Their Significance for the Origin of Life
Abstract
1. Introduction
2. Inventory of Meteoritic N-Heterocycles
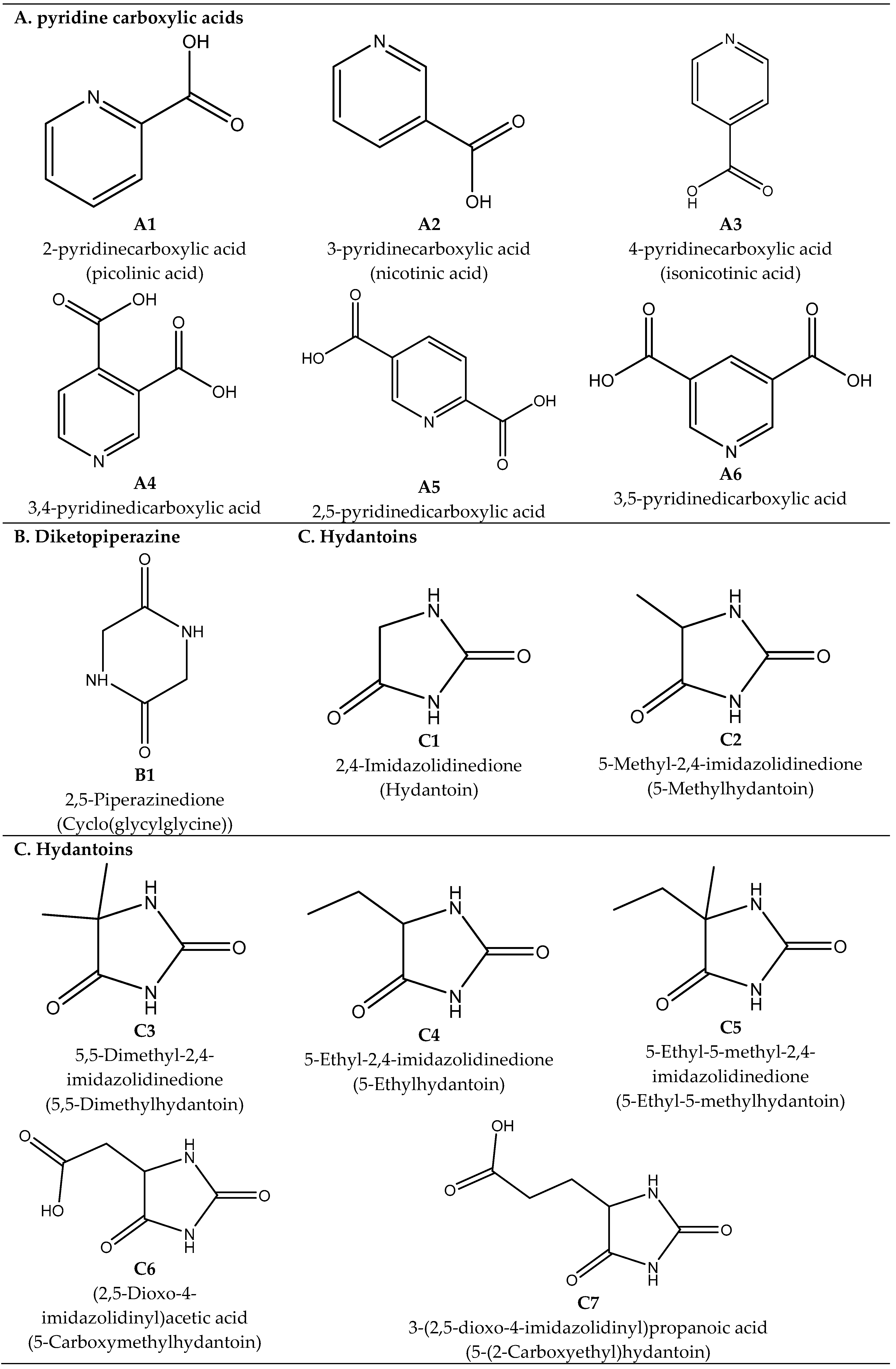
2.1. Pyridine Carboxylic Acids
Synthesis of Pyridine Carboxylic Acids
2.2. Diketopiperazine and Hydantoins
Synthesis of Diketopiperazine and Hydantoins
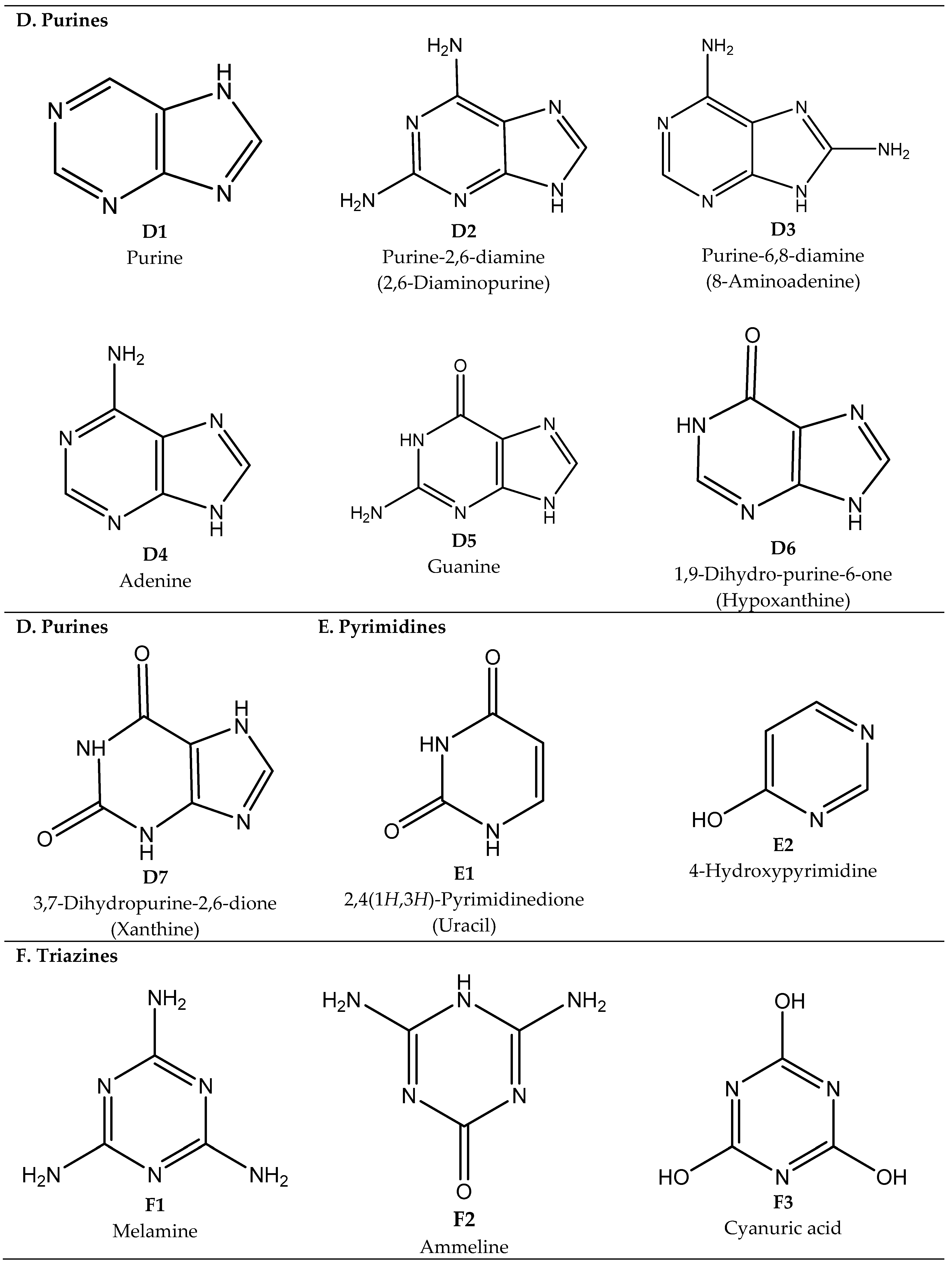
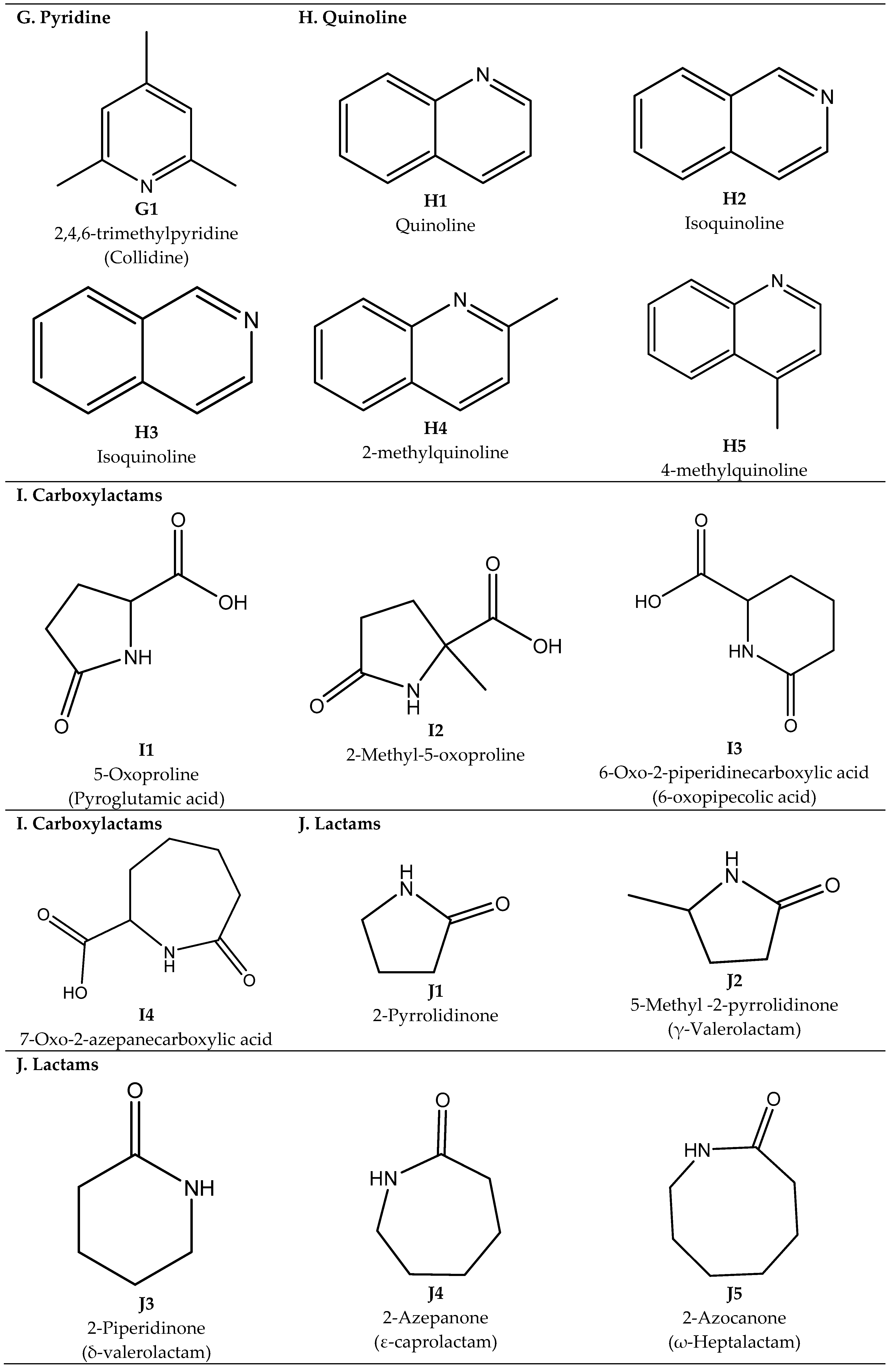
2.3. Purines, Pyrimidines, Triazines, Pyridines, and Quinolines
Synthesis of Purines, Pyrimidines, Pyridines, and Quinolines
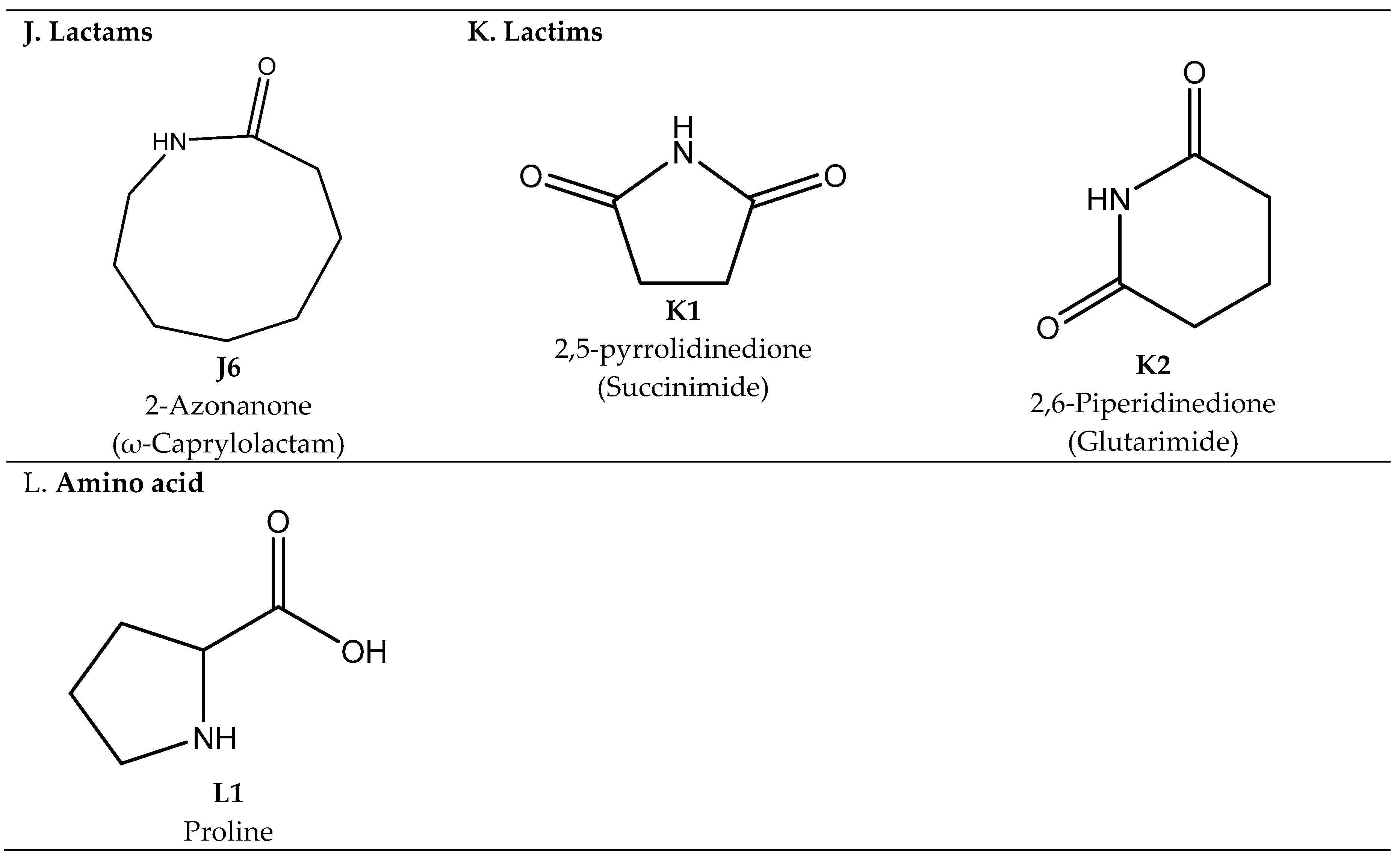
2.4. Carboxylactams, Lactams, and Lactims
Synthesis of Carboxylactams, Lactams, and Lactims
2.5. Amino acid Proline
Synthesis of the Amino Acid Proline
3. Astrophysical and Astrobiological Implications of Meteoritic N-Heterocycles
4. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- McSween, H.Y., Jr.; Huss, G.R. Cosmochemistry; McSween, H.Y., Jr., Huss, G.R., Eds.; Cambridge University Press: Cambridge, UK, 2015. [Google Scholar]
- Bischoff, A.; Vogel, N.; Roszjar, J. The Rumuruti chondrite group. Chem. Erde Geochem. 2011, 71, 101–133. [Google Scholar] [CrossRef]
- Krot, A.N.; Keil, K.; Scott, E.R.D.; Goodrich, C.A.; Weisberg, M.K. Classification of meteorites and their genetic relationships. In Meteorites and Cosmochemical Processes; Davis, A.M., Ed.; Volume 1 of Treatise on Geochemistry; Elsevier: New York, NY, USA, 2014; pp. 1–63. [Google Scholar]
- Lodders, K. Solar system abundances and condensation temperatures of the elements. Astrophys. J. 2003, 591, 1220–1247. [Google Scholar] [CrossRef]
- Choe, W.H.; Huber, H.; Rubin, A.E.; Kallemeyn, G.W.; Wasson, J.T. Compositions and taxonomy of 15 unusual carbonaceous chondrites. Meteorit. Planet. Sci. 2010, 45, 531–554. [Google Scholar] [CrossRef]
- McSween, H.Y. Alteration in CM carbonaceous chondrites inferred from modal and chemical variations in matrix. Geochim. Cosmochim. Acta 1979, 43, 1761–1770. [Google Scholar] [CrossRef]
- Browning, L.B.; McSween, H.Y.; Zolensky, M.E. Correlated alteration effects in CM carbonaceous chondrites. Geochim. Cosmochim. Acta 1996, 60, 2621–2633. [Google Scholar] [CrossRef]
- Palmer, E.E.; Lauretta, D.S. Aqueous alteration of kamacite in CM chondrites. Meteorit. Planet. Sci. 2011, 46, 1587–1607. [Google Scholar] [CrossRef]
- Vinogradoff, V.; Le Guillou, C.; Bernard, S.; Binet, L.; Cartigny, P.; Brearley, A.J.; Remusat, L. Paris vs. Murchison: Impact of hydrothermal alteration on organic matter in CM chondrites. Geochim. Cosmochim. Acta 2017, 212, 234–252. [Google Scholar] [CrossRef]
- Alexander, C.M.O.D.; Nittler, L.R.; Davidson, J.; Ciesla, F.J. Measuring the level of interstellar inheritance in the solar protoplanetary disk. Meteorit. Planet. Sci. 2017, 52, 1797–1821. [Google Scholar] [CrossRef]
- Smith, J.W.; Kaplan, I.R. Endogenous carbon in carbonaceous meteorites. Science 1970, 167, 1367–1370. [Google Scholar] [CrossRef] [PubMed]
- Anders, E.; Zinner, E. Interstellar grains in primitive meteorites—Diamond, silicon carbide, and graphite. Meteoritics 1993, 28, 490–514. [Google Scholar] [CrossRef]
- Benedix, G.K.; Leshin, L.A.; Farquhar, J.; Jackson, T.; Thiemens, M.H. Carbonates in CM2 chondrites: Constraints on alteration conditions from oxygen isotopic compositions and petrographic observations. Geochim. Cosmochim. Acta 2003, 67, 1577–1588. [Google Scholar] [CrossRef]
- Gardinier, A.; Derenne, S.; Robert, F.; Behar, F.; Largeau, C.; Maquet, J. Solid state CP/MAS 13C NMR of the insoluble organic matter of the Orgueil and Murchison meteorites: Quantitative study. Earth Planet. Sci. Lett. 2000, 184, 9–21. [Google Scholar] [CrossRef]
- Cody, G.D.; Alexander, C.M.O.; Tera, F. Solid-state (1H and 13C) nuclear magnetic resonance spectroscopy of insoluble organic residue in the Murchison meteorite: A self-consistent quantitative analysis. Geochim. Cosmochim. Acta 2002, 66, 1851–1865. [Google Scholar] [CrossRef]
- Cody, G.D.; Alexander, C.M.O. NMR studies of chemical structural variation of insoluble organic matter from different carbonaceous chondrites groups. Geochim. Cosmochim. Acta 2005, 69, 1085–1097. [Google Scholar] [CrossRef]
- Cronin, J.R.; Chang, S. Organic matter in meteorites: Molecular and isotopic analyses of the Murchison meteorites. In The Chemistry of Life’s Origin; Greenberg, J.M., Mendoza-Gomez, C.X., Pirronello, V., Eds.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 1993; pp. 209–258. [Google Scholar]
- Martins, Z.; Sephton, M.A. Extraterrestrial amino acids. In Amino Acids, Peptides and Proteins in Organic Chemistry; Hughes, A.B., Ed.; Wiley: Hoboken, NJ, USA, 2009; pp. 3–42. [Google Scholar]
- Martins, Z. Organic chemistry of carbonaceous meteorites. Elements 2011, 7, 35–40. [Google Scholar] [CrossRef]
- Joule, J.A.; Mills, K. Heterocyclic Chemistry; John Wiley & Sons: Hoboken, NJ, USA, 2013. [Google Scholar]
- Schidlowski, M. A 3800-million-year isotopic record of life from carbon in sedimentary rocks. Nature 1988, 333, 313–318. [Google Scholar] [CrossRef]
- Rosing, M.T. 13C-Depleted carbon microparticles in >3700-Ma sea-floor sedimentary rocks from west Greenland. Science 1999, 283, 674–676. [Google Scholar] [CrossRef] [PubMed]
- Furnes, H.; Banerjee, N.R.; Muehlenbachs, K.; Staudigel, H.; de Wit, M. Early Life Recorded in Archean Pillow Lavas. Science 2004, 304, 578–581. [Google Scholar] [CrossRef] [PubMed]
- Rosing, M.T.; Frei, R. U-rich Archaean sea-floor sediments from Greenland—Indications of >3700 Ma oxygenic photosynthesis. Earth Planet. Sci. Lett. 2004, 217, 237–244. [Google Scholar] [CrossRef]
- Westall, F.; de Vries, S.T.; Nijman, W.; Rouchon, V.; Orberger, B.; Pearson, V.; Watson, J.; Verdosvsky, A.; Wright, I.; Rouzaud, J.N.; et al. The 3.466 Ga “Kitty’s Gap Chert,” an Early Archaean microbial ecosystem. In Processes on the Early Earth; Reimold, W.U., Gibson, R.L., Eds.; Geological Society America: Boulder, CO, USA, 2006; pp. 105–131. [Google Scholar]
- Westall, F.; Cavalazzi, B.; Lemelle, L.; Marrocchi, Y.; Rouzaud, J.-N.; Simionovici, A.; Salomé, M.; Mostefaoui, S.; Andreazza, C.; Foucher, F.; et al. Implications of in situ calcification for photosynthesis in a ~3.3-Ga-old microbial biofilm from the Barberton greenstone belt, South Africa. Earth Planet. Sci. Lett. 2011, 310, 468–479. [Google Scholar] [CrossRef]
- Westall, F.; Foucher, F.; Cavalazzi, B.; de Vries, S.T.; Nijman, W.; Pearson, V.; Watson, J.; Verchovsky, A.; Wright, I.; Rouzaud, J.-N.; et al. Early life on Earth and Mars: A case study from ~3.5 Ga-old rocks from the Pilbara, Australia. Planet. Space Sci. 2011, 59, 1093–1106. [Google Scholar] [CrossRef]
- Sugitani, K.; Grey, K.; Nagaoka, T.; Mimura, K.; Walter, M.R. Taxonomy and biogenicity of Archaean spheroidal microfossils (ca. 3.0 Ga) from the Mount Goldsworthy-Mount Grant area in the northeastern Pilbara Craton, Western Australia. Precambrian Res. 2009, 173, 50–59. [Google Scholar] [CrossRef]
- Javaux, E.J.; Marshall, C.P.; Bekker, A. Organic-walled microfossils in 3.2-billion-year-old shallow-marine siliciclastic deposits. Nature 2010, 463, 934–938. [Google Scholar] [CrossRef] [PubMed]
- Wacey, D.; McLoughlin, N.; Whitehouse, M.J.; Kilburn, M.R. Two coexisting sulfur metabolisms in a ca. 3400 Ma sandstone. Geology 2010, 38, 1115–1118. [Google Scholar] [CrossRef]
- Chyba, C.; Sagan, C. Endogenous production, exogenous delivery and impact-shock synthesis of organic molecules: An inventory for the origins of life. Nature 1992, 355, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Schopf, J.W. Microfossils of the Early Archean: New evidence of the antiquity of life. Science 1993, 260, 640–646. [Google Scholar] [CrossRef] [PubMed]
- Yuen, G.; Blair, N.; Des Marais, D.J.; Chang, S. Carbon isotope composition of low molecular weight hydrocarbons and monocarboxylic acids from Murchison meteorite. Nature 1984, 307, 252–254. [Google Scholar] [CrossRef] [PubMed]
- Krishnamurthy, R.V.; Epstein, S.; Cronin, J.R.; Pizzarello, S.; Yuen, G.U. Isotopic and molecular analyses of hydrocarbons and monocarboxylic acids of the Murchison meteorite. Geochim. Cosmochim. Acta 1992, 56, 4045–4058. [Google Scholar] [CrossRef]
- Cronin, J.R.; Pizzarello, S.; Epstein, S.; Krishnamurthy, R.V. Molecular and isotopic analyses of the hydroxy acids, dicarboxylic acids, and hydroxydicarboxylic acids of the Murchison meteorite. Geochim. Cosmochim. Acta 1993, 57, 4745–4752. [Google Scholar] [CrossRef]
- Tielens, A.G.G.M. Surface chemistry of deuterated molecules. Astron. Astrophys. 1983, 119, 177–184. [Google Scholar]
- Yang, J.; Epstein, S. Interstellar organic matter in meteorites. Geochim. Cosmochim. Acta 1983, 47, 2199–2216. [Google Scholar] [CrossRef]
- Terzieva, R.; Herbst, E. The possibility of nitrogen isotopic fractionation in interstellar clouds. Mon. Not. R. Astron. Soc. 2000, 317, 563–568. [Google Scholar] [CrossRef]
- Sandford, S.A.; Bernstein, M.P.; Dworkin, J.P. Assessment of the interstellar processes leading to deuterium enrichment in meteoritic organics. Meteorit. Planet. Sci. 2001, 36, 1117–1133. [Google Scholar] [CrossRef]
- Robert, F. The D/H ratio in chondrites. Space Sci. Rev. 2003, 106, 87–101. [Google Scholar] [CrossRef]
- Aléon, J.; Robert, F. Interstellar chemistry recorded by nitrogen isotopes in Solar System organic matter. Icarus 2004, 167, 424–430. [Google Scholar] [CrossRef]
- Grimm, R.E.; McSween, H.Y. Water and the thermal evolution of carbonaceous chondrite parent bodies. Icarus 1989, 82, 244–280. [Google Scholar] [CrossRef]
- Pizzarello, S.; Huang, Y.; Becker, L.; Poreda, R.J.; Nieman, R.A.; Cooper, G.; Williams, M. The organic content of the Tagish Lake meteorite. Science 2001, 293, 2236–2239. [Google Scholar] [CrossRef] [PubMed]
- Pizzarello, S.; Huang, Y.; Fuller, M. The carbon isotopic distribution of Murchison amino acids. Geochim. Cosmochim. Acta 2004, 68, 4963–4969. [Google Scholar] [CrossRef]
- Pizzarello, S.; Huang, Y. The deuterium enrichment of individual amino acids in carbonaceous meteorites: A case for the presolar distribution of biomolecule precursors. Geochim. Cosmochim. Acta 2005, 69, 599–605. [Google Scholar] [CrossRef]
- Smith, K.E.; Callahan, M.P.; Gerakines, P.A.; Dworkin, J.P.; House, C.H. Investigation of pyridine carboxylic acids in CM2 carbonaceous chondrites: Potential precursor molecules for ancient coenzymes. Geochim. Cosmochim. Acta 2014, 136, 1–12. [Google Scholar] [CrossRef]
- Stoks, P.G.; Schwartz, A.W. Basic nitrogen-heterocyclic compounds in the Murchison meteorite. Geochim. Cosmochim. Acta 1982, 46, 309–315. [Google Scholar] [CrossRef]
- Shimoyama, A.; Ogasawara, R. Dipeptides and diketopiperazines in the Yamato-791198 and Murchison carbonaceous chondrites. Orig. Life Evol. Biosph. 2002, 32, 165–179. [Google Scholar] [CrossRef] [PubMed]
- Cooper, G.W.; Cronin, J.R. Linear and cyclic aliphatic carboxamides of the Murchison meteorite: Hydrolyzable derivatives of amino acids and other carboxylic acids. Geochim. Cosmochim. Acta 1995, 59, 1003–1015. [Google Scholar] [CrossRef]
- Bujdak, J.; Rode, B.M. Silica, alumina, and clay-catalyzed alanine peptide bond formation. J. Mol. Evol. 1997, 45, 457–466. [Google Scholar] [CrossRef]
- Bujdak, J.; Rode, B.M. Silica, alumina and clay catalyzed peptide bond formation: Enhanced efficiency of alumina catalyst. Orig. Life Evol. Biosph. 1999, 29, 451–461. [Google Scholar] [CrossRef] [PubMed]
- Lahav, N.; White, D.; Chang, S. Peptide formation in the prebiotic era: Thermal condensation of glycine in fluctuating clay environments. Science 1978, 201, 67–69. [Google Scholar] [CrossRef] [PubMed]
- Lawless, J.G.; Levi, N. The role of metal ions in chemical evolution: Polymerization of alanine and glycine in a cation-exchanged clay environment. J. Mol. Evol. 1979, 13, 281–286. [Google Scholar] [CrossRef] [PubMed]
- de Marcellus, P.; Bertrand, M.; Nuevo, M.; Westall, F.; Le Sergeant d’Hendecourt, L. Prebiotic significance of extraterrestrial ice photochemistry: Detection of hydantoin in organic residues. Astrobiology 2011, 11, 847–854. [Google Scholar] [CrossRef] [PubMed]
- Hayatsu, R. Orgueil meteorite: Organic nitrogen contents. Science 1964, 146, 1291–1293. [Google Scholar] [CrossRef] [PubMed]
- Hayatsu, R.; Studier, M.H.; Oda, A.; Fuse, K.; Anders, E. Origin of organic matter in early solar system—II. Nitrogen compounds. Geochim. Cosmochim. Acta 1968, 32, 175–190. [Google Scholar] [CrossRef]
- Hayatsu, R.; Studier, M.H.; Moore, L.P.; Anders, E. Purines and triazines in the Murchison meteorite. Geochim. Cosmochim. Acta 1975, 39, 471–488. [Google Scholar] [CrossRef]
- Folsome, C.E.; Lawless, J.; Romiez, M.; Ponnamperuma, C. Heterocyclic compounds indigenous to the Murchison meteorite. Nature 1971, 232, 108–109. [Google Scholar] [CrossRef] [PubMed]
- Folsome, C.E.; Lawless, J.; Romiez, M.; Ponnamperuma, C. Heterocyclic compounds recovered from carbonaceous chondrite. Geochim. Cosmochim. Acta 1973, 37, 455–465. [Google Scholar] [CrossRef]
- Lawless, J.G.; Folsome, C.E.; Kvenvolden, K.A. Organic matter in meteorites. Sci. Am. 1972, 226, 38–46. [Google Scholar] [CrossRef]
- Van der Velden, W.; Schwartz, A.W. Search for purines and pyrimidines in Murchison meteorite. Geochim. Cosmochim. Acta 1977, 41, 961–968. [Google Scholar] [CrossRef]
- Stoks, P.G.; Schwartz, A.W. Uracil in carbonaceous meteorites. Nature 1979, 282, 709–710. [Google Scholar] [CrossRef]
- Stoks, P.G.; Schwartz, A.W. Nitrogen-heterocyclic compounds in meteorites: Significance and mechanisms of formation. Geochim. Cosmochim. Acta 1981, 45, 563–569. [Google Scholar] [CrossRef]
- Shimoyama, A.; Hagishita, S.; Harada, K. Search for nucleic acid bases in carbonaceous chondrites from Antarctica. Geochem. J. 1990, 24, 343–348. [Google Scholar] [CrossRef]
- Martins, Z.; Botta, O.; Fogel, M.L.; Sephton, M.A.; Glavin, D.P.; Watson, J.S.; Dworkin, J.P.; Schwartz, A.W.; Ehrenfreund, P. Extraterrestrial nucleobases in the Murchison meteorite. Earth Planet. Sci. Lett. 2008, 270, 130–136. [Google Scholar] [CrossRef]
- Callahan, M.P.; Smith, K.E.; Cleaves, H.J.; Ruzicka, J.; Stern, J.C.; Glavin, D.P.; House, C.H.; Dworkin, J.P. Carbonaceous meteorites contain a wide range of extraterrestrial nucleobases. Proc. Natl. Acad. Sci. USA 2011, 108, 13995–13998. [Google Scholar] [CrossRef] [PubMed]
- Simon, M.N.; Simon, M. Search for interstellar acrylonitrile, pyrimidine, and pyridine. Astrophys. J. 1973, 184, 757–762. [Google Scholar] [CrossRef]
- Kuan, Y.-J.; Charnley, S.B.; Huang, H.-C.; Kisiel, Z.; Ehrenfreund, P.; Tseng, W.-L.; Yan, C.-H. Searches for interstellar molecules of potential prebiotic importance. Adv. Space Res. 2004, 33, 31–39. [Google Scholar] [CrossRef]
- Charnley, S.B.; Kuan, Y.-J.; Huang, H.-C.; Botta, O.; Butner, H.M.; Cox, N.; Despois, D.; Ehrenfreund, P.; Kisiel, Z.; Lee, Y.-Y.; et al. Astronomical searches for nitrogen heterocycles. Adv. Space Res. 2005, 36, 137–145. [Google Scholar] [CrossRef]
- Brünken, S.; McCarthy, M.C.; Thaddeus, P.; Godfrey, P.D.; Brown, R.D. Improved line frequencies for the nucleic acid base uracil for a radioastronomical search. Astron. Astrophys. 2006, 459, 317–320. [Google Scholar] [CrossRef]
- Peeters, Z.; Botta, O.; Charnley, S.B.; Ruiterkamp, R.; Ehrenfreund, P. The astrobiology of nucleobases. Astrophys. J. 2003, 593, L129–L132. [Google Scholar] [CrossRef]
- Peeters, Z.; Botta, O.; Charnley, S.B.; Kisiel, Z.; Kuan, Y.-J.; Ehrenfreund, P. Formation and photostability of N-heterocycles in space. I. The effect of nitrogen on the photostability of small aromatic molecules. Astron. Astrophys. 2005, 433, 583–590. [Google Scholar] [CrossRef]
- Nuevo, M.; Milam, S.N.; Sandford, S.A.; Elsila, J.E.; Dworkin, J.P. Formation of uracil from the ultraviolet photo-irradiation of pyrimidine in pure H2O ices. Astrobiology 2009, 9, 683–695. [Google Scholar] [CrossRef] [PubMed]
- Nuevo, M.; Materese, C.K.; Sandford, S.A. The photochemistry of pyrimidine in realistic astrophysical ices and the production of nucleobases. Astrophys. J. 2014, 793, 125. [Google Scholar] [CrossRef]
- Nuevo, M.; Milam, S.N.; Sandford, S.A. Nucleobases and prebiotic molecules in organic residues produced from the ultraviolet photo-irradiation of pyrimidine in NH3 and H2O + NH3 ices. Astrobiology 2012, 12, 295–314. [Google Scholar] [CrossRef] [PubMed]
- Materese, C.K.; Nuevo, M.; Bera, P.P.; Lee, T.J.; Sandford, S.A. Thymine and other prebiotic molecules produced from the ultraviolet photo-irradiation of pyrimidine in simple astrophysical ice analogs. Astrobiology 2013, 13, 948–962. [Google Scholar] [CrossRef] [PubMed]
- Materese, C.K.; Nuevo, M.; Sandford, S.A. The formation of nucleobases from the Ultraviolet photoirradiation of purine in simple astrophysical ice analogues. Astrobiology 2017, 17, 761–770. [Google Scholar] [CrossRef] [PubMed]
- Bera, P.P.; Stein, T.; Head-Gordon, M.; Lee, T.J. Mechanisms of the formation of adenine, guanine, and their analogues in UV-irradiated mixed NH3:H2O molecular ices containing purine. Astrobiology 2017, 17, 771–785. [Google Scholar] [CrossRef] [PubMed]
- Oró, J. Synthesis of adenine from ammonium cyanide. Biochem. Biophys. Res. Commun. 1960, 2, 407–412. [Google Scholar] [CrossRef]
- Oró, J. Mechanism of synthesis of adenine from hydrogen cyanide under possible primitive Earth conditions. Nature 1961, 191, 1193–1194. [Google Scholar] [CrossRef] [PubMed]
- Oró, J.; Kimball, A.P. Synthesis of purines under possible primitive Earth conditions I. Adenine from hydrogen cyanide. Arch. Biochem. Biophys. 1961, 94, 217–227. [Google Scholar] [CrossRef]
- Sanchez, R.A.; Ferris, J.P.; Orgel, L.E. Studies in prebiotic synthesis. II. Synthesis of purine precursors and amino acids from aqueous hydrogen cyanide. J. Mol. Biol. 1967, 30, 223–253. [Google Scholar] [PubMed]
- Ferris, J.P.; Joshi, P.C.; Edelson, E.H.; Lawless, J.G. HCN: A plausible source of purines, pyrimidines and amino acids on the primitive Earth. J. Mol. Evol. 1978, 11, 293–311. [Google Scholar] [CrossRef] [PubMed]
- Voet, A.B.; Schwartz, A.W. Uracil synthesis via HCN oligomerization. Orig. Life 1982, 12, 45–49. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, A.W.; Bakker, C.G. Was adenine the first purine? Science 1989, 245, 1102–1104. [Google Scholar] [CrossRef] [PubMed]
- Pesce-Rodriguez, R.A.; Liebman, S.A.; Matthews, C.N. Characterization of hydrogen cyanide polymers and the Murchison meteorite: Implications for prebiotic and extraterrestrial chemistry. Orig. Life Evol. Biosph. 1994, 24, 123–124. [Google Scholar]
- Minard, R.D.; Hatcher, P.G.; Gourley, R.C.; Matthews, C.N. Structural investigations of hydrogen cyanide polymers: New insights using TMAH thermochemolysis/GC-MS. Orig. Life Evol. Biosph. 1998, 28, 461–473. [Google Scholar] [CrossRef] [PubMed]
- Levy, M.; Miller, S.L.; Oró, J. Production of guanine from NH4CN polymerizations. J. Mol. Evol. 1999, 49, 165–168. [Google Scholar] [CrossRef] [PubMed]
- Miyakawa, S.; Cleaves, H.J.; Miller, S.L. The cold origin of life: B. Implications based on pyrimidines and purines produced from frozen ammonium cyanide solutions. Orig. Life Evol. Biosph. 2002, 32, 209–218. [Google Scholar] [CrossRef] [PubMed]
- Pearce, B.K.D.; Pudritz, R.E. Meteorites and the RNA World: A thermodynamic model of nucleobase synthesis within planetesimals. Astrobiology 2016, 16, 853–872. [Google Scholar] [CrossRef] [PubMed]
- Materese, C.K.; Nuevo, M.; Sandford, S.A. N- and O-heterocycles produced from the irradiation of benzene and naphthalene in H2O/NH3-containing ices. Astrophys. J. 2015, 800, 116. [Google Scholar] [CrossRef]
- Kvenvolden, K.; Lawless, J.; Pering, K.; Peterson, E.; Flores, J.; Ponnamperuma, C. Evidence for extraterrestrial amino acids and hydrocarbons in the Murchison meteorite. Nature 1970, 228, 923–926. [Google Scholar] [CrossRef] [PubMed]
- Cronin, J.R.; Moore, C.B. Amino acid analyses of the Murchison, Murray, and Allende carbonaceous chondrites. Science 1971, 172, 1327–1329. [Google Scholar] [CrossRef] [PubMed]
- Kvenvolden, K.A.; Lawless, J.G.; Ponnamperuma, C. Nonprotein amino acids in the Murchison meteorite. Proc. Natl. Acad. Sci. USA 1971, 68, 486–490. [Google Scholar] [CrossRef] [PubMed]
- Cronin, J.R. Acid-labile amino acid precursors in the Murchison meteorite. II. A search for peptides and amino acyl amides. Orig. Life 1976, 7, 343–348. [Google Scholar] [CrossRef] [PubMed]
- Engel, M.H.; Nagy, B. Distribution and enantiomeric composition of amino acids in the Murchison meteorite. Nature 1982, 296, 837–840. [Google Scholar] [CrossRef]
- Engel, M.H.; Macko, S.A. Isotopic evidence for extraterrestrial non-racemic amino acids in the Murchison meteorite. Nature 1997, 389, 265–268. [Google Scholar] [CrossRef] [PubMed]
- Bernstein, M.P.; Dworkin, J.P.; Sandford, S.A.; Cooper, G.W.; Allamandola, L.J. Racemic amino acids from the ultraviolet photolysis of interstellar ice analogues. Nature 2002, 416, 401–403. [Google Scholar] [CrossRef] [PubMed]
- Muñoz Caro, G.M.; Meierhenrich, U.J.; Schutte, W.A.; Barbier, B.; Arcones Segovia, A.; Rosenbauer, H.; Thiemann, W.H.-P.; Brack, A.; Greenberg, J.M. Amino acids from ultraviolet irradiation of interstellar ice analogues. Nature 2002, 416, 403–406. [Google Scholar] [CrossRef] [PubMed]
- Woon, D.E. Pathways to glycine and other amino acids in ultraviolet-irradiated astrophysical ices determined via quantum chemical modeling. Astrophys. J. 2002, 571, L177–L180. [Google Scholar] [CrossRef]
- Elsila, J.E.; Dworkin, J.P.; Bernstein, M.P.; Martin, M.P.; Sandford, S.A. Mechanisms of amino acid formation in interstellar ice analogs. Astrophys. J. 2007, 660, 911–918. [Google Scholar] [CrossRef]
- Förstel, M.; Bergantini, A.; Maksyutenko, P.; Góbi, S.; Kaiser, R.I. Formation of methylamine and ethylamine in extraterrestrial ices and their role as fundamental building blocks of proteinogenic α-amino acids. Astrophys. J. 2017, 845, 1–12. [Google Scholar] [CrossRef]
- Fuller, W.D.; Sanchez, R.A.; Orgel, L.E. Studies in prebiotic synthesis VI. Synthesis of purine nucleosides. J. Mol. Biol. 1972, 67, 25–33. [Google Scholar] [CrossRef]
- Orgel, L.E. Prebiotic chemistry and the origin of the RNA world. Crit. Rev. Biochem. Mol. Biol. 2004, 39, 99–123. [Google Scholar] [PubMed]
| Murchison | Murray | |||
|---|---|---|---|---|
| δD | δ13C | δD | δ13C | |
| Nicotinic acid | +129 ± 1 | +20.3 ± 1.7 | - | - |
| Nicotinic methyl homologue | - | +20.3 ± 1.2 | +621 ± 43 | - |
| WIS 91600 | DOM 03183 | DOM 08003 | ALH 85013 | EET 96016 | LAP 02333 | LAP 02336 | LEW 85311 | |
|---|---|---|---|---|---|---|---|---|
| Picolinic Acid | 25.1 ± 2 | 70.2 ± 7 | 482.2 ± 48 | 98.8 ± 10 | 322.0 ± 32 | 197.1 ± 20 | 318.4 ± 32 | 510.7 ± 51 |
| Nicotinic Acid | 96.3 ± 10 | 121.9 ± 12 | 221.0 ± 22 | 139.6 ± 14 | 265.1 ± 26 | 246.8 ± 25 | 332.1 ± 33 | 571.8 ± 57 |
| Isonicotinic Acid | 42.0 ± 42 | 70.8 ± 7 | 153.7 ± 15 | 67.4 ± 7 | 116.7 ± 12 | 161.5 ± 16 | 256.9 ± 26 | 294.1 ± 29 |
| Yamato-791198 | Murchison | |
|---|---|---|
| Cyclo(glycylglycine) | 2.1 | 2.6 |
| Hydantoin | 6.5 | 7.3 |
| 5-Methylhydantoin | 5.5 | 11.9 |
| 5,5-Dimethylhydantoin | 5.6 | 9.0 |
| 5-Ethylhydantoin | 1.0 | 1.5 |
| 5-Ethyl-5-methylhydantoin | 3.4 | 6.7 |
| 5-Carboxymethylhydantoin | n.d. | 0.9 |
| 5-(2-Carboxyethyl)hydantoin | n.d. | 1.4 |
| Orgueil | SCO 06043 | MET 01070 | GRO 95577 | ALH 83100 | Murchison | LEW 90500 | LON 94102 | GRA 95229 | EET 92042 | QUE 99177 | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Guanine | 20 | (2) | 29 | <2 † | 21 | 56 | 167 | 244 | 4 | <2 † | <2 † |
| Hypoxanthine | (5) | (4) | <3 † | <3 † | 4 | 26 | 23 | 94 | (4) | <3 | <3 † |
| Xanthine | <10 † | <10 † | <10 † | <10 † | (4) | 60 | 22 | 77 | <10 † | <10 † | <10 † |
| Adenine | 7 | 4 | 5 | <0.5 | 1 | 5 | 10 | 30 | 21 | 5 | 11 |
| Purine | 5 | <1 † | <1 † | <1 † | <0.1 † | 3 | 1 | 6 | 9 | (4) | 7 |
| 2,6-Diamonopurine | <2 † | <2 † | <2 † | <2 | <0.2 † | + | <0.2 † | 5 | <2 † | <2 † | <2 † |
| Uracil | 27 | n.d. | n.d. | n.d. | n.d | 33 | n.d. | n.d. | n.d. | n.d. | n.d. |
| Murchison | |
|---|---|
| Uracil | +44.5 ± 2.3 |
| Xanthine | +37.7 ± 1.6 |
© 2018 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martins, Z. The Nitrogen Heterocycle Content of Meteorites and Their Significance for the Origin of Life. Life 2018, 8, 28. https://doi.org/10.3390/life8030028
Martins Z. The Nitrogen Heterocycle Content of Meteorites and Their Significance for the Origin of Life. Life. 2018; 8(3):28. https://doi.org/10.3390/life8030028
Chicago/Turabian StyleMartins, Zita. 2018. "The Nitrogen Heterocycle Content of Meteorites and Their Significance for the Origin of Life" Life 8, no. 3: 28. https://doi.org/10.3390/life8030028
APA StyleMartins, Z. (2018). The Nitrogen Heterocycle Content of Meteorites and Their Significance for the Origin of Life. Life, 8(3), 28. https://doi.org/10.3390/life8030028





