Monosaccharides and Their Derivatives in Carbonaceous Meteorites: A Scenario for Their Synthesis and Onset of Enantiomeric Excesses
Abstract
1. Introduction
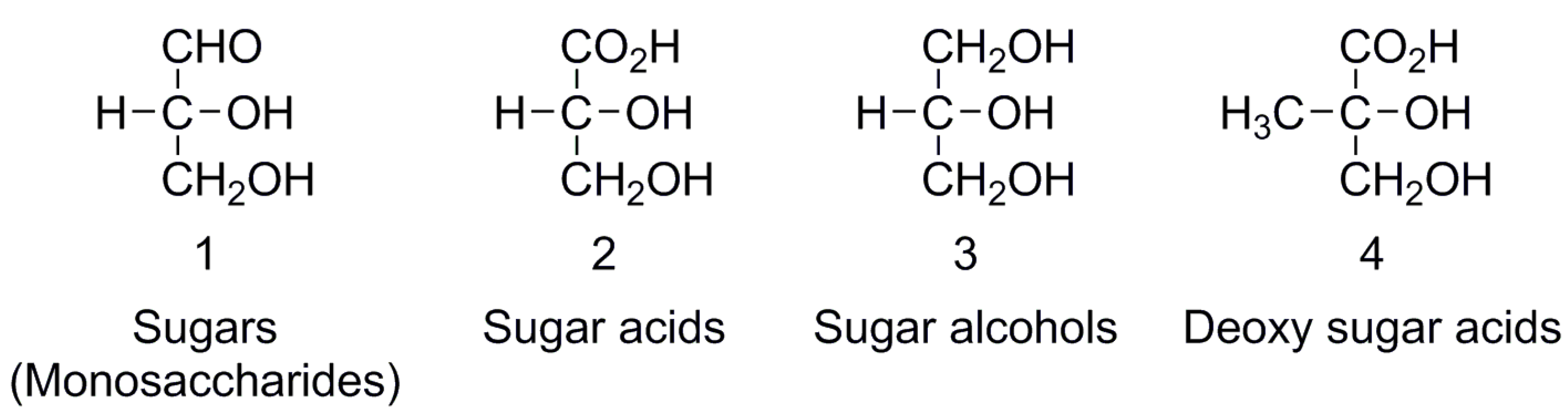
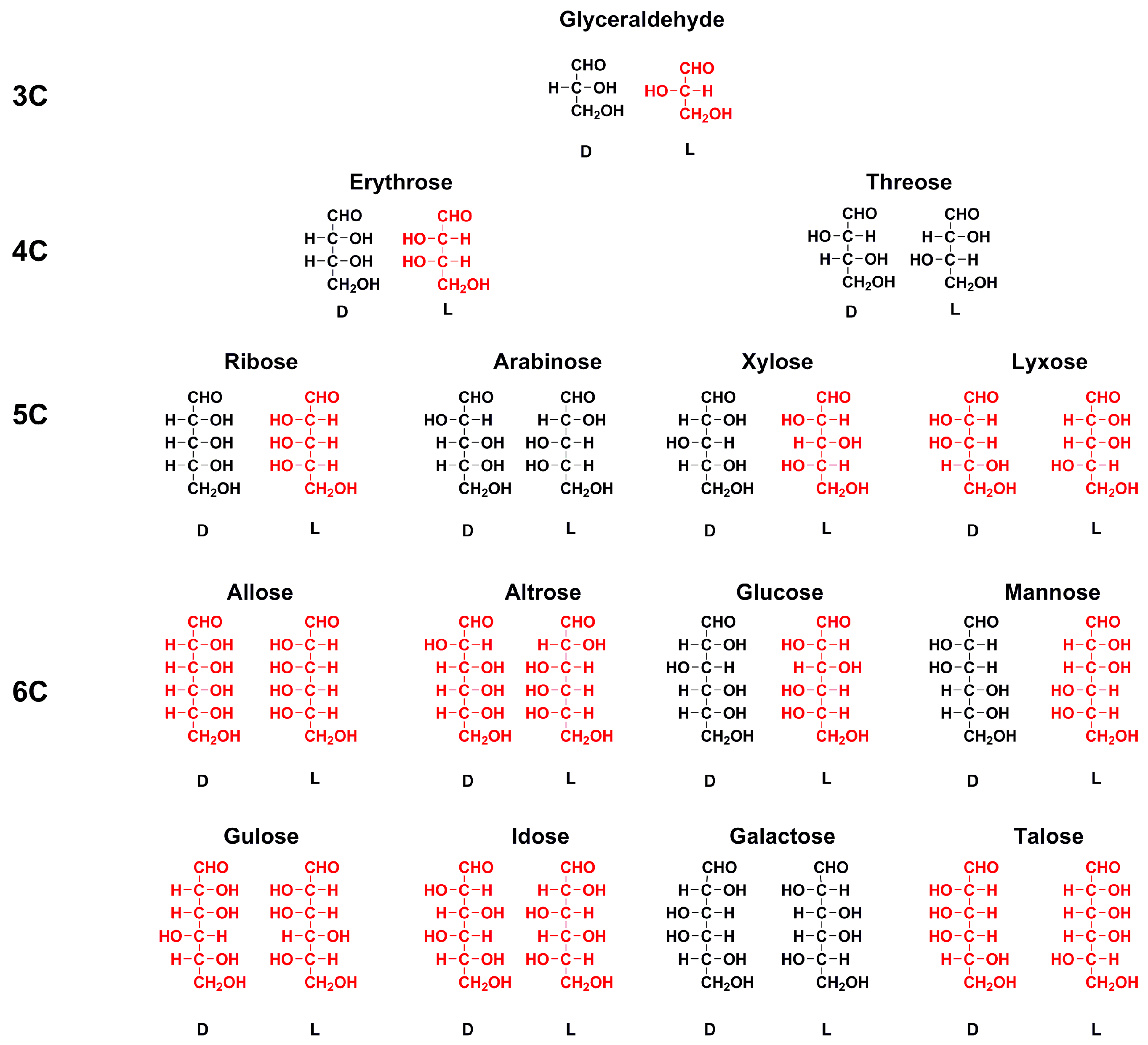
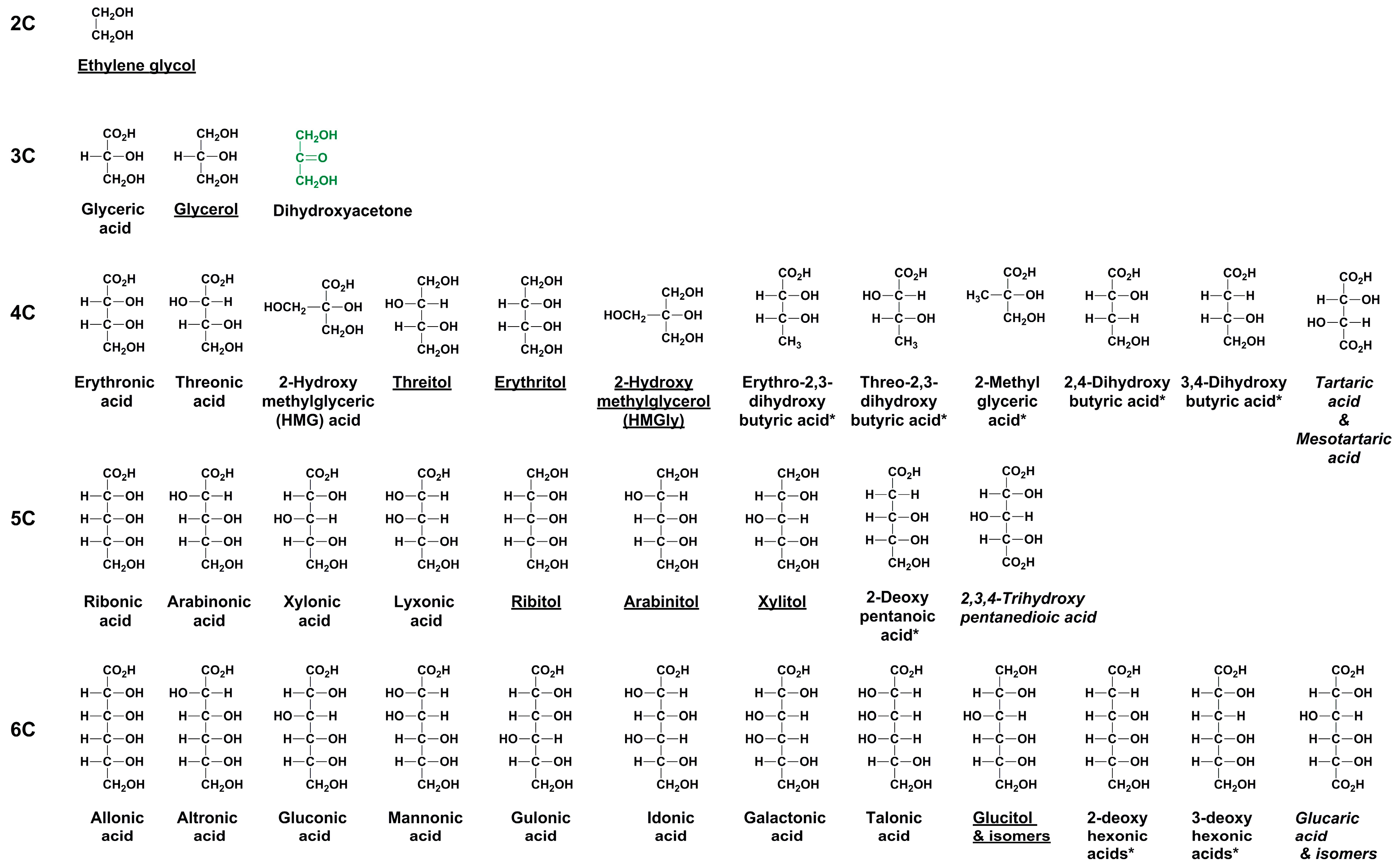
Enantiomer Properties of Polyols and Their Relevance to the Origin of Life
2. The Reported History of Meteoritic Polyol Research
2.1. Early Reports of Meteoritic Sugars
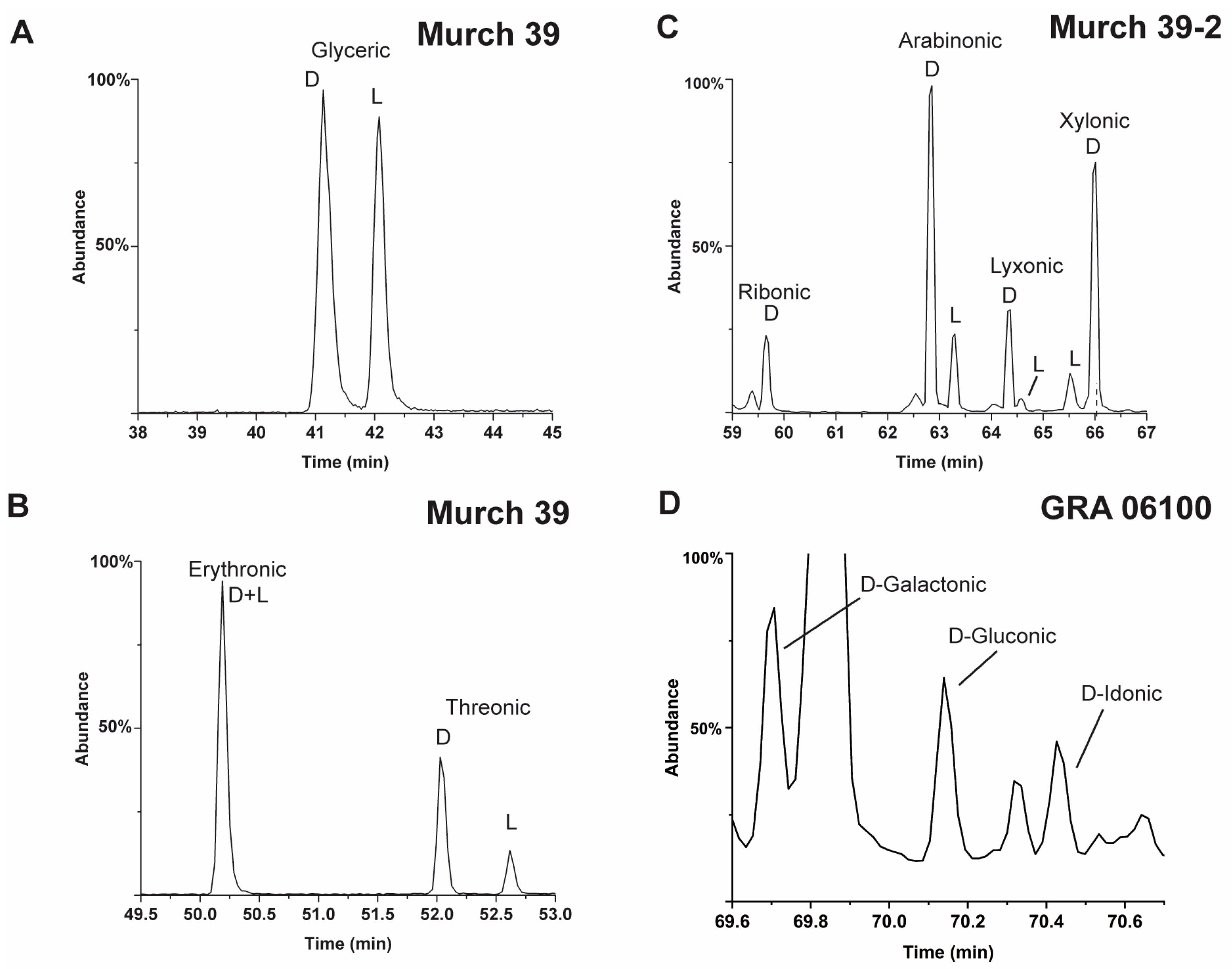
2.2. Recent Reports of Meteoritic Polyols and Their Enantiomer Properties
3. The Synthesis of Meteoritic Polyols
3.1. Meteorite Parent Body (Aqueous) Synthesis of Polyols
3.2. Interstellar Irradiation Synthesis of Polyols
3.3. Formation of Meteoritic Sugar Alcohols: Parent Body (Aqueous) versus Cold Interstellar Synthesis: Are Meteoritic Sugar Alcohols Primarily Products of Cold Interstellar Synthesis?
3.3.1. Sugar Alcohol Production in Standard (Aqueous) Formose Reactions
3.3.2. Sugar Alcohol Production by Low-Temperature Photolytic/Grain Surface Reactions
3.3.3. Deoxy Polyol Synthesis: Aqueous Reactions versus Low-Temperature Interstellar Synthesis
4. Potential Contributions of Polyols and Precursors from Comets
5. Possible Mechanisms for Polyol Enantiomeric Excess Production: Laboratory Results
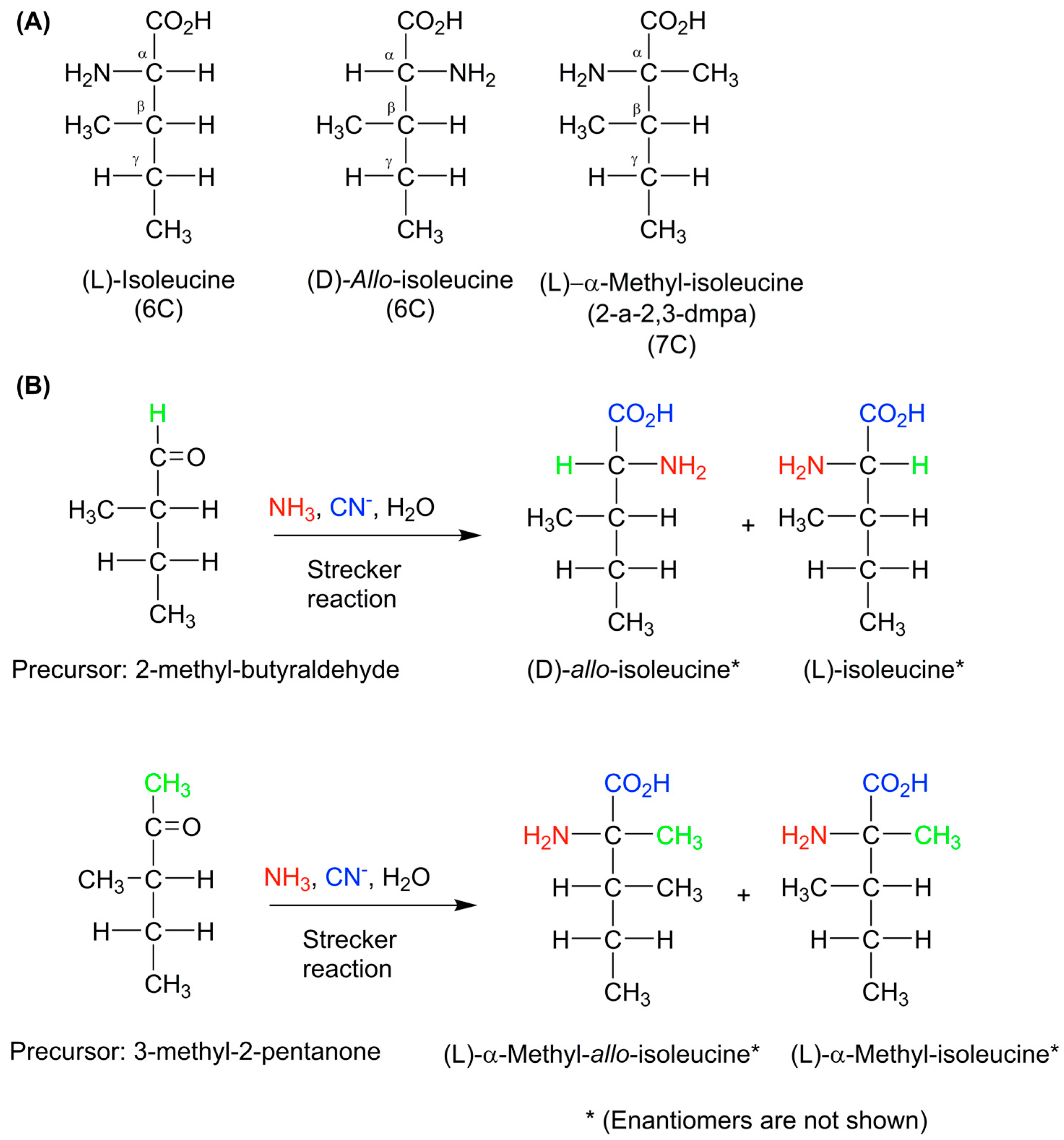
6. Lessons and Conclusions from Meteoritic Amino Acid Research
7. Summary and Conclusions on Meteoritic Amino Acids
8. Meteoritic Enantiomeric Excesses: Summarized Facts and Predictions
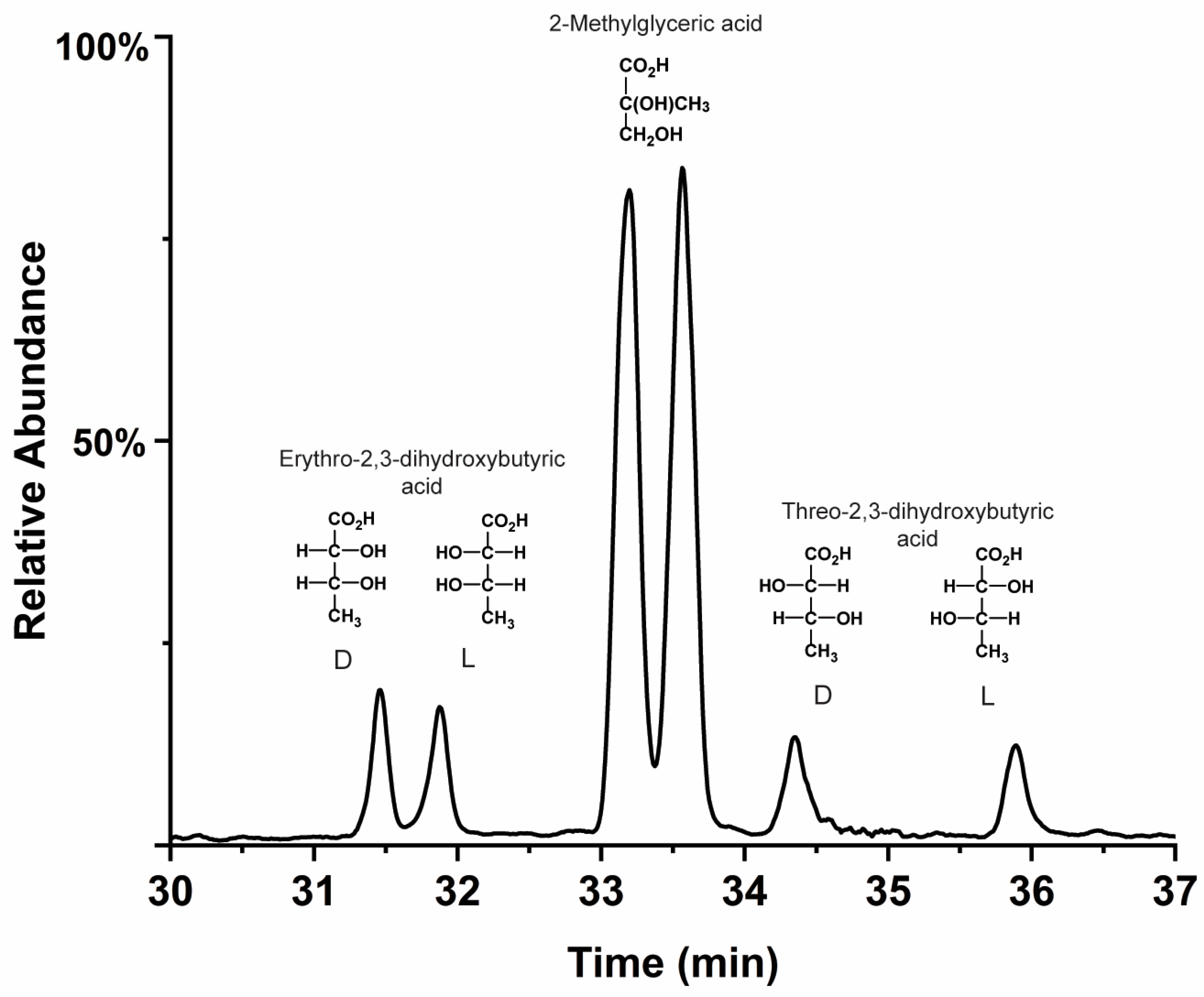
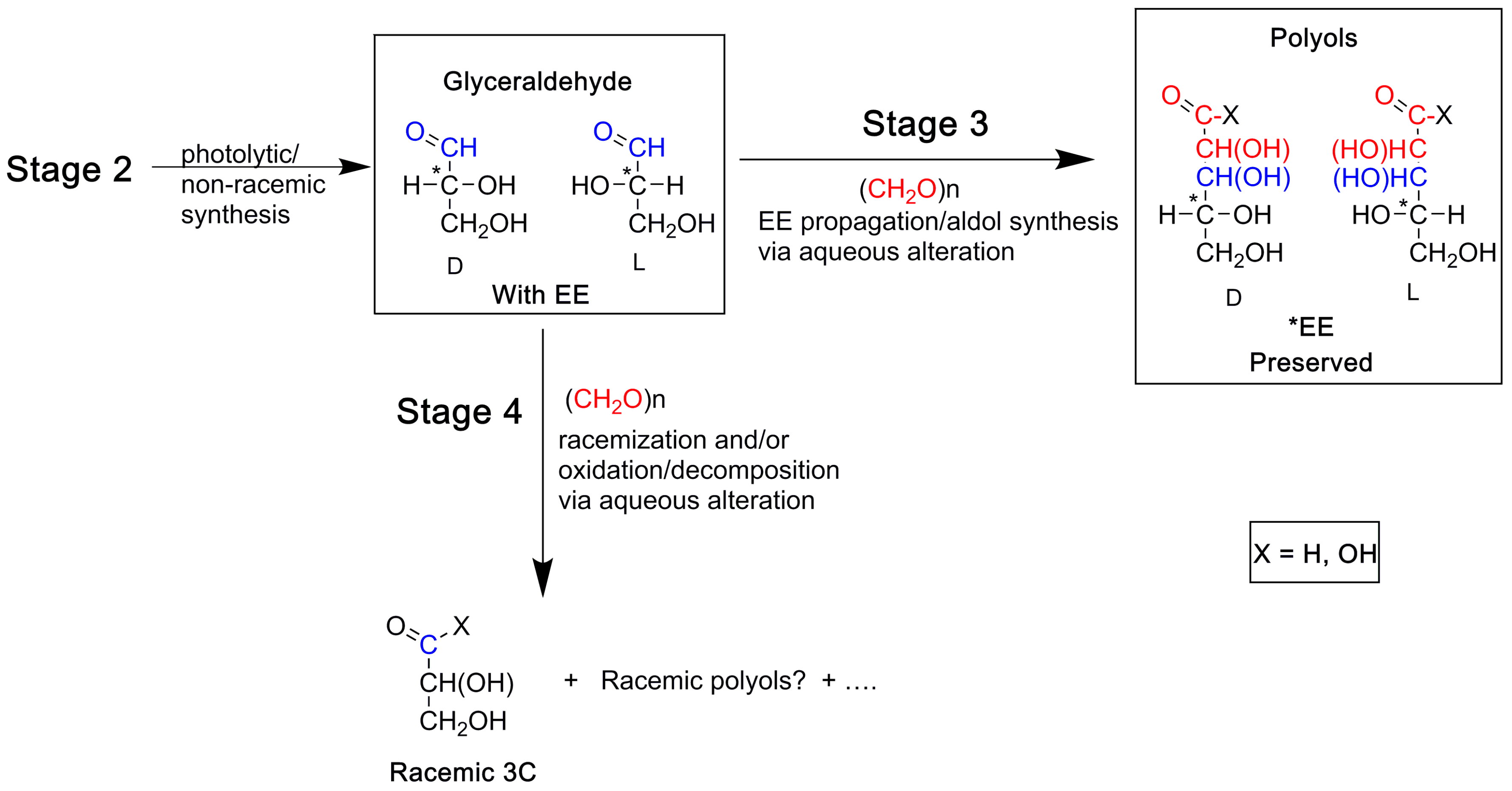
- The racemic (and lower mass) meteoritic polyols, deoxy sugar acids, sugar alcohols, and glyceric acid are racemic (or nearly so). These compounds are readily produced by low-temperature photo-irradiation and possibly cold grain chemistry. However, with the possible exception of glyceric acid, they are either not produced or, at best, produced in very low abundances, via weak (i.e., carbonate-catalyzed) non-photolytic formose-type reactions under conditions of carbonaceous meteorite parent bodies.
- Exposure of interstellar grains and their ice coatings to the ambient radiation UV field [102,103] and cold grain chemistry [69] predated the formation of the solar disk, therefore significant portions of racemic 3C and 4C polyols could have been produced much earlier than their higher mass homologs.
- There is evidence that aqueous reactions on (the later formed) meteorite parent bodies increased the ee of isovaline, a rare meteoritic amino acid [95]. On the other hand, observations of pristine meteorites lead to the conclusion that extended aqueous alteration on meteorite parent bodies was not necessary for either the formation or ee ratios of certain other compounds, i.e., both occurred at an earlier period [47].
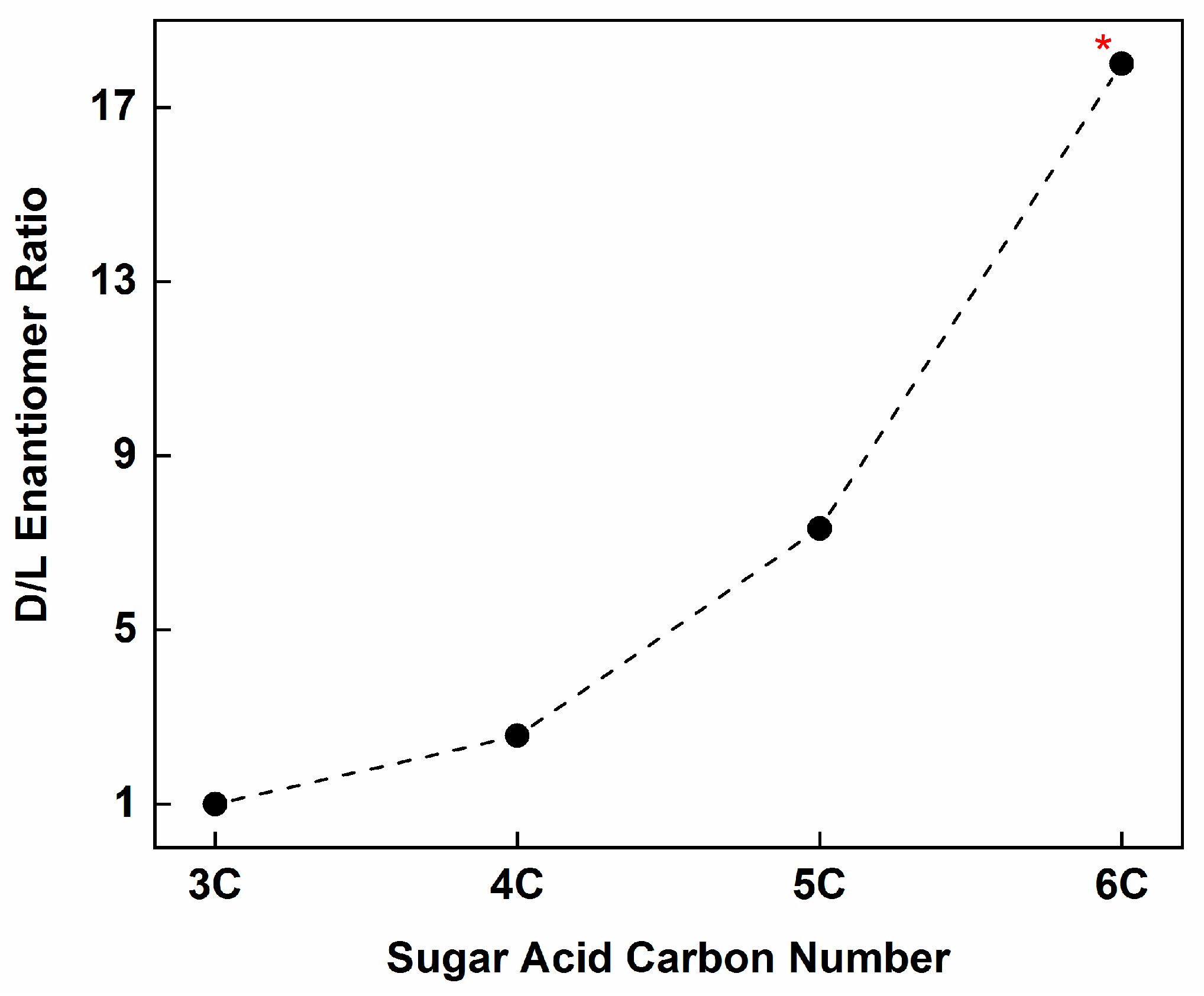
- However, meteoritic sugar acids (i.e., excluding deoxy acids) with higher masses (>3C) carry relatively large ee which increase with increasing carbon number (Figure 4), as if successive compounds were being constructed from their immediate precursors in a liquid-type medium. Importantly, these sugar acids are found in relatively low amounts (or not reported at all) in the vast majority of past photolysis experiments.
- If polyols were racemic during an initial synthesis via photo-irradiation or reactions on grains (Item 2) while some compounds contain ee in pristine meteorites (along with ee propagation during later-stage aqueous alteration, Item 4), the early solar disk is indicated as the time and locale of ee production.
- Magnetochiral effects could be greatly enhanced during the formation of a disk of material, i.e., as the magnetic field intensity in the disk is expected to increase together with increasing density and additive motions of electrically charged ions and grains. In general, the rate and product distribution of chemical reactions involving radicals are known to be affected by magnetic fields [104]. Organic radicals play a significant role in laboratory interstellar ice analog chemistry [59,65,66,69] and such species may have been influenced (directed) by magnetic fields at low disk temperatures. Inorganic (OH) radicals were likely present [11] and may have also interacted with organic species. Although there could be multidirectional magnetic fields in a planetary disk [105], a specific combination of parallel-aligned radiation and magnetic field [85] could theoretically produce enantiomeric excesses. Radicals would play a lesser role in the chemistry of organic compounds, as they (and their precursors) would gradually become shielded from radiation inside of larger objects. The above discussion does not exclude CPL [92] and other mechanisms from ee production.
- During aqueous alteration, some of the ee-carrying compounds could have acted as catalysts that induced ee into other forming compounds (ee amplification). As shown in prebiotically plausible conditions, non-racemic amino acids are capable of acting as catalysts in inducing ee into sugars during aqueous reactions [106]. However, the singular enantiomer (d) enrichment of meteoritic aldonic acids and nearly complete l enrichment of amino acids would have to be explained by such a mechanism.
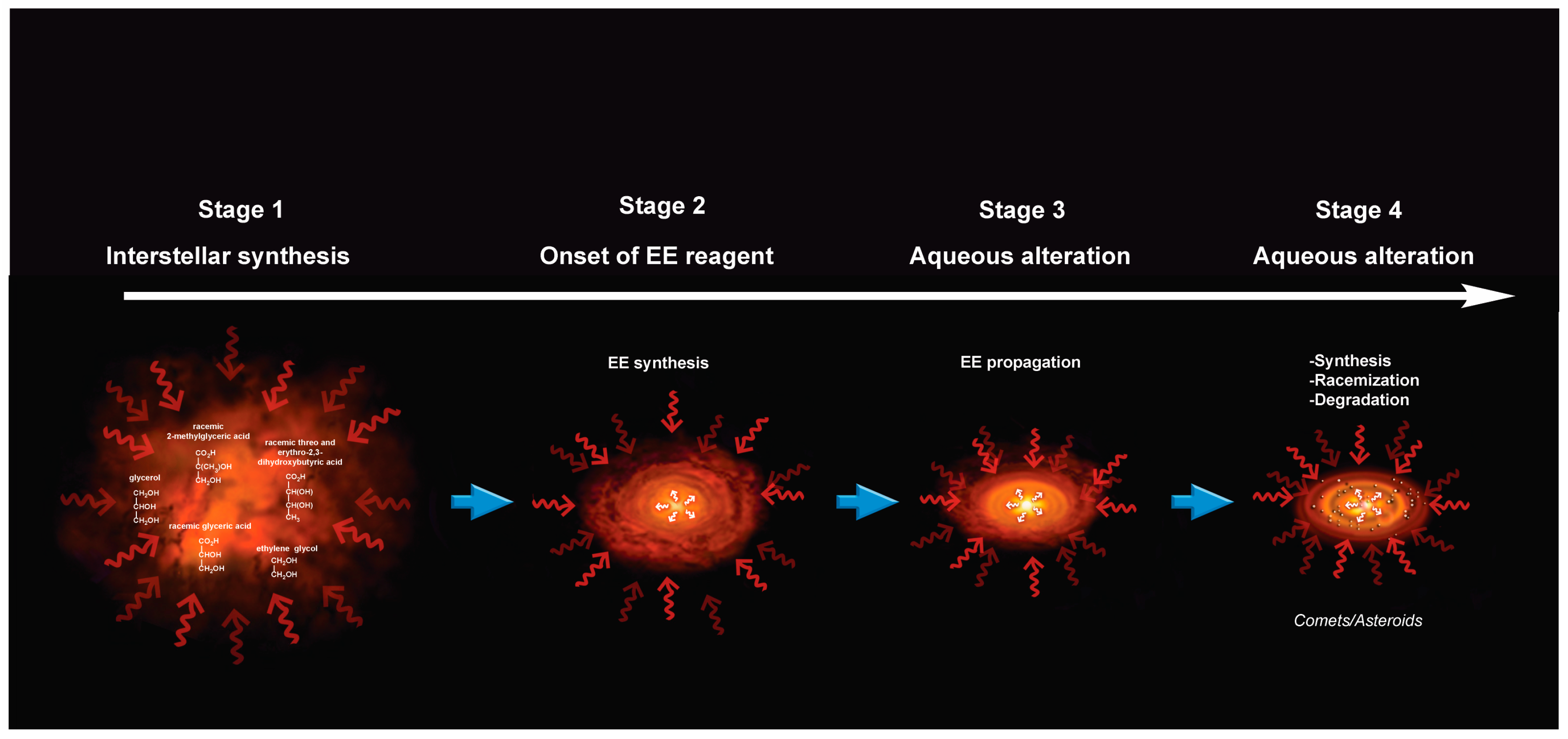
9. A Chronology for Molecular and Enantiomeric Excess Production of Polyols
A Note on the Possible Role of Glyceraldehyde in the Enantiomeric Excesses of Meteoritic Polyols
10. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Alexander, C.M.; Howard, K.T.; Bowden, R.; Fogel, M.L. The classification of CM and CR chondrites using bulk H, C and N abundances and isotopic compositions. Geochim. Cosmochim. Acta 2013, 123, 244–260. [Google Scholar] [CrossRef]
- Zinner, E. Presolar grains. In Meteorites and Cosmochemical Processes, Volume 1 of Treatise On Geochemistry; Davis, A.M., Ed.; Elsevier: Amsterdam, The Netherlands, 2014; Volume 1, pp. 181–213. [Google Scholar]
- MacPherson, G.J.; Boss, A. Cosmochemical evidence for astrophysical processes during the formation of our solar system. Proc. Natl. Acad. Sci. USA 2011, 108, 19152–19158. [Google Scholar] [CrossRef] [PubMed]
- Amari, S.; Zinner, E.; Lewis, R.S. Large 18O excesses in circumstellar graphite grains from the Murchison meteorite: Indication of a massive-star origin. Astrophys. J. Lett. 1995, 447, L147–L150. [Google Scholar] [CrossRef]
- Hoppe, P.; Pignatari, M.; Kodolányi, J.; Gröner, E.; Amari, S. Nanosims isotope studies of rare types of presolar silicon carbide grains from the murchison meteorite: Implications for supernova models and the role of 14C. Geochim. Cosmochim. Acta 2018, 221, 182–199. [Google Scholar] [CrossRef]
- Telus, M.; Huss, G.R.; Ogliore, R.C.; Nagashima, K.; Howard, D.L.; Newville, M.G.; Tomkins, A.G. Mobility of iron and nickel at low temperatures: Implications for 60Fe–60Ni systematics of chondrules from unequilibrated ordinary chondrites. Geochim. Cosmochim. Acta 2016, 178, 87–105. [Google Scholar] [CrossRef]
- Gyngard, F.; Jadhav, M.; Nittler, L.R.; Stroud, R.M.; Zinner, E. Bonanza: An extremely large dust grain from a supernova. Geochim. Cosmochim. Acta 2018, 221, 60–86. [Google Scholar] [CrossRef]
- Nittler, L.R.; Alexander, C.M.; Davidson, J.; Riebe, M.E.I.; Stroud, R.M.; Wang, J. High abundances of presolar grains and 15N-rich organic matter in CO3.0 chondrite dominion range 08006. Geochim. Cosmochim. Acta 2018, 226, 107–131. [Google Scholar] [CrossRef] [PubMed]
- Pizzarello, S.; Cooper, G.W.; Flynn, G.J. The nature and distribution of the organic material in carbonaceous chondrites and interplanetary dust particles. In Meteorites and the Early Solar System II; Lauretta, D., Leshin, L.A., McSween, H.Y., Jr., Eds.; University of Arizona Press: Tucson, AZ, USA, 2006; pp. 625–651. [Google Scholar]
- Sephton, M.A. Organic compounds in carbonaceous meteorites. Nat. Prod. Rep. 2002, 19, 292–311. [Google Scholar] [CrossRef] [PubMed]
- Cody, G.D.; Alexander, C.M. NMR studies of chemical structural variation of insoluble organic matter from different carbonaceous chondrite groups. Geochim. Cosmochim. Acta 2005, 69, 1085–1097. [Google Scholar] [CrossRef]
- Alexander, C.M.; Newsome, S.D.; Fogel, M.L.; Nittler, L.R.; Busemann, H.; Cody, G.D. Deuterium enrichments in chondritic macromolecular material-implications for the origin and evolution of organics, water and asteroids. Geochim. Cosmochim. Acta 2010, 74, 4417–4437. [Google Scholar] [CrossRef]
- Pizzarello, S. Molecular asymmetry in prebiotic chemistry: An account from meteorites. Life 2016, 6, 18. [Google Scholar] [CrossRef] [PubMed]
- Elsila, J.E.; Charnley, S.B.; Burton, A.S.; Glavin, D.P.; Dworkin, J.P. Compound-specific carbon, nitrogen, and hydrogen isotopic ratios for amino acids in CM and CR chondrites and their use in evaluating potential formation pathways. Meteorit. Planet. Sci. 2012, 47, 1517–1536. [Google Scholar] [CrossRef]
- Stoks, P.G.; Schwartz, A.W. Basic nitrogen-heterocyclic compounds in the murchison meteorite. Geochim. Cosmochim. Acta 1982, 46, 309–315. [Google Scholar] [CrossRef]
- Callahan, M.P.; Smith, K.E.; Cleaves, H.J.; Ruzicka, J.; Stern, J.C.; Glavin, D.P.; House, C.H.; Dworkin, J.P. Carbonaceous meteorites contain a wide range of extraterrestrial nucleobases. Proc. Natl. Acad. Sci. USA 2011, 108, 13995–13998. [Google Scholar] [CrossRef] [PubMed]
- Cooper, G.; Kimmich, N.; Belisle, W.; Sarinana, J.; Brabham, K.; Garrel, L. Carbonaceous meteorites as a source of sugar-related organic compounds for the early earth. Nature 2001, 414, 879–883. [Google Scholar] [CrossRef] [PubMed]
- Chiba, H.; Sasaki, R. Functions, of 2,3-bisphosphoglycerate and its metabolism. Curr. Top. Cell. Regul. 1978, 14, 75–116. [Google Scholar] [PubMed]
- Haenecour, P.; Floss, C.; Zega, T.J.; Croat, T.K.; Wang, A.; Jolliff, B.L.; Carpenter, P. Presolar silicates in the matrix and fine-grained rims around chondrules in primitive CO3.0 chondrites: Evidence for pre-accretionary aqueous alteration of the rims in the solar nebula. Geochim. Cosmochim. Acta 2018, 221, 379–405. [Google Scholar] [CrossRef]
- Le Guillou, C.; Brearley, A. Relationships between organics, water and early stages of aqueous alteration in the pristine CR3.0 chondrite MET 00426. Geochim. Cosmochim. Acta 2014, 131, 344–367. [Google Scholar] [CrossRef]
- Blackmond, D.G. The origin of biological homochirality. Philos. Trans. R. Soc. B Biol. Sci. 2011, 366, 2878–2884. [Google Scholar] [CrossRef] [PubMed]
- Joyce, G.F.; Visser, G.M.; van Boeckel, C.A.A.; van Boom, J.H.; Orgel, L.E.; van Westrenen, J. Chiral selection in poly(C)-directed synthesis of oligo(G). Nature 1984, 310, 602–604. [Google Scholar] [CrossRef] [PubMed]
- David Barron, L. True and false chirality and absolute enantioselection. Rend. Fis. Acc. Lincei 2013, 24, 179–189. [Google Scholar] [CrossRef]
- Meierhenrich, U. Amino Acids and the Asymmetry of Life; Brack, A., McKay, C.P., Horneck, G., Stan-Lotter, H., Eds.; Springer: Berlin, Germany, 2008; pp. 103–124. [Google Scholar]
- Aponte, J.C.; Tarozo, R.; Alexandre, M.R.; Alexander, C.M.; Charnley, S.B.; Hallmann, C.; Summons, R.E.; Huang, Y. Chirality of meteoritic free and iom-derived monocarboxylic acids and implications for prebiotic organic synthesis. Geochim. Cosmochim. Acta 2014, 131, 1–12. [Google Scholar] [CrossRef]
- Burton, A.; Berger, E. Insights into abiotically-generated amino acid enantiomeric excesses found in meteorites. Life 2018, 8, 14. [Google Scholar] [CrossRef] [PubMed]
- Degens, E.T.; Bajor, M. Amino acids and sugars in the Bruderheim and Murray meteorite. Naturwissenschaften 1962, 49, 605–606. [Google Scholar] [CrossRef]
- Kaplan, I.R.; Degens, E.T.; Reuter, J.H. Organic compounds in stony meteorites. Geochim. Cosmochim. Acta 1963, 27, 805–834. [Google Scholar] [CrossRef]
- Burton, A.S.; Elsila, J.E.; Hein, J.E.; Glavin, D.P.; Dworkin, J.P. Extraterrestrial amino acids identified in metal-rich CH and CB carbonaceous chondrites from antarctica. Meteorit. Planet. Sci. 2013, 48, 390–402. [Google Scholar] [CrossRef]
- Hayes, J.M. Organic constituents of meteorites—A review. Geochim. Cosmochim. Acta 1967, 31, 1395–1440. [Google Scholar] [CrossRef]
- Cooper, G.; Rios, A.C. Enantiomer excesses of rare and common sugar derivatives in carbonaceous meteorites. Proc. Natl. Acad. Sci. USA 2016, 113, E3322–E3331. [Google Scholar] [CrossRef] [PubMed]
- Partridge, R.D.; Weiss, A.H.; Todd, D. Branched-chain carbohydrate structures resulting from formaldehyde condensation. Carbohydr. Res. 1972, 24, 29–44. [Google Scholar] [CrossRef]
- Decker, P.; Schweer, H.; Pohlamnn, R. Bioids: X. Identification of formose sugars, presumable prebiotic metabolites, using capillary gas chromatography/gas chromatography—Mass spectrometry of n-butoxime trifluoroacetates on OV-225. J. Chromatogr. A 1982, 244, 281–291. [Google Scholar] [CrossRef]
- Kawasaki, T.; Hatase, K.; Fujii, Y.; Jo, K.; Soai, K.; Pizzarello, S. The distribution of chiral asymmetry in meteorites: An investigation using asymmetric autocatalytic chiral sensors. Geochim. Cosmochim. Acta 2006, 70, 5395–5402. [Google Scholar] [CrossRef]
- Cleaves, H.J., II. A hypothesis for a unified mechanism of formation and enantioenrichment of polyols and aldaric, aldonic, amino, hydroxy and sugar acids in carbonaceous chondrites. In Origins of Life: The Primal Self-Organization; Egel, R., Lankenau, D.-H., Mulkidjanian, A.Y., Eds.; Springer: Berlin/Heidelberg, Germany, 2011; pp. 37–55. [Google Scholar]
- Mizuno, T.; Weiss, A.H. Synthesis and utilization of formose sugars. In Advances in Carbohydrate Chemistry and Biochemistry; Tipson, R.S., Horton, D., Eds.; Academic Press: Cambridge, MA, USA, 1974; Volume 29, pp. 173–227. [Google Scholar]
- Meinert, C.; Myrgorodska, I.; de Marcellus, P.; Buhse, T.; Nahon, L.; Hoffmann, S.V.; d’Hendecourt, L.L.S.; Meierhenrich, U.J. Ribose and related sugars from ultraviolet irradiation of interstellar ice analogs. Science 2016, 352, 208–212. [Google Scholar] [CrossRef] [PubMed]
- Delpoux, O.; Gourier, D.; Vezin, H.; Binet, L.; Derenne, S.; Robert, F. Biradical character of D-rich carriers in the insoluble organic matter of carbonaceous chondrites: A relic of the protoplanetary disk chemistry. Geochim. Cosmochim. Acta 2011, 75, 326–336. [Google Scholar] [CrossRef]
- Butlerow, A.M. Formation synthétique d’une substance sucrée. Compt. Rendus Acad. Sci. 1861, 53, 145–147. [Google Scholar]
- Walker, J.F. Formaldehyde, 3rd ed.; Reinhold: New York, NY, USA, 1964; Volume xxvi, 701p. [Google Scholar]
- Delidovich, I.V.; Simonov, A.N.; Taran, O.P.; Parmon, V.N. Catalytic formation of monosaccharides: From the formose reaction towards selective synthesis. ChemSusChem 2014, 7, 1833–1846. [Google Scholar] [CrossRef] [PubMed]
- Socha, R.F.; Weiss, A.H.; Sakharov, M.M. Autocatalysis in the formose reaction. React. Kinet. Catal. Lett. 1980, 14, 119–128. [Google Scholar] [CrossRef]
- Hollis, J.M.; Lovas, F.J.; Jewell, P.R. Interstellar glycolaldehyde: The first sugar. Astrophys. J. 2000, 540, L107–L110. [Google Scholar] [CrossRef]
- Milam, S.N.; Remijan, A.J.; Womack, M.; Abrell, L.; Ziurys, L.M.; Wyckoff, S.; Apponi, A.J.; Friedel, D.N.; Snyder, L.E.; Veal, J.M.; et al. Formaldehyde in comets C/1995 O1 (Hale-Bopp), C/2002 T7 (LINEAR), and C/2001 Q4 (NEAT): Investigating the cometary origin of H2CO. Astrophys. J. 2006, 649, 1169–1177. [Google Scholar] [CrossRef]
- List of Interstellar and Circumstellar Molecules. Available online: https://www.astro.uni-koeln.de/cdms/molecules (accessed on 24 May 2018).
- Lerner, N.R.; Cooper, G.W. Iminodicarboxylic acids in the murchison meteorite: Evidence of strecker reactions. Geochim. Cosmochim. Acta 2005, 69, 2901–2906. [Google Scholar] [CrossRef]
- Pizzarello, S.; Schrader, D.L.; Monroe, A.A.; Lauretta, D.S. Large enantiomeric excesses in primitive meteorites and the diverse effects of water in cosmochemical evolution. Proc. Natl. Acad. Sci. USA 2012, 109, 11949–11954. [Google Scholar] [CrossRef] [PubMed]
- Brearley, A.; Hutcheon, I.; Browning, L. Compositional zoning and Mn-Cr systematics in carbonates from the Y791198 CM2 carbonaceous chondrite. In Proceedings of the 32nd Lunar and Planetary Science XXXII, Houston, TX, USA, 12–16 March 2001. [Google Scholar]
- De Leuw, S.; Rubin, A.E.; Wasson, J.T. Carbonates in cm chondrites: Complex formational histories and comparison to carbonates in CI chondrites. Meteorit. Planet. Sci. 2010, 45, 513–530. [Google Scholar] [CrossRef]
- Lee, M.R.; Lindgren, P.; Sofe, M.R. Aragonite, breunnerite, calcite and dolomite in the CM carbonaceous chondrites: High fidelity recorders of progressive parent body aqueous alteration. Geochim. Cosmochim. Acta 2014, 144, 126–156. [Google Scholar] [CrossRef]
- Brearley, A.J. The action of water. In Meteorites and the Early Solar System II; Lauretta, D., Leshin, L.A., McSween, H.Y., Jr., Eds.; University of Arizona Press: Tucson, AZ, USA, 2006; pp. 584–624. [Google Scholar]
- Guo, W.; Eiler, J.M. Temperatures of aqueous alteration and evidence for methane generation on the parent bodies of the CM chondrites. Geochim. Cosmochim. Acta 2007, 71, 5565–5575. [Google Scholar] [CrossRef]
- Fujiya, W. Oxygen isotopic ratios of primordial water in carbonaceous chondrites. Earth Planet. Sci. Lett. 2018, 481, 264–272. [Google Scholar] [CrossRef]
- Harju, E.R.; Rubin, A.E.; Ahn, I.; Choi, B.-G.; Ziegler, K.; Wasson, J.T. Progressive aqueous alteration of CR carbonaceous chondrites. Geochim. Cosmochim. Acta 2014, 139, 267–292. [Google Scholar] [CrossRef]
- Bose, M.; Floss, C.; Stadermann, F.J.; Stroud, R.M.; Speck, A.K. Circumstellar and interstellar material in the CO3 chondrite ALHA77307: An isotopic and elemental investigation. Geochim. Cosmochim. Acta 2012, 93, 77–101. [Google Scholar] [CrossRef]
- Agarwal, V.K.; Schutte, W.; Greenberg, J.M.; Ferris, J.P.; Briggs, R.; Connor, S.; Vandebult, C.P.E.M.; Baas, F. Photochemical-reactions in interstellar grains photolysis of CO, NH3, and H2O. Orig. Life Evol. Biosph. 1985, 16, 21–40. [Google Scholar] [CrossRef] [PubMed]
- Bernstein, M.P.; Dworkin, J.P.; Sandford, S.A.; Cooper, G.W.; Allamandola, L.J. Racemic amino acids from the ultraviolet photolysis of interstellar ice analogues. Nature 2002, 416, 401–403. [Google Scholar] [CrossRef] [PubMed]
- Muñoz Caro, G.M.; Meierhenrich, U.J.; Schutte, W.A.; Barbier, B.; Segovia, A.A.; Rosenbauer, H.; Thiemann, W.H.P.; Brack, A.; Greenberg, J.M. Amino acids from ultraviolet irradiation of interstellar ice analogues. Nature 2002, 416, 403–406. [Google Scholar] [CrossRef] [PubMed]
- Nuevo, M.; Auger, G.; Blanot, D.; d’Hendecourt, L. A detailed study of the amino acids produced from the vacuum UV irradiation of interstellar ice analogs. Orig. Life Evol. Biosph. 2008, 38, 37–56. [Google Scholar] [CrossRef] [PubMed]
- Nuevo, M.; Materese, C.K.; Sandford, S.A. The photochemistry of pyrimidine in realistic astrophysical ices and the production of nucleobases. Astrophys. J. 2014, 793, 125. [Google Scholar] [CrossRef]
- Materese, C.K.; Nuevo, M.; Sandford, S.A. The formation of nucleobases from the ultraviolet photoirradiation of purine in simple astrophysical ice analogues. Astrobiology 2017, 17, 761–770. [Google Scholar] [CrossRef] [PubMed]
- De Marcellus, P.; Bertrand, M.; Nuevo, M.; Westall, F.; Le Sergeant d’Hendecourt, L. Prebiotic significance of extraterrestrial ice photochemistry: Detection of hydantoin in organic residues. Astrobiology 2011, 11, 847–854. [Google Scholar] [CrossRef] [PubMed]
- De Marcellus, P.; Meinert, C.; Myrgorodska, I.; Nahon, L.; Buhse, T.; Le Sergeant d’Hendecourt, L.; Meierhenrich, U.J. Aldehydes and sugars from evolved precometary ice analogs: Importance of ices in astrochemical and prebiotic evolution. Proc. Natl. Acad. Sci. USA 2015, 112, 965–970. [Google Scholar] [CrossRef] [PubMed]
- McDonald, G.D.; Whited, L.J.; DeRuiter, C.; Khare, B.N.; Patnaik, A.; Sagan, C. Production and chemical analysis of cometary ice tholins. Icarus 1996, 122, 107–117. [Google Scholar] [CrossRef]
- Nuevo, M.; Bredehoft, J.H.; Meierhenrich, U.J.; d’Hendecourt, L.; Thiemann, W.H.P. Urea, glycolic acid, and glycerol in an organic residue produced by ultraviolet irradiation of interstellar/pre-cometary ice analogs. Astrobiology 2010, 10, 245–256. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, R.I.; Maity, S.; Jones, B.M. Synthesis of prebiotic glycerol in interstellar ices. Angew. Chem. Int. Ed. 2015, 54, 195–200. [Google Scholar] [CrossRef] [PubMed]
- Henderson, B.L.; Gudipati, M.S. Direct detection of complex organic products in ultraviolet (Lyα) and electron-irradiated astrophysical and cometary ice analogs using two-step laser ablation and ionization mass spectrometry. Astrophys. J. 2015, 800, 66. [Google Scholar] [CrossRef]
- Maity, S.; Kaiser, R.I.; Jones, B.M. Formation of complex organic molecules in methanol and methanol–carbon monoxide ices exposed to ionizing radiation—A combined FTIR and reflectron time-of-flight mass spectrometry study. Phys. Chem. Chem. Phys. 2015, 17, 3081–3114. [Google Scholar] [CrossRef] [PubMed]
- Fedoseev, G.; Chuang, K.J.; Ioppolo, S.; Qasim, D.; van Dishoeck, E.F.; Linnartz, H. Formation of glycerol through hydrogenation of CO ice under prestellar core conditions. Astrophys. J. 2017, 842, 52–60. [Google Scholar] [CrossRef]
- Nuevo, M.; Cooper, G.; Sanders, J.M.; Buffo, C.E.; Materese, C.K.; Sandford, S.A. Detailed study of the formation of sugar derivatives produced from the uv irradiation of astrophysical ice analogs. In Proceedings of the American Chemical Society Spring Meeting, New Orleans, LA, USA, 19–22 March 2018. Abstract No. 2849788. [Google Scholar]
- Cooper, G.W.; Thiemens, M.H.; Jackson, T.; Chang, S. Sulfur and hydrogen isotope anomalies in meteoritic sulfonic acids. Science 1997, 277, 1072–1074. [Google Scholar] [CrossRef] [PubMed]
- Thiemens, M.H.; Shaheen, R. Mass-independent isotopic composition of terrestrial and extraterrestrial materials. Treatise Geochem. 2014, 5, 151–177. [Google Scholar]
- Kopetzki, D.; Antonietti, M. Hydrothermal formose reaction. New J. Chem. 2011, 35, 1787–1794. [Google Scholar] [CrossRef]
- De Bruijn, J.M.; Kieboom, A.P.G.; Van Bekkum, H. Reactions of monosaccharides in aqueous alkaline solutions. Sugar Technol. Rev. 1986, 13, 21–52. [Google Scholar]
- Lambert, J.B.; Gurusamy-Thangavelu, S.A.; Ma, K. The Silicate-Mediated formose reaction: Bottom-up synthesis of sugar silicates. Science 2010, 327, 984–986. [Google Scholar] [CrossRef] [PubMed]
- Pizzarello, S.; Cronin, J.R. Non-racemic amino acids in the Murray and Murchison meteorites. Geochim. Cosmochim. Acta 2000, 64, 329–338. [Google Scholar] [CrossRef]
- Goesmann, F.; Rosenbauer, H.; Bredehöft, J.H.; Cabane, M.; Ehrenfreund, P.; Gautier, T.; Giri, C.; Krüger, H.; Le Roy, L.; MacDermott, A.J.; et al. Organic compounds on comet 67P/Churyumov-Gerasimenko revealed by COSAC mass spectrometry. Science 2015, 349, aab0689. [Google Scholar] [CrossRef] [PubMed]
- Wright, I.P.; Sheridan, S.; Barber, S.J.; Morgan, G.H.; Andrews, D.J.; Morse, A.D. Cho-bearing organic compounds at the surface of 67P/Churyumov-Gerasimenko revealed by Ptolemy. Science 2015, 349, aab0673. [Google Scholar] [CrossRef] [PubMed]
- Altwegg, K.; Balsiger, H.; Berthelier, J.J.; Bieler, A.; Calmonte, U.; Fuselier, S.A.; Goesmann, F.; Gasc, S.; Gombosi, T.I.; Le Roy, L.; et al. Organics in comet 67P—A first comparative analysis of mass spectra from ROSINA–DFMS, cosac and ptolemy. Mon. Not. R. Astron. Soc. 2017, 469, S130–S141. [Google Scholar] [CrossRef]
- Reinhard, R. The Giotto encounter with comet Halley. Nature 1986, 321, 313–318. [Google Scholar] [CrossRef]
- Huebner, W.F.; Boice, D.C.; Sharp, C.M. Polyoxymethylene in Comet Halley. Astrophys. J. 1987, 320, L149–L152. [Google Scholar] [CrossRef]
- Butscher, T.; Duvernay, F.; Danger, G.; Chiavassa, T. Radical-induced chemistry from VUV photolysis of interstellar ice analogues containing formaldehyde. Astron. Asrophys. 2016, 593, A60. [Google Scholar] [CrossRef]
- Yanlong, G.; Joël, B.; François, J. Glycerol as an efficient promoting medium for organic reactions. Adv. Synth. Catal. 2008, 350, 2007–2012. [Google Scholar]
- Chyba, C.; Sagan, C. Endogenous production, exogenous delivery and impact-shock synthesis of organic molecules: An inventory for the origins of life. Nature 1992, 355, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Rikken, G.L.J.A.; Raupach, E. Enantioselective magnetochiral photochemistry. Nature 2000, 405, 932–935. [Google Scholar] [CrossRef] [PubMed]
- Kleindienst, P.; Wagnière, G.H. Interferometric detection of magnetochiral birefringence. Chem. Phys. Lett. 1998, 288, 89–97. [Google Scholar] [CrossRef]
- Banerjee-Ghosh, K.; Ben Dor, O.; Tassinari, F.; Capua, E.; Yochelis, S.; Capua, A.; Yang, S.-H.; Parkin, S.S.P.; Sarkar, S.; Kronik, L.; et al. Separation of enantiomers by their enantiospecific interaction with achiral magnetic substrates. Science 2018. [Google Scholar] [CrossRef] [PubMed]
- Flores, J.J.; Bonner, W.A.; Massey, G.A. Asymmetric photolysis of (rs)-leucine with circularly polarized UV light. J. Am. Chem. Soc. 1977, 99, 3622–3625. [Google Scholar] [CrossRef] [PubMed]
- Takano, Y.; Takahashi, J.; Kaneko, T.; Marumo, K.; Kobayashi, K. Asymmetric synthesis of amino acid precursors in interstellar complex organics by circularly polarized light. Earth Planet. Sci. Lett. 2007, 254, 106–114. [Google Scholar] [CrossRef]
- Nuevo, M.; Meierhenrich, U.J.; Muñoz Caro, G.M.; Dartois, E.; d’Hendecourt, L.; Deboffle, D.; Auger, G.; Blanot, D.; Bredehöft, J.H.; Nahon, L. The effects of circularly polarized light on amino acid enantiomers produced by the UV irradiation of interstellar ice analogs. Astron. Astrophys. 2006, 457, 741–751. [Google Scholar] [CrossRef]
- De Marcellus, P.; Meinert, C.; Nuevo, M.; Filippi, J.-J.; Danger, G.; Deboffle, D.; Nahon, L.; Le Sergeant d’Hendecourt, L.; Meierhenrich, U.J. Non-racemic amino acid production by UV irradiation of achiral interstellar ice analogs with circularly polarized light. Astrophys. J. Lett. 2011, 727, L27. [Google Scholar] [CrossRef]
- Modica, P.; Meinert, C.; de Marcellus, P.; Nahon, L.; Meierhenrich, U.J.; d’Hendecourt, L.L. Enantiomeric excesses induced in amino acids by ultraviolet circularly polarized light irradiation of extraterrestrial ice analogs: A possible source of asymmetry for prebiotic chemistry. Astrophys. J. 2014, 788, 79. [Google Scholar] [CrossRef]
- Lucas, P.W.; Hough, J.H.; Bailey, J.; Chrysostomou, A.; Gledhill, T.M.; McCall, A. UV circular polarisation in star formation regions: The origin of homochirality? Orig. Life Evol. Biosph. 2005, 35, 29–60. [Google Scholar] [CrossRef] [PubMed]
- Chuang, K.J.; Fedoseev, G.; Qasim, D.; Ioppolo, S.; van Dishoeck, E.F.; Linnartz, H. Production of complex organic molecules: H-atom addition versus UV irradiation. Mon. Not. R. Astron. Soc. 2017, 467, 2552–2565. [Google Scholar]
- Glavin, D.P.; Dworkin, J.P. Enrichment of the amino acid L-isovaline by aqueous alteration on CI and CM meteorite parent bodies. Proc. Natl. Acad. Sci. USA 2009, 106, 5487–5492. [Google Scholar] [CrossRef] [PubMed]
- Martins, Z.; Modica, P.; Zanda, B.; Le Sergeant d’Hendecourt, L. The amino acid and hydrocarbon contents of the Paris meteorite: Insights into the most primitive CM chondrite. Meteorit. Planet. Sci. 2015, 50, 926–943. [Google Scholar] [CrossRef]
- Pizzarello, S.; Huang, Y.; Alexandre, M.R. Molecular asymmetry in extraterrestrial chemistry: Insights from a pristine meteorite. Proc. Natl. Acad. Sci. USA 2008, 105, 3700–3704. [Google Scholar] [CrossRef] [PubMed]
- Peltzer, E.T.; Bada, J.L.; Schlesinger, G.; Miller, S.L. The chemical conditions on the parent body of the murchison meteorite: Some conclusions based on amino, hydroxy and dicarboxylic acids. Adv. Space Res. 1984, 4, 69–74. [Google Scholar] [CrossRef]
- Cronin, J.R.; Pizzarello, S. Enantiomeric excesses in meteoritic amino acids. Science 1997, 275, 951–955. [Google Scholar] [CrossRef] [PubMed]
- Nuevo, M.; Meierhenrich, U.J.; d’Hendecourt, L.; Muñoz Caro, G.M.; Dartois, E.; Deboffle, D.; Thiemann, W.H.P.; Bredehöft, J.H.; Nahon, L. Enantiomeric separation of complex organic molecules produced from irradiation of interstellar/circumstellar ice analogs. Adv. Space Res. 2007, 39, 400–404. [Google Scholar] [CrossRef]
- Nuevo, M.; Chen, Y.J.; Yih, T.S.; Ip, W.H.; Fung, H.S.; Cheng, C.Y.; Tsai, H.R.; Wu, C.Y.R. Amino acids formed from the uv/euv irradiation of inorganic ices of astrophysical interest. Adv. Space Res. 2007, 40, 1628–1633. [Google Scholar] [CrossRef]
- Throop, H.B. UV photolysis, organic molecules in young disks, and the origin of meteoritic amino acids. Icarus 2011, 212, 885–895. [Google Scholar] [CrossRef]
- Ciesla, F.J.; Sandford, S.A. Organic synthesis via irradiation and warming of ice grains in the solar nebula. Science 2012, 336, 452–454. [Google Scholar] [CrossRef] [PubMed]
- Rodgers, C.T. Magnetic field effects in chemical systems. Pure Appl. Chem. 2009, 81, 19–43. [Google Scholar] [CrossRef]
- Adams, F.C.; Gregory, S.G. Magnetically controlled accretion flows onto young stellar objects. Astrophys. J. 2012, 744, 55. [Google Scholar] [CrossRef]
- Pizzarello, S.; Weber, A.L. Prebiotic amino acids as asymmetric catalysts. Science 2004, 303, 1151. [Google Scholar] [CrossRef] [PubMed]
- Carey, F.A.; Sundberg, R. Advanced Organic Chemistry, Part A: Structure and Mechanisms, 4th ed.; Kluwer: New York, NY, USA, 2000; pp. 128–131. [Google Scholar]
- Martins, Z.; Alexander, C.M.; Orzechowska, G.E.; Fogel, M.L.; Ehrenfreund, P. Indigenous amino acids in primitive cr meteorites. Meteorit. Planet. Sci. 2007, 42, 2125–2136. [Google Scholar] [CrossRef]

| Degree of Aqueous Alteration | |||||||
|---|---|---|---|---|---|---|---|
 | |||||||
| Meteorite (Petrologic Type) | GRO 95577 (1.3) | PCA 91082 (2.3) | QUE 99177 (2.4) | Murchison (2.5) | EET 92042 (2.5) | MET 00426 (2.6) | MIL 07525 (2.8) |
| Glycerol/erythritol | 89 | 24 | 74 | 100 | 171 | 104 | 70 |
| 5C Alcohols abundance | tr | tr/nf | nf | tr | tr | tr/nf | tr/nf |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cooper, G.; Rios, A.C.; Nuevo, M. Monosaccharides and Their Derivatives in Carbonaceous Meteorites: A Scenario for Their Synthesis and Onset of Enantiomeric Excesses. Life 2018, 8, 36. https://doi.org/10.3390/life8030036
Cooper G, Rios AC, Nuevo M. Monosaccharides and Their Derivatives in Carbonaceous Meteorites: A Scenario for Their Synthesis and Onset of Enantiomeric Excesses. Life. 2018; 8(3):36. https://doi.org/10.3390/life8030036
Chicago/Turabian StyleCooper, George, Andro C. Rios, and Michel Nuevo. 2018. "Monosaccharides and Their Derivatives in Carbonaceous Meteorites: A Scenario for Their Synthesis and Onset of Enantiomeric Excesses" Life 8, no. 3: 36. https://doi.org/10.3390/life8030036
APA StyleCooper, G., Rios, A. C., & Nuevo, M. (2018). Monosaccharides and Their Derivatives in Carbonaceous Meteorites: A Scenario for Their Synthesis and Onset of Enantiomeric Excesses. Life, 8(3), 36. https://doi.org/10.3390/life8030036





