Abstract
Carbonaceous Chondrite (CC) meteorites are fragments of asteroids, solar planetesimals that never became large enough to separate matter by their density, like terrestrial planets. CC contains various amounts of organic carbon and carry a record of chemical evolution as it came to be in the Solar System, at the time the Earth was formed and before the origins of life. We review this record as it pertains to the chiral asymmetry determined for several organic compounds in CC, which reaches a broad molecular distribution and enantiomeric excesses of up to 50%–60%. Because homochirality is an indispensable attribute of extant polymers and these meteoritic enantiomeric excesses are still, to date, the only case of chiral asymmetry in organic molecules measured outside the biosphere, the possibility of an exogenous delivery of primed prebiotic compounds to early Earth from meteorites is often proposed. Whether this exogenous delivery held a chiral advantage in molecular evolution remains an open question, as many others regarding the origins of life are.
1. Carbon-Containing Meteorites and Cosmochemical Evolution
One of the paradigms of chemical evolution proposes that the abiotic formation of increasingly complex molecules throughout cosmochemical and Earthly processes could eventually lead to life’s precursor molecules. It finds its rationale in the observation of phenomena, both leading to, and proceeding from, the origin of life, such as the formation of complex organic molecules in interstellar media (e.g., [1]), and a terrestrial phylogeny that evolved from much simpler organisms (e.g., [2]).
To date, such chemical evolution has found direct experimental scrutiny, solely through the analyses of Carbonaceous Chondrites (CC) meteorites, stony fragments of asteroids containing abundant organic carbon, ranging in complexity from kerogen-like macromolecular materials to simple soluble compounds, several of which are identical to terrestrial biomolecules. Because CC Asteroidal parent bodies are planetesimals found between Mars and Jupiter that never accreted large enough to undergo the extensive differentiation involved in planet formation, their meteoritic fragments uniquely offer a pristine record of abiotic organic chemistry in the early Solar System, as found in a planetary setting, at a unique juncture in the long history of the biogenic element, and at its closest to the onset of life. Their studies, therefore, have offered an understanding, not only of how compounds identical to life’s molecules were formed abiotically, but also about the organic inventory accreted by early Earth, e.g., through its heavy bombardment from comets and meteorites [3,4].
Comprehensive molecular and isotopic analyses for over forty-five years, and continuing today, have revealed that CCs may contain a complex organic suite of compounds, which are seen as the products of the synthetic capabilities of both solar and pre-solar environments [5]. Their complex range varies from polar compounds, such as amino acids, to non-polar hydrocarbons (e.g., [5,6,7]) and, when detected simply by their elemental compositions, may include thousands of molecular species [8]. From the perspective of a possible continuity between cosmochemical and prebiotic evolution, this diverse suite would not appear to indicate any selective synthetic trend and, in fact, indicate a fundamental distinction between abiotic and biological chemical processes in their gaining of molecular complexity. To say it with the words of Shakespeare, therefore, a meteoritic organic input of thousands H, C, N, O containing molecules would have offered “too much of a good thing” for an evolutionary transition towards the origins of life.
Nevertheless, within this evident heterogeneity, several meteoritic compounds have been found to contain L-enantiomeric excesses (ee), i.e., to be more abundant in the l-chiral form like the amino acids of terrestrial proteins [9], offering the first unequivocal indication that a purely chemical evolutionary process, as the one believed to be responsible for the formation of meteorite organics, may also have been at work in chiral selection, a property known since the time of Pasteur to be intimately associated with life.
The following review reports on ee distributions found within meteorite organics so far, the possible venues that might have lead to their formation, and the effect that the input of such selected molecules might have had in prebiotic chemistry.
2. Enantiomeric Excesses in Meteoritic Compounds
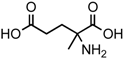
The search for chiral asymmetry in meteorites was first undertaken by Engel and Nagy [10] and, although carried out carefully, was controversial [11] because of the pervasive distribution of homochirality in the biosphere and the possibility of terrestrial contamination in carbonaceous meteorites resulting from bacteria, molds, or their remnants. However, ee were ultimately detected in the Murchison meteorite for some amino acids not common in terrestrial proteins and abundant in meteorites, the 2-methyl, 2-aminoacids (2maa), and to have the same configuration (L) as protein amino acids [9]. To date, L-ee have been detected for a number of amino acids, as well as other compounds, and their inventory is summarized in Table 1 [12]. As shown, the larger number comprises the amino acids of several meteorites and involves molecular species that lack an α-H, with the exception of the diastereomers of isoleucine, as further detailed below, and the one-time finding for aspartic acid [13]. Lactic acid is the only hydroxy acid to display ee [14], while two recent communications have reported their detection in several sugar acids derivatives [15] and two amines, sec-butyl- and 1-methylbutyl amines [16]. These latter studies, appear to point at the possibility of diverse asymmetric effects in cosmochemistry. These meteoritic enantiomeric excesses are still, to date, the only case of molecular asymmetry measured outside the biosphere.

Table 1.
Amines and amino-, hydroxy, and sugar-acids displaying enantiomeric excesses in carbonaceous chondrites.
The overall extent of ee in 2maa varies significantly, between 0% and 18%, and even within short distances in the same meteorite stone [17,18]. For Isovaline (2-amino, 2-methylbutanoic acid), the most abundant of the acids, the absence of any contribution from terrestrial contaminants within this chiral heterogeneity was validated by Murchison, using 13C-, and D isotopic analyses [17,18,19], where the two enantiomers were found to have comparable enrichments. Isovaline δD values were particularly high, +3600‰ [19], and near to those measured spectroscopically for organic molecules in the interstellar medium.
It should be noted that racemization, the conversion of one enantiomer into another, is possible for 2H-amino acids in water through a repeat statistical abstraction and reacquisition of the α-H and because of its vicinity to the electron-withdrawing carboxyl group. Racemization, with time and proper conditions, will lead to racemic solutions (equal amounts of the two enantiomers). The fact that all amino acids found non-racemic in meteorites have a 2-methyl in place of a 2-H and cannot racemize, easily raises the question of whether the twelve racemizable meteoritic amino acids detected having terrestrial protein counterparts might have initially carried ee but racemized during the water phases known to have affected CC parent bodies. The understanding of the processes involved in meteoritic amino acids’ syntheses and the apparently odd ee findings for diastereomer amino acids in several meteorites may be able to address the question.
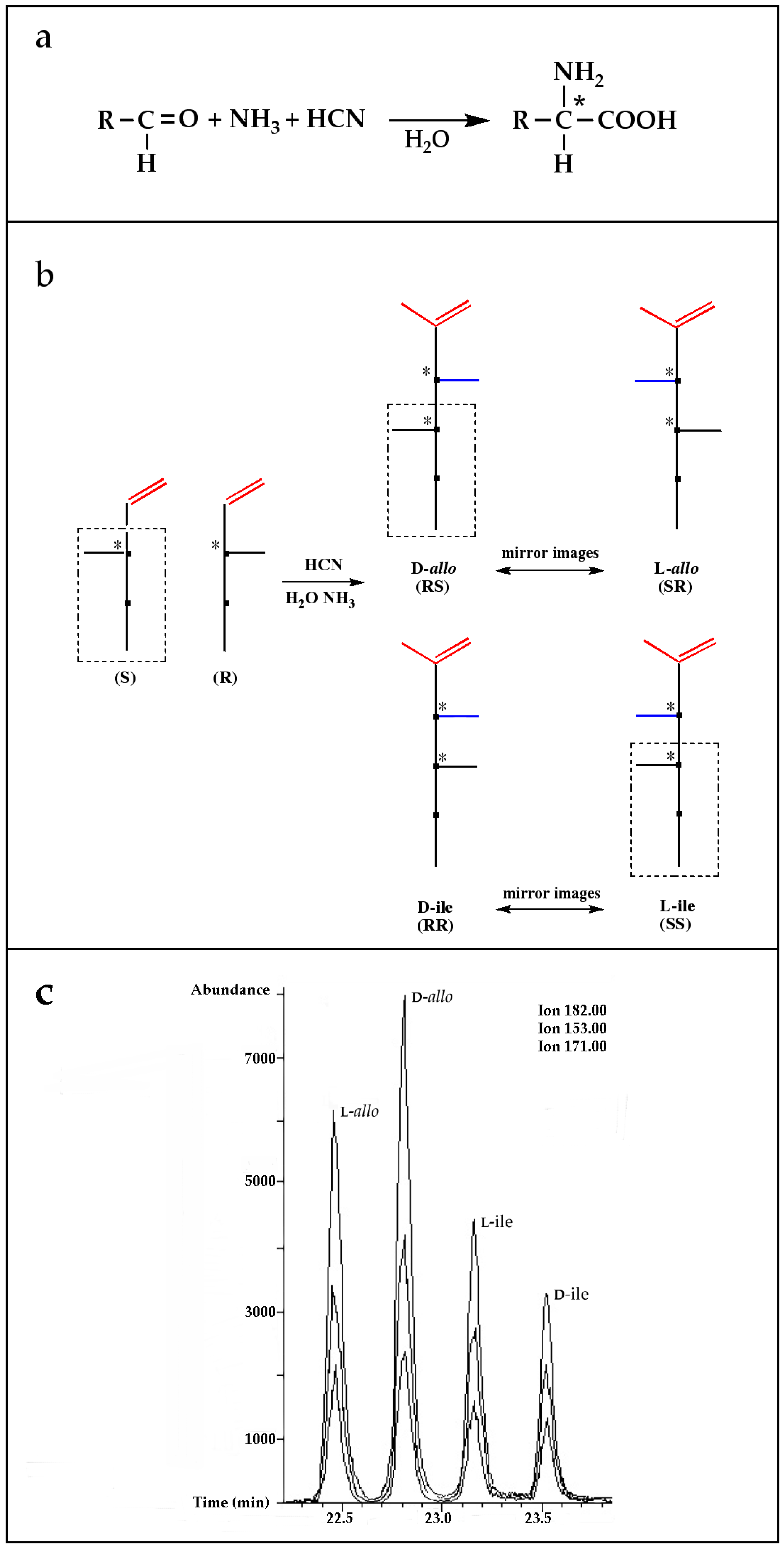
A possible pathway proposed for the formation of 2-amino-, and 2-hydroxy acids in meteorites is the addition of ammonia and HCN to ketones and aldehydes in the presence of water (Figure 1a, Reference [12] and the references therein). This is a reasonable hypothesis, for at least some of the amino acids because all the needed reagents are abundant components of interstellar ices and likely constituents of primitive asteroids [20,21]. Although producing an asymmetric carbon and, therefore, a chiral molecule in most cases, this type of synthesis is non-stereospecific because the HCN addition would be random and give equal amounts of D-, and L-enantiomers.
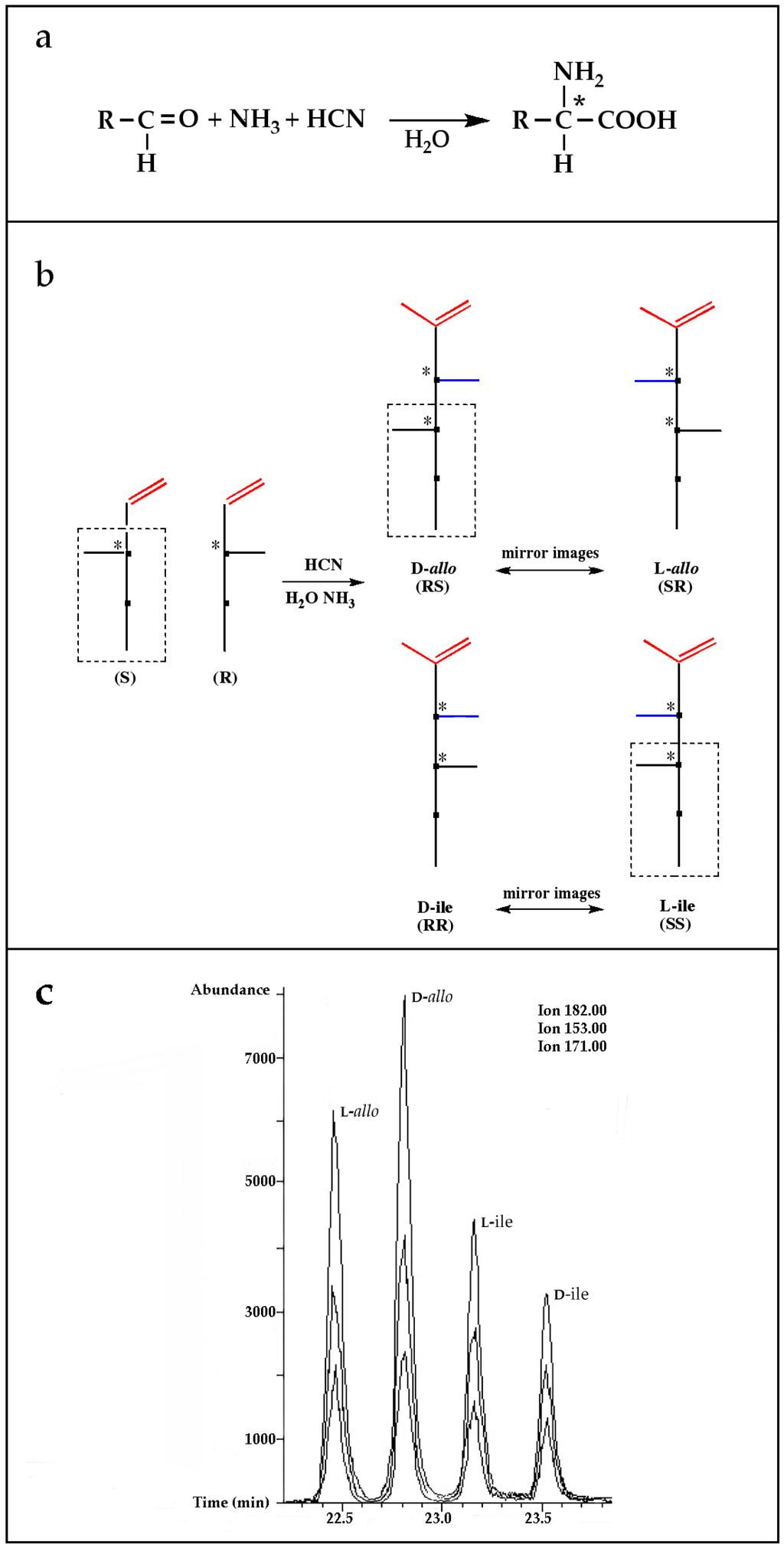
Figure 1.
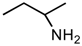
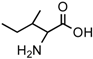
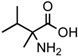
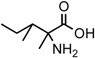
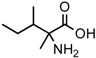
(a) The Strecker synthesis for the possible formation of amino acids in meteorites ([12] and references therein); (b) Structural relation of the alloisoleucine and isoleucine diastereomers; (c) Enantiomeric excesses detected for the isoleucine diastereomers in the GRA 95229 meteorites using Gas Chromatography-Mass Spectrometry, single ion traces [22].
However, reaction products become more complex for longer aldehydes that already contain an asymmetric carbon, such as in the synthesis of the 6-C isoleucine (ile) and alloisolucine (allo) diastereomers (Figure 1b) from DL 2-methylbutanal [12]. In this case, were an ee already present in the precursor aldehyde, e.g., of the (S) configuration, those diastereomeric product amino acids that carried the (S) portion of the precursor through their synthesis will be more abundant than their respective enantiomers and any original ee would be revealed in different compounds.
In the above example, this would be the (RS) allo and (SS) ile compounds or, in the formalism used for amino acids, D-allo and L-ile. As can be seen, these two sets of diastereomers are also each other’s racemization product (called epimerization in this case,) and, because the 3C is too far removed from the carboxyl and will racemize much more slowly, if at all, original ee may be preserved much longer.
Such was the distribution found in the extracts of a group of pristine meteorites collected in Antarctica (Renazzo-type or CR) (Figure 1c, [22,23]) and, based on the above formative premise, as well as upon confirming the indigeneity of all four individual enantiomers by 13C isotopic analyses [22], the findings were interpreted to signify that their precursor aldehyde carried an original S-asymmetry to the meteorite’s parent body. CR meteorites’ organic composition, therefore, offers a new exciting account of cosmochemical evolution’s capabilities toward the selective production of very large abiotic ee, of up to 60% in the case of the isoleucine diastereomers, in some meteorites (Table 1).
3. The Likely Locals for Asymmetric Syntheses
The origin of ee in the amino acids of meteorites has been long debated since their initial detection, and is still not known (e.g., [24]). The first hypotheses to be put forward proposed their possible asymmetric decomposition upon UV photolysis [25] and, following the hint of amino acids’ high isotopic enrichments in deuterium, the likelihood of their syntheses in cold, deuterium-enriching cosmic environments [7]. The two enantiomers of a chiral molecule have identical chemical properties, but differ in some physico-chemical features, e.g., polarizability [26], and, as a result, may react/interact differently with other chiral molecules or entities (a common illustration could be that of the distinct ways two people can join hands for different aims).
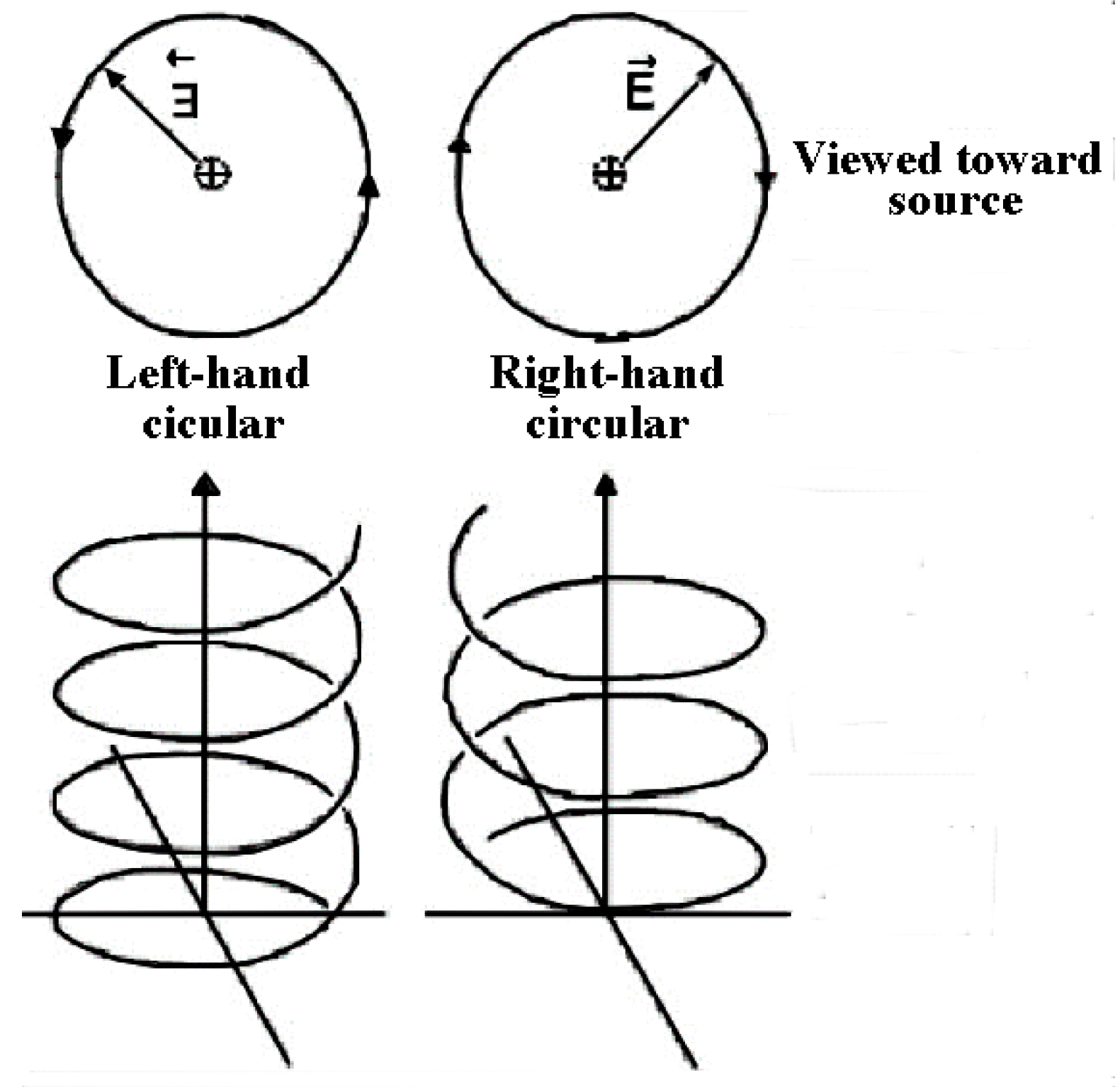
The effects of UV circularly polarized light (CPL) irradiation, which can have right-handed or left-handed helical paths of propagation (Figure 2), were discussed and studied in detail [27] and for amino acids in particular. Several experiments did show UVCPL irradiation generating adsorption bands by the compounds with the same sign as the irradiating CPL [28], and even over a large wavelength range [29], however, the maximum ee achieved were found to be, at most, 9% (at an unlikely pH 1 [30]), i.e., far lower than found in meteorites.
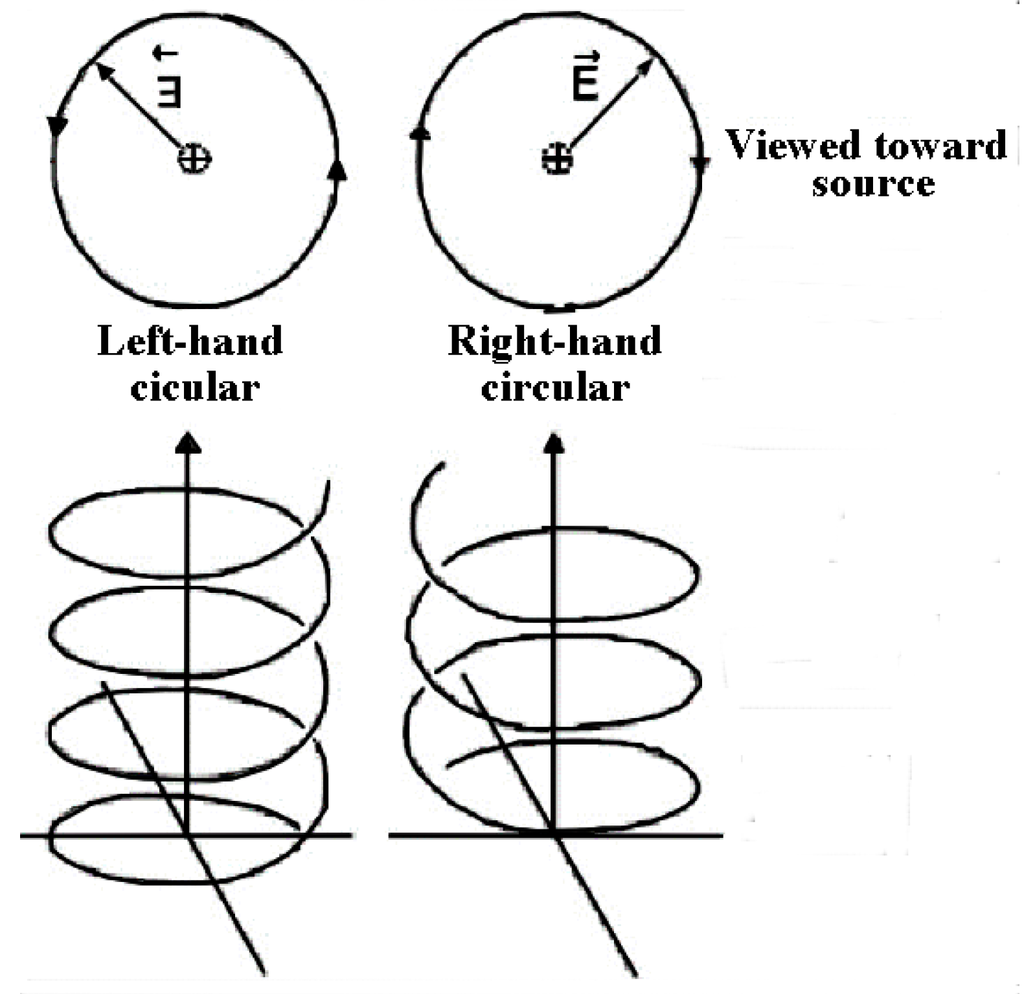
Figure 2.
Graphic representation of Left and Right Circularly Polarized Light.
These experimental data leave open the possibility that ee were first formed in precursor molecules, such as the aldehydes mentioned above, which would also justify the findings for ile and allo. Aldehydes have not been studied in this regard, e.g., their circular dichroism is not known, nor is their behavior upon CPL irradiation, and the field is, in fact, yet open to inquiry. However, the proposal of their participation as precursors to the syntheses of amino acids in meteorites would draw the important inference that, because chiral aldehydes racemize quickly in water, in fact, much more rapidly than amino acids, the synthetic window for amino acids in meteorites from non-racemic aldehydes during the asteroidal water processes should have been short and icy [22]. Early parent body aggregates or protoplanetary disc environments would fit the scenario, with ammonia abundance possibly providing a lower freezing point of water.
Although the scenario also implies that meteoritic ee would be rare and could be detected for 2-amino acids only in the special cases, such as for the diastereomer compounds described above, the hypothesis does not explain the ee for 2maa, of which precursors would be ketones that cannot racemize. Additionally, the large heterogeneity of ee values demonstrated throughout these studies of meteoritic chiral compounds is very puzzling and has brought other proposals that the formation of ee could be related to asteroidal parent body effects during aqueous stages [12,31] and, in particular, catalysis from their mineral phases.
This was first addressed to justify the large variability of isovaline ee between contiguous fragments of the Murchison meteorite and, by their X-ray diffraction analyses, showed a possible correlation between isovaline ee and hydrous silicates abundances [16]. In the case of CR meteorites mentioned above, that their aqueous processes are found to be variable within the meteorite matrix [31] would also satisfy the observation of varying ee detected between meteorites’ samples.
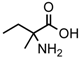
The recent findings of two non-racemic amines in several CR2 meteorites with ee as high as 60% [15] were able to further this discussion. Because, as for amino acids, amines’ circular dichroism [15] and anisotropy spectra [32] do not encourage suggestions of possible asymmetric effects by UV CPL, it has been proposed that the ketones precursor to these amines, while not chiral, could have benefited by adsorption upon mineral phases possessing asymmetry. Such minerals, e.g., magnetite seen to have in meteorites helical structures [33], could then provide chiral induction during syntheses. For 2-methylamines, it could have been a reductive amination process made possible by the large abundance of ammonia known to be present in this type of meteorites, e.g., [34]. If further confirmed experimentally, processes like these would point to possible important roles for Asteroidal bodies, their aqueous history and the minerals ensuing from warm hydrous stages in the development of chiral asymmetry in prebiotic molecules.
4. Prebiotic Chiral Asymmetry and the Origins of Life
The origins of life are utterly unknown, have been debated for close to a century, and the only achieved consensus is one of life likely emerging from inanimate molecules, about 3.5 billion years ago and by diverse possible scenarios, such as syntheses in the early atmosphere, the deep Earth or by input of organics materials from comets and meteorites (e.g., [5,35,36]). As mentioned already, and as Cronin and Chang [37] remarked pointedly, the known meteoritic inventory discussed above “…is clearly not the recipe for constructing the progenote…and a basic challenge resides in finding…where diversity that characterizes chemical evolution…gives way to the selectivity of life”. The question therefore is: Has the molecular asymmetry of meteoritic compounds changed the odds of an exobiology?
One distinguishing characteristic of Earth life is that the structure and functions of biopolymers rely on the exclusive one-handedness of their monomeric components, i.e., most protein amino acids have an L-configuration while sugars in RNA and DNA have a D-configuration and substitutions along the polymers with enantiomers of opposite handedness usually result in loss of function. That the trait of molecular asymmetry could be, or become by evolution, so pervasive as to define life processes and that several meteoritic compounds may have ee of the same L-configuration as terrestrial proteins do seem to provide a new dimension to this debate.
However, the overall data also pose a caveat. As we have seen, the asteroidal aqueous environments, as recorded in the mineralogy of all meteorites containing organic compounds of prebiotic interest, tend to racemize either the α-H amino acids or their aldehyde precursors, making ee for these amino acids scarce in the mature parent bodies. The α-substituted species that do not racemize would be more abundant but possibly less reactive. On the other hand, their ketone precursors do not racemize at all and could, in fact, have been very desirable prebiotic reactants. Moreover, some of the CC meteorites, such as the Renazzo-type, contain, not only abundant amino acids, but also large abundance of ammonia [38,39,40], an indispensable reactant for their syntheses. The combination of mineral catalysts with exogenously delivered key ingredients and chiral compounds, therefore, may still represent a very good evolutionary chance in prebiotic chemistry.
Acknowledgments
The author is grateful for the near forty years of funding from the NASA Exobiology and Astrobiology Division and the current support from the National Aeronautics and Space Administration under Agreement No. NNX15AD94G for the program “Earths in Other Solar Systems”.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Herbst, E.; Van Dishoeck, E.F. Complex organic interstellar molecules. Annu. Rev. Astron. Astrophys. 2009, 47, 427–480. [Google Scholar] [CrossRef]
- Schopf, J.W. Fossil evidence of Archaean life. Philos. Trans. R. Soc. B 2006, 361, 869–885. [Google Scholar] [CrossRef] [PubMed]
- Gomes, R.; Levison, H.F.; Tsiganis, K.; Morbidelli, A. Origin of the cataclysmic Late Heavy Bombardment period of the terrestrial planets. Nature 2005, 435, 466–469. [Google Scholar] [CrossRef] [PubMed]
- Strom, R.G.; Malhotra, R.; Takashi, I.; Yoshida, F.; Kring, D.A. The origin of planetary impactors in the Early Solar System. Science 2005, 309, 1847–1850. [Google Scholar] [CrossRef] [PubMed]
- Pizzarello, S.; Cooper, G.W.; Flynn, G.J. The nature and distribution of the organic material in carbonaceous chondrites and interplanetary dust particles. In Meteorites and the Early Solar System II; Lauretta, D., McSween, H.Y., Eds.; University of Arizona Press: Tucson, AZ, USA, 2006; pp. 625–651. [Google Scholar]
- Sephton, M.A. Organic compounds in carbonaceous meteorites. Nat. Prod. Rep. 2002, 19, 292–311. [Google Scholar] [CrossRef] [PubMed]
- Pizzarello, S.; Shock, E. The organic composition of carbonaceous meteorites: The evolutionary story ahead of biochemistry. Cold Spring Harb. Perspect. Biol. 2010, 2, a002105. [Google Scholar] [CrossRef] [PubMed]
- Schmitt-Kopplin, P.; Gabelicab, Z.; Gougeonc, R.D.; Feketea, A.; Kanawatia, B.; Harira, M.; Gebefuegia, I.; Eckeld, G.; Hertkorn, N. High molecular diversity of extraterrestrial organic matter in Murchison meteorite revealed 40 years after its fall. Proc. Nat. Acad. Sci. USA 2010, 107, 2763–2768. [Google Scholar] [CrossRef] [PubMed]
- Cronin, J.R.; Pizzarello, S. Enantiomeric excesses in meteoritic amino acids. Science 1997, 275, 951–955. [Google Scholar] [CrossRef] [PubMed]
- Engel, M.H.; Nagy, B. Distribution and enantiomeric composition of amino acids in the Murchison meteorite. Nature 1982, 296, 837–840. [Google Scholar] [CrossRef]
- Bada, J.L.; Cronin, J.R.; Ho, M.-S.; Kvenvolden, K.A.; Lawless, J.G.; Miller, S.L.; Oro, J.; Steinberg, S. On the reported optical-activity of amino acids in the Murchison meteorite. Nature 1983, 301, 494–496. [Google Scholar] [CrossRef]
- Pizzarello, S.; Groy, T.L. Molecular asymmetry in extraterrestrial organic chemistry: An analytical perspective. Geochim. Cosmochim. Acta 2011, 75, 645–656. [Google Scholar] [CrossRef]
- Glavin, L.P.; Elsila, J.E.; Burton, A.S.; Callahan, M.P.; Dworkin, J.P.; Hilts, R.W.; Herd, C.D.K. Unusual nonterrestrial l-proteinogenic amino acid excesses in the Tagish Lake meteorite. Met. Planet. Sci. 2012, 47, 1347–1364. [Google Scholar] [CrossRef]
- Pizzarello, S.; Wang, Y.; Chaban, G.M. A comparative study of the hydroxy acids from the Murchison, GRA 95229 and LAP 02342 meteorites. Geochim. Cosmochim. Acta 2010, 74, 6206–6217. [Google Scholar] [CrossRef]
- Cooper, G.; Rios, A.C. Meteoritic sugar derivatives: Enantiomer excesses and laboratory attempts at duplication. In Lunar Planetary Science Conference, Houston, TX, USA, 16-20 March 2015. Abstract #2993.
- Pizzarello, S.; Yarnes, C.T. Enantiomeric excesses of chiral amines in ammonia-rich carbonaceous meteorites. Earth Planet. Sci. Lett. 2016, 443, 176–184. [Google Scholar] [CrossRef]
- Pizzarello, S.; Zolensky, M.; Turk, K.A. Non racemic isovaline in the Murchison meteorite: Chiral distribution and mineral association. Geochim. Cosmochim. Acta 2003, 67, 1589–1595. [Google Scholar] [CrossRef]
- Glavin, D.P.; Dworkin, J.P. Enrichment of the amino acid l-isovaline by aqueous alteration on CI and CM meteorite parent bodies. Proc. Natl. Acad. Sci. USA 2009, 106, 5487–5492. [Google Scholar] [CrossRef] [PubMed]
- Pizzarello, S.; Huang, Y. The deuterium enrichment of individual amino acids in carbonaceous meteorites: A case for the presolar distribution of biomolecules precursors. Geochim. Cosmochim. Acta 2005, 69, 599–605. [Google Scholar] [CrossRef]
- De Marcellus, P.; Meinert, C.; Myrgorodska, I.; Nahon, L.; Buhse, T.; Le Sargent d’Hencourt, L.; Meierhenrich, U.J. Aldehydes and sugars from evolved precometary ice analogs: Importance of ices in astrochemical and prebiotic evolution. Proc. Natl. Acad. Sci. USA 2015, 112, 965–970. [Google Scholar] [CrossRef] [PubMed]
- Goesmann, F.; Rosenbauer, H.T.; Bredehoeft, J.H.; Cabane, M.; Ehrenfreund, P.; Gautier, T.; Giri, C.; Kruger, H.; le Roy, L.; MacDermott, A.J.; et al. Organic compounds on comet 67P/Churyumov-Gerasimenko revealed by COSAC mass spectrometry. Science 2015, 349. [Google Scholar] [CrossRef] [PubMed]
- Pizzarello, S.; Huang, Y.; Alexandre, M.R. Molecular asymmetry in extraterrestrial chemistry: Insights from a pristine meteorite. Proc. Natl. Acad. Sci. USA 2008, 105, 3700–3704. [Google Scholar] [CrossRef] [PubMed]
- Pizzarello, S.; Schrader, D.L.; Monroe, A.A.; Lauretta, D.S. Large enantiomeric excesses in primitive meteorites and the diverse effects of water in chemical evolution. Proc. Natl. Acad. Sci. USA 2012, 109, 11949–11954. [Google Scholar] [CrossRef] [PubMed]
- Meierhenrich, U.J. Amino Acids and the Asymmetry of Life; Springer: Heidelberg, Germany, 2008. [Google Scholar]
- Rubenstein, E.; Bonner, W.A.; Noyes, H.P.; Brown, G.S. Supernovae and life. Nature 1983, 306, 118. [Google Scholar] [CrossRef]
- Quack, M. How important is parity violation for molecular and biomolecular chirality? Angew. Chem. Int. Ed. 2002, 41, 4618–4630. [Google Scholar] [CrossRef] [PubMed]
- Balavoine, G.; Moradpour, A.; Kagan, H.B. Preparation of chiral compounds with high optical purity by irradiation with Circularly Polarized Light, a model reaction for the prebiotic generation of optical activity. J. Am. Chem. Soc. 1974, 96, 5152–5158. [Google Scholar] [CrossRef]
- Flores, J.J.; Bonner, W.A.; Massey, G.A. Asymmetric photolysis of (RS)-leucine with circularly polarized light. J. Am. Chem. Soc. 1977, 99, 3622–3625. [Google Scholar] [CrossRef] [PubMed]
- Meierhenrich, U.J.; Filippi, J.J.; Meinart, C.; Bredehöft, J.H.; Tkahashi, J.; Nahon, L.; Jones, N.C.; Sóren, V.H. Circular dichroism of amino acids in the vacuum-ultraviolet region. Angew. Chem. Int. Ed. 2010, 49, 7799–7802. [Google Scholar] [CrossRef] [PubMed]
- Nishino, H.; Kosaka, A.; Hembury, G.A.; Matsushima, K.; Inoue, Y. The pH dependence of the anisotropy factors of essential amino acids. J. Chem. Soc. Perkin Trans. 2002, 23, 582–590. [Google Scholar] [CrossRef]
- Schrader, D.L.; Connolly, H.C., Jr.; Lauretta, D.S.; Zega, T.J.; Davidson, J.; Domanik, K.J. The formation and alteration of Renazzo-like carbonaceous chondrites III: Toward understanding the genesis of ferromagnesian chondrules. Meteorit. Planet. Sci. 2015, 50, 15–50. [Google Scholar] [CrossRef]
- Meinert, C.; Jones, N.C.; Hoffman, S.V.; Meierhenrich, U.J. Anysotropy spectroscopy of chiral alcohols, amines and monocarboxylic acids: Implication for the analyses of extraterrestrial samples. J. Photochem. Photobiol. A Chem. 2016. [Google Scholar] [CrossRef]
- Hua, X.; Buseck, P.R. Unusual forms of magnetite in the Orgueil carbonaceous chondrite. Meteorit. Planet. Sci. 1998, 33, A215–A220. [Google Scholar] [CrossRef]
- Pizzarello, S.; Williams, L.B. Ammonia in the early Solar System: An account from carbonaceous meteorites. Astrophys. J. 2012, 749, 161–166. [Google Scholar] [CrossRef]
- Sandford, S.A.; Aléon, J.; Alexander, C.M.; Araki, T.; Bajt, S.; Baratta, G.A.; Borg, J.; Bradley, J.P.; Brownlee, D.E.; Brucato, J.R.; et al. Organics captured from Comet 81P/Wild 2 by the Stardust Spacecraft. Science 2006, 340, 1720–1724. [Google Scholar] [CrossRef] [PubMed]
- Orgel, L.E. The origin of life: A review of facts and speculation. In The Nature of Life: Classical and Contemporary Perspective from Philosophy and Science; Bedau, M.D., Clealand, C.E., Eds.; Cambridge University Press: Cambridge, UK, 2010; pp. 121–128. [Google Scholar]
- Cronin, J.R.; Chang, S. Organic matter in meteorites: molecular and isotopic analyses of the Murchison meteorite. In The Chemistry of Life’s Origins; Greenberg, J.M., Mendoza-Gómez, C.X., Pirronello, V., Eds.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 1993; pp. 209–258. [Google Scholar]
- Pizzarello, S.; Williams, L.B.; Lehman, J.; Holland, G.P.; Yarger, J.L. Abundant ammonia in primitive asteroids and the case for a possible exobiology. Proc. Natl. Acad. Sci. USA 2011, 108, 4303–4306. [Google Scholar] [CrossRef] [PubMed]
- Pizzarello, S.; Bose, M. The path of reduced nitrogen towards early Earth: The cosmic trail and its solar shortcuts. Astrophs. J. 2015, 814, 107–1015. [Google Scholar] [CrossRef]
- Pizzarello, S.; Holmes, W. Nitrogen-containing compounds in two CR meteorites: 15N composition, molecular distribution and precursor molecules. Geochim. Cosmochim. Acta 2009, 73, 2150–2162. [Google Scholar] [CrossRef]
© 2016 by the author; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).