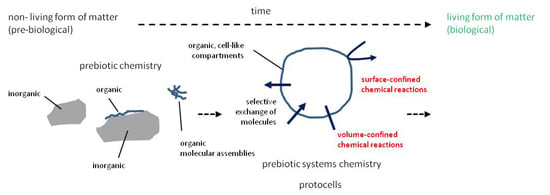
Current Ideas about Prebiological Compartmentalization
Abstract
:1. Introduction
2. Models for Prebiological Compartmentalization
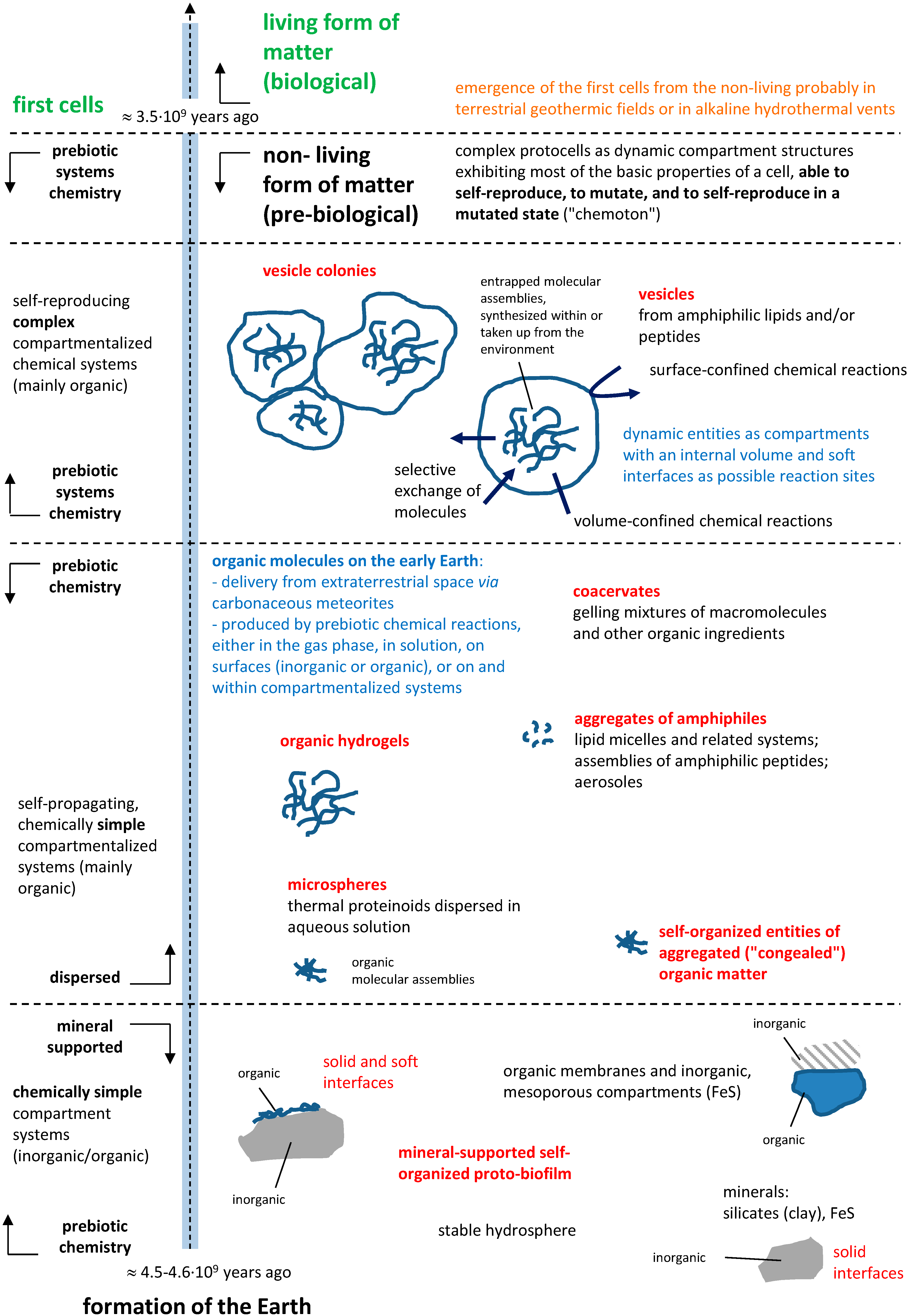
3. Sources of Potentially Prebiotic Compartment Building Blocks
4. Experimental Approaches to Potentially Prebiological Compartmentalization
4.1. Assemblies of Lipidic Amphiphiles
4.2. Assemblies of Macromolecules
4.3. Mineral Surfaces and Related Inorganic Systems
4.4. Mixed Compartment Systems
5. Possible Roles of Prebiotic Compartments
| Cellular compartments | Potentially prebiological compartments | References |
|---|---|---|
| Ensure cell integrity by encapsulation | Ensure some form of integrity by co-localization and encapsulation | [13,31,136] |
| Define the system compositional identity and heritable traits | [137] | |
| Support metabolism by providing an heterogeneous medium | Promote catalytic reactions | [37,48,79] |
| Stabilize reaction intermediates through incorporation into compartment boundaries | [138] | |
| Regulate exchanges with the environment by use of complex exchange systems and simple permeability | Regulate exchanges of molecules with the environment by association and simple permeability | [40,75,139,140] |
| Support energy uptake and conversion | Support energy uptake and conversion | [119,123,141,142] |
| Trigger self-reproduction | [143] | |
| Impart evolutionary advantages due to its composition | [112,144,145] |
5.1. Co-Localization versus Encapsulation and Its Impact on Prebiological Catalytic Abilities
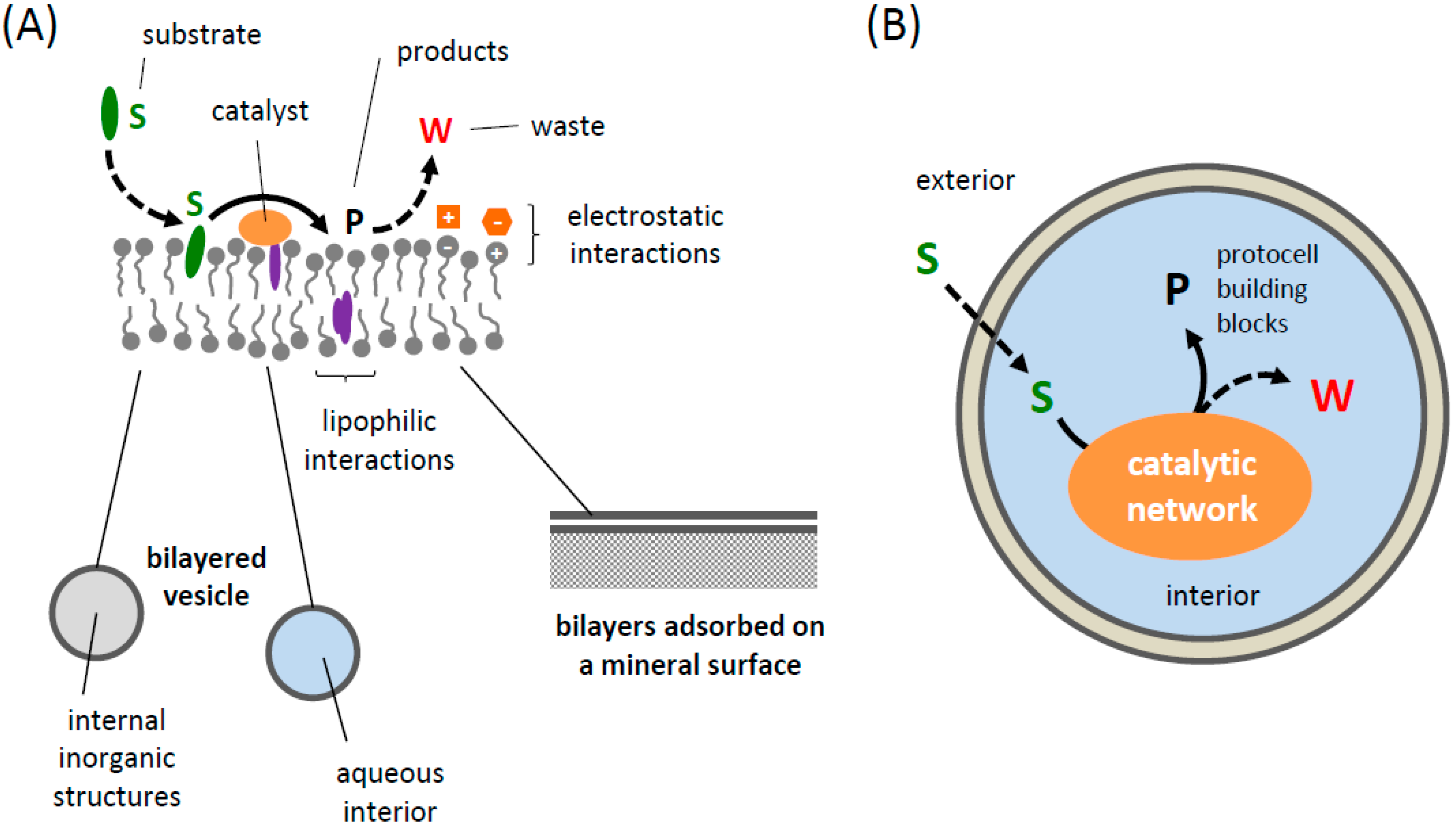
5.2. Compartment Reproduction
5.3. Specific Compartment Functions
6. Implications of the Protocell Model Development
7. Conclusions and Outlook
Acknowledgments
Conflicts of Interest
References
- Lazcano, A. Origin of Life. In Encyclopedia of Astrobiology; Springer: Berlin/Heidelberg, Germany, 2011; pp. 1183–1190. [Google Scholar]
- Luisi, P.L. About various definitions of Life. Orig. Life Evol. Biosph. 1998, 28, 613–622. [Google Scholar] [CrossRef]
- Alberts, B.; Johnson, A.; Lewis, J.; Morgan, D.; Raff, M.; Roberts, K.; Walter, P. Molecular Biology of the Cell, 6th ed.; Garland Science: New York, NY, USA, 2015. [Google Scholar]
- Caetano-Anollés, G.; Seufferheld, M.J. The Coevolutionary Roots of Biochemistry and Cellular Organization Challenge the RNA World Paradigm. J. Mol. Microbiol. Biotechnol. 2013, 23, 152–177. [Google Scholar] [CrossRef] [PubMed]
- Deamer, D.W. On the origin of systems. Systems biology, synthetic biology and the origin of life. EMBO Rep. 2009, 10, S1–S4. [Google Scholar] [CrossRef] [PubMed]
- Luisi, P.L. The Emergence of Life. From Chemical Origins to Synthetic Biology; Cambridge University Press: Cambridge, UK, 2006. [Google Scholar]
- Egel, R. Origins and Emergent Evolution of Life: The Colloid Microsphere Hypothesis Revisited. Orig. Life Evol. Biosph. 2014, 44, 87–110. [Google Scholar] [CrossRef] [PubMed]
- Koonin, E.V. The origins of cellular life. Antoine van Leeuwenhoek 2014, 106, 27–41. [Google Scholar] [CrossRef]
- Kurland, C.G. The RNA dreamtime. Bioessays 2010, 32, 866–871. [Google Scholar] [CrossRef]
- Egel, R. Peptide-dominated membranes preceding the genetic takeover by RNA: Latest thinking on classic controversy. Bioessays 2009, 31, 1100–1109. [Google Scholar] [CrossRef] [PubMed]
- Van der Gulik, P.T.S.; Speijer, D. How Amino Acids and Peptides Shaped the RNA World. Life 2015, 5, 230–246. [Google Scholar] [CrossRef] [PubMed]
- Wilson, M.A.; Wei, C.; Pohorille, A. Towards Co-Evolution of Membrane Proteins and Metabolism. Orig. Life Evol. Biosph. 2015. [Google Scholar] [CrossRef]
- Szostak, J.W.; Bartel, D.P.; Luisi, P.L. Synthesizing life. Nature 2001, 409, 387–390. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, S.; Chen, L.; Deamer, D.; Krakauer, D.C.; Packard, N.H.; Stadler, P.F.; Bedau, M.A. Transitions from nonliving to living matter. Science 2004, 303, 963–965. [Google Scholar] [CrossRef] [PubMed]
- Ludlow, R.F.; Otto, S. Systems Chemistry. Chem. Soc. Rev. 2008, 37, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Mirazo, K.; Briones, C.; Dela Escosura, A. Prebiotic System Chemistry: New Perspectives for the Origins of Life. Chem. Rev. 2013, 114, 285–366. [Google Scholar] [CrossRef] [PubMed]
- Kauffman, S.A. Approaches to the Origin of Life on Earth. Life 2011, 1, 34–48. [Google Scholar] [CrossRef] [PubMed]
- Svetina, S. Vesicle Budding and the Origin of Cellular Life. ChemPhysChem 2009, 10, 2769–2776. [Google Scholar] [CrossRef] [PubMed]
- Morowitz, H.J.; Heinz, B.; Deamer, D.W. The Chemical Logic of a Minimum Protocell. Orig. Life Evol. Biosph. 1988, 18, 281–287. [Google Scholar] [CrossRef] [PubMed]
- Deamer, D. First life: Discovering the Connections between Stars, Cells and How Life Began; University of California Press: Berkeley, CA, USA, 2011. [Google Scholar]
- Chen, I.A.; Walde, P. From Self-Assembled Vesicles to Protocells. Cold Spring Harb. Perspect Biol. 2010, 2, a002170. [Google Scholar] [CrossRef] [PubMed]
- Meierhenrich, U.J.; Filippi, J.-J.; Meinert, C.; Vierling, P.; Dworkin, J.P. On the Origin of Primitive Cells: From Nutrient Intake to Elongation of Encapsulated Nucleotides. Angew. Chem. Int. Ed. 2010, 49, 3738–3750. [Google Scholar] [CrossRef]
- Kandler, O. Cell wall biochemistry in Archaea and its phylogenetic implications. J. Biol. Phys. 1994, 20, 165–169. [Google Scholar] [CrossRef]
- Wächtershäuser, G. From pre-cells to Eukarya—a tale of two lipids. Mol. Microbiol. 2003, 47, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Tan, C. The engineering of artificial cellular systems using synthetic biology approaches. WIREs NanoMed. Nanobiotechnol. 2014, 6, 369–383. [Google Scholar] [CrossRef]
- Kamat, N.P.; Katz, J.S.; Hammer, D.A. Engineering Polymersome Protocells. J. Phys. Chem. Lett. 2011, 2, 1612–1623. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Jakobsson, E.; Wang, Y.; Brinker, C.J. Nanoporous Silica-Based Protocells at Multiple Scales for Designs of Life and Nanomedicine. Life 2015, 5, 214–229. [Google Scholar] [CrossRef] [PubMed]
- Yeates, T.O.; Kerfeld, C.A.; Heinhorst, S.; Cannon, G.C.; Shively, J.M. Protein-based organelles in bacteria: Carboxysomes and related microcompartments. Nat. Rev. Microbiol. 2008, 6, 681–691. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, C.; Sinha, A. Diverse Bacterial Microcompartment Organelles. Microbiol. Mol. Biol. Rev. 2014, 78, 438–468. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Huang, X.; Tang, T.-Y.D.; Mann, S. Synthetic cellularity based on non-lipid micro-compartments and protocell models. Curr. Opin. Chem. Biol. 2014, 22, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Monnard, P.-A.; Deamer, D.W. Membrane self-assembly processes: Steps towards the first cellular life. Anat. Rec. 2002, 268, 196–207. [Google Scholar] [CrossRef] [PubMed]
- Walde, P. Surfactant assemblies and their various possible role for the origin(s) of Life. Orig. Life Evol. Biosph. 2006, 36, 109–150. [Google Scholar] [CrossRef] [PubMed]
- Nakatani, Y.; Ribeiro, N.; Streiff, S.; Gotoh, M.; Pozzi, G.; Désaubry, L.; Milon, A. Search for the Most ‘primitive’ Membranes and Their Reinforcers: A Review of the Polyprenyl Phosphates Theory. Orig. Life Evol. Biosph. 2014, 44, 197–208. [Google Scholar] [CrossRef] [PubMed]
- Budin, I.; Szostak, J.W. Expanding Roles for Diverse Physical Phenomena during the Origin of Life. Annu. Rev. Biophys. 2010, 39, 245–263. [Google Scholar] [CrossRef] [PubMed]
- Fishjis, M. Steps Towards the Formation of a Protocell: The possible Role of Short Peptides. Orig. Life Evol. Biosph. 2007, 37, 537–553. [Google Scholar] [CrossRef] [PubMed]
- Cronin, L. Inorganic Molecular Capsules: From Structure to Function. Angew. Chem. Int. Ed. 2006, 45, 3576–3578. [Google Scholar] [CrossRef]
- Li, M.; Huang, X.; Mann, S. Spontaneous Growth and Division in Self-reproducing Inorganic Colloidosomes. Small 2014, 10, 3291–3298. [Google Scholar] [CrossRef] [PubMed]
- Weber, A.L. Growth of organic microspherules in sugar-ammonia reactions. Orig. Life Evol. Biosph. 2005, 35, 523–536. [Google Scholar] [CrossRef] [PubMed]
- Apel, C.L.; Mautner, M.N.; Deamer, D.W. Self-assembled vesicles of monocarboxylic acids and alcohols: Conditions for stability and for encapsulation of biopolymers. Biochim. Biophys. Acta 2002, 1559, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Maurer, S.E.; Deamer, D.W.; Boncella, J.M.; Monnard, P.A. Chemical evolution of amphiphiles: Glycerol monoacyl derivatives stabilize plausible prebiotic membranes. Astrobiology 2009, 9, 979–987. [Google Scholar] [CrossRef] [PubMed]
- Sacerdote, M.G.; Szostak, J.W. Semipermeable lipid bilayers exhibit diastereoselectivity favoring ribose. Proc. Natl. Acad. Sci. USA 2005, 102, 6004–6008. [Google Scholar] [CrossRef] [PubMed]
- Mansy, S.S.; Szostak, J.W. Thermostability of model protocell membranes. Proc. Natl Acad. Sci. USA 2008, 105, 13351–13355. [Google Scholar] [CrossRef] [PubMed]
- McKay, C.P. Requirements and limits for life in the context of exoplanets. Proc. Natl. Acad. Sci. USA 2014, 111, 12628–12633. [Google Scholar] [CrossRef] [PubMed]
- Keller, M.; Blöchl, E.; Wächtershäuser, G.; Stetter, K.O. Formation of amide bonds without a condensation agent and implications for origin of life. Nature 1994, 368, 836–838. [Google Scholar] [CrossRef] [PubMed]
- Cody, G.D.; Boctor, N.Z.; Filley, T.R.; Hazen, R.M.; Scott, J.H.; Sharma, A.; Yoder, H.S., Jr. Primordial carbonylated iron-sulfur compounds and synthesis of pyruvate. Science 2000, 289, 1337–1340. [Google Scholar] [CrossRef] [PubMed]
- Ferris, J.P.; Hill, A.R., Jr.; Liu, R.; Orgel, L.E. Synthesis of long prebiotic oligomers on mineral surfaces. Nature 1996, 381, 59–61. [Google Scholar] [CrossRef] [PubMed]
- Hanczyc, M.M.; Mansy, S.S.; Szostak, J.W. Mineral surface directed membrane assembly. Orig. Life Evol. Biosph. 2006, 37, 67–82. [Google Scholar] [CrossRef] [PubMed]
- Maurer, S.E.; DeClue, M.S.; Albertsen, A.N.; Dörr, M.; Kuiper, D.S.; Ziock, H.; Rasmussen, S.; Boncella, J.M.; Monnard, P.-A. Interactions between catalyst and amphiphilic structures and their implications for a protocell model. ChemPhysChem 2011, 12, 828–835. [Google Scholar] [CrossRef] [PubMed]
- Wamberg, M.C.; Wieczorek, R.; Bo Brier, S.; de Vries, J.W.; Kwak, M.; Herrmann, A.; Monnard, P.-A. Functionalization of Fatty Acid Vesicles through Newly Synthesized Bolaamphiphile-DNA Conjugates. Bioconjugate Chem. 2014, 25, 1678–1688. [Google Scholar] [CrossRef]
- Tessera, M. Life Began When Evolution Began: A Lipidic Vesicle-Based Scenario. Orig. Life Evol. Biosph. 2009, 39, 559–564. [Google Scholar] [CrossRef] [PubMed]
- Koga, S.; Williams, D.S.; Perriman, A.W.; Mann, S. Peptide–nucleotide microdroplets as a step towards a membrane-free protocell model. Nat. Chem. 2011, 3, 720–724. [Google Scholar] [CrossRef] [PubMed]
- Lunine, J.I. Earth: Evolution of a Habitable World; Cambridge University Press: Cambridge, UK, 1999. [Google Scholar]
- Watson, E.B.; Harrison, T.M. Zircon thermometer reveals minimum melting conditions on earliest Earth. Science 2005, 308, 841–844. [Google Scholar] [CrossRef] [PubMed]
- Alexander, C.M.O’D.; Bowden, R.; Fogel, M.L.; Howard, K.T.; Herd, C.D.K.; Nittler, L.R. The Provenances of Asteroids, and Their Contributions to the Volatile Inventories of the Terrestrial Planets. Science 2012, 337, 721–723. [Google Scholar] [CrossRef] [PubMed]
- Mulkidjanian, A.Y.; Bychkov, A.Y.; Bibrova, D.V.; Galperin, M.Y.; Koonin, E.V. Origin of first cells at terrestrial, anoxic geothermal fields. Proc. Natl. Acad. Sci. USA 2012, 109, E821–E830. [Google Scholar] [CrossRef] [PubMed]
- Lane, N.; Martin, W.F. The Origin of Membrane Bioenergetics. Cell 2012, 151, 1406–1416. [Google Scholar] [CrossRef] [PubMed]
- Miller, S.L. A Production of Amino Acids under Possible Primitive Earth Conditions. Science 1953, 117, 528–529. [Google Scholar] [CrossRef] [PubMed]
- Wächtershäuser, G. Groundworks for an evolutionary biochemistry: The iron-sulfur world. Prog. Biophys. Mol. Biol. 1992, 58, 85–201. [Google Scholar] [CrossRef] [PubMed]
- Jheeta, S.; Joshi, P.C. Prebiotic RNA Synthesis by Montmorillonite Catalysis. Life 2014, 4, 318–330. [Google Scholar] [CrossRef] [PubMed]
- Lazcano, A. Primordial Soup. In Encyclopedia of Astrobiology; Springer: Berlin/Heidelberg, Germany, 2011; pp. 1334–1337. [Google Scholar]
- Gilbert, W. The RNA world. Nature 1986, 319. [Google Scholar] [CrossRef]
- Joyce, G.F. RNA evolution and the origins of life. Nature 1989, 338, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Oró, J. Synthesis of adenine from ammonium cyanide. Biochem. Biophys. Res. Commun. 1960, 2, 407–412. [Google Scholar] [CrossRef]
- Oró, J.; Kimball, A.P. Synthesis of Purines under Possible Primitive Earth Conditions. Arch. Biochem. Biophys. 1962, 96, 293–313. [Google Scholar] [CrossRef] [PubMed]
- Orgel, L.E. Prebiotic Chemistry and the Origin of the RNA World. Crit. Rev. Biochem. Mol. Biol. 2004, 39, 99–123. [Google Scholar] [CrossRef] [PubMed]
- Powner, M.W.; Sutherland, J.D.; Szostak, J.W. The Origins of Nucleotides. Synlett 2011, 14, 1956–1964. [Google Scholar] [CrossRef]
- Engelhart, A.E.; Powner, M.W.; Szostak, J.W. Functional RNAs exhibit tolerance for non-heritable 2'-5' versus 3'-5' backbone heterogeneity. Nat. Chem. 2013, 5, 390–394. [Google Scholar] [CrossRef] [PubMed]
- Patel, B.H.; Percivalle, C.; Ritson, D.J.; Duffy, C.D.; Sutherland, J.D. Common origins of RNA, protein and lipid precursors in a cyanosulfidic protometabolism. Nat. Chem. 2015, 7, 301–307. [Google Scholar] [CrossRef] [PubMed]
- Fox, S.W.; Dose, K. Molecular Evolution and the Origin of Life; W. H. Freeman and Company: San Francisco, CA, USA, 1972. [Google Scholar]
- Segré, D.; Ben-Eli, D.; Deamer, D.W.; Lancet, D. The Lipid World. Orig. Life Evol. Biosph. 2001, 31, 119–145. [Google Scholar] [CrossRef] [PubMed]
- Damer, B.; Deamer, D. Coupled Phases and Combinatorial Selection in Fluctuating Hydrothermal Pools: A Scenario to Guide Experimental Approaches to the Origin of Cellular Life. Life 2015, 5, 872–887. [Google Scholar] [CrossRef] [PubMed]
- Sturgis, J.N. Peptide-Dominated Vesicles: Bacterial Internal Membrane Compartments as Model Systems for Prebiotic Evolution. In Origins of Life: The Primal Self-Organization; Egel, R., Lankenau, D.-H., Mulkidjanian, A.Y., Eds.; Springer-Verlag: Berlin/Heidelberg, Germany, 2011; pp. 167–181. [Google Scholar]
- Dobson, C.M.; Ellison, G.B.; Tuck, A.F.; Vaida, V. Atmospheric aerosols as prebiotic chemical reactors. Proc. Natl. Acad. Sci. USA 2000, 97, 11864–11868. [Google Scholar] [CrossRef] [PubMed]
- Cleaves, H.J., II. Prebiotic Chemistry: Geochemical Context and Reaction Screening. Life 2013, 3, 331–345. [Google Scholar] [CrossRef] [PubMed]
- Walde, P.; Goto, A.; Monnard, P.-A.; Wessicken, M.; Luisi, P.L. Oparin’s reaction revisited: Enzymatic synthesis of poly(adenyl acid) in micelles and self-reproducing vesicles. J. Am. Chem. Soc. 1994, 116, 7541–7547. [Google Scholar] [CrossRef]
- Ruiz-Mirazo, K.; Mavelli, F. Question 7: Modelling Minimal ‘Lipid-Peptide’ Cells. Orig. Life Evol. Biosph. 2007, 37, 433–437. [Google Scholar] [CrossRef] [PubMed]
- Maurer, S.E.; Monnard, P.-A. Primitive Membrane Formation, Characteristics and Roles in Emergent Properties of a Protocell. Entropy 2011, 13, 466–484. [Google Scholar] [CrossRef]
- Walde, P.; Umakoshi, H.; Stano, P.; Mavelli, F. Emergent properties arising from the assembly of amphiphiles. Artificial vesicle membranes as reaction promoters and regulators. Chem. Commun. 2014, 50, 10177–10197. [Google Scholar] [CrossRef]
- Walde, P.; Wick, R.; Fresta, M.; Mangone, A.; Luisi, P.L. Autopoietic Self-Reproduction of Fatty Acid Vesicles. J. Am. Chem. Soc. 1994, 116, 11649–11654. [Google Scholar] [CrossRef]
- Carrara, P.; Stano, P.; Luisi, P.L. Giant Vesicles “Colonies”: A Model for Primitive Cell Communities. ChemBioChem 2012, 13, 1497–1502. [Google Scholar] [CrossRef] [PubMed]
- Paleos, C.M.; Tsiourvas, D.; Sideratou, T. Interaction of Vesicles: Adhesion, Fusion and Multicompartment Systems Formation. ChemBioChem 2011, 12, 512–521. [Google Scholar] [CrossRef]
- Gánti, T. The Principles of Life; Oxford University Press: Oxford, UK, 2003. [Google Scholar]
- Dworkin, L.P.; Deamer, D.W.; Sandford, S.A.; Allamandola, L.J. Self-assembling amphiphilic molecules: Synthesis in simulated interstellar/precometary ices. Proc. Natl. Acad. Sci. USA 2001, 98, 815–819. [Google Scholar] [CrossRef] [PubMed]
- Mautner, M.N.; Leonard, R.L.; Deamer, D.W. Meteorite Organics in Planetary Environments: Hydrothermal Release, Surface-Activity, and Microbial Utilization. Planet. Space Sci. 1995, 43, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Cooper, G.W.; Onwo, W.M.; Cronin, J.R. Alkyl phosphonic acids and sulfonic acids in the Murchison meteorite. Geochim. Cosmochim. Acta 1992, 56, 4109–4115. [Google Scholar] [CrossRef] [PubMed]
- Degraaf, R.M.; Visscher, J.; Schwartz, A.W. A plausibly prebiotic synthesis of phosphonic-acids. Nature 1995, 378, 474–477. [Google Scholar] [CrossRef] [PubMed]
- Pizzarello, S.; Holmes, W. Nitrogen-containing compounds in two CR2 meteorites: N-15 composition, molecular distribution and precursor molecules. Geochim. Cosmochim. Acta 2009, 73, 2150–2162. [Google Scholar] [CrossRef]
- Pasek, M.A.; Lauretta, D.S. Aqueous corrosion of phosphide minerals from iron meteorites: A highly reactive source of prebiotic phosphorus on the surface of the early Earth. Astrobiology 2005, 5, 515–535. [Google Scholar] [CrossRef] [PubMed]
- Pasek, M.A.; Lauretta, D.S. Extraterrestrial flux of potentially prebiotic C, N, and P to the early Earth. Orig. Life Evol. Biosph. 2008, 38, 5–21. [Google Scholar] [CrossRef] [PubMed]
- McCollom, T.M.; Ritter, G.; Simoneit, B.R.T. Lipid synthesis under hydrothermal conditions by Fischer-Tropsch-type reactions. Orig. Life Evol. Biosph. 1999, 29, 153–166. [Google Scholar] [CrossRef] [PubMed]
- Rushdi, A.I.; Simoneit, B.R.T. Lipid formation by aqueous Fischer-Tropsch-type synthesis over a temperature range of 100 to 400 degrees C. Orig. Life Evol. Biosph. 2001, 31, 103–118. [Google Scholar] [CrossRef] [PubMed]
- Apel, C.L.; Deamer, D.W. The formation of glycerol monodecanoate by a dehydration/condensation reaction: Increasing the chemical complexity of amphiphiles on the early earth. Orig. Life Evol. Biosph. 2005, 35, 323–332. [Google Scholar] [CrossRef] [PubMed]
- Simoneit, B.R.T.; Rushdi, I.A.; Deamer, D.W. Abiotic formation of acylglycerols under simulated hydrothermal conditions and self-assembly properties of such lipid products. Adv. Space Res. 2007, 40, 1649–1656. [Google Scholar] [CrossRef]
- Miller, S.L.; Urey, H.C. Organic Compound Synthesis on the Primitive Earth. Science 1959, 130, 245–251. [Google Scholar] [CrossRef] [PubMed]
- Kopetzki, D.; Antonietti, M. Hydrothermal formose reaction. New J. Chem. 2011, 35, 1787–1794. [Google Scholar] [CrossRef]
- Weber, A.L. Formation of pyrophosphate on hydroxyapatite with thioesters as condensing agents. Biosystems 1982, 15, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Morigaki, K.; Walde, P. Fatty Acid Vesicles. Curr. Opin. Colloid Interf. Sci. 2007, 12, 75–80. [Google Scholar] [CrossRef]
- Powner, M.W.; Sutherland, J.D. Prebiotic chemistry: A new modus operandi. Phil. Trans. R. Soc. B 2011, 366, 2870–2877. [Google Scholar] [CrossRef] [PubMed]
- Syren, R.M.; Sanjur, A.; Fox, S.W. Proteinoid microspheres more stable in hot than in cold water. Biosystems 1985, 17, 275–280. [Google Scholar] [CrossRef] [PubMed]
- Jakschitz, T.A.E.; Rode, B.M. Chemical evolution from simple inorganic compounds to chiral peptides. Chem. Soc. Rev. 2012, 41, 5484–5489. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Pan, F.; Xu, H.; Yaseen, M.; Shan, H.; Hauser, C.A.; Zhang, S.; Lu, J.R. Molecular self-assembly and applications of designer peptide amphiphiles. Chem. Soc. Rev. 2010, 39, 3480–3498. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Li, M.; Green, D.C.; Williams, D.S.; Patil, A.J.; Mann, S. Interfacial assembly of protein–polymer nano-conjugates into stimulus-responsive biomimetic protocells. Nat. Commun. 2013, 4. [Google Scholar] [CrossRef]
- Bernal, J. The physical basis of life. Proc. Phys. Soc. Lond. Sect. A 1949, 62, 537–558. [Google Scholar] [CrossRef]
- Russell, M.J.; Hall, A.J. The emergence of Life from iron monosulphide bubbles at a submarine hydrothermal redox and pH front. J. Geol. Soc. 1997, 154, 377–402. [Google Scholar] [CrossRef]
- Baaske, P.; Weinert, F.M.; Duhr, S.; Lemke, K.H.; Russell, M.J.; Braun, D. Extreme accumulation of nucleotides in simulated hydrothermal pore systems. Proc. Natl. Acad. Sci. USA 2007, 104, 9346–9351. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Green, D.C.; Anderson, J.L.R.; Binks, B.P.; Mann, S. In vitro gene expression and enzyme catalysis in bio-inorganic protocells. Chem. Sci. 2011, 2, 1739–1745. [Google Scholar] [CrossRef]
- Black, R.A.; Blosser, M.C.; Stottrup, B.L.; Tavakley, R.; Deamer, D.W.; Keller, S.L. Nucleobases bind to and stabilize aggregates of a prebiotic amphiphile, providing a viable mechanism for the emergence of protocells. Proc. Natl. Acad. Sci. USA 2013, 110, 13272–13276. [Google Scholar] [CrossRef] [PubMed]
- Gebicki, J.M.; Hicks, M. Ufasomes are stable particles surrounded by unsaturated fatty acid membranes. Nature 1973, 243, 232–234. [Google Scholar] [CrossRef] [PubMed]
- Gebicki, J.M.; Hicks, M. Preparation and properties of vesicles enclosed by fatty acid membranes. Chem. Phys. Lipids 1976, 16, 142–160. [Google Scholar] [CrossRef] [PubMed]
- Monnard, P.-A.; Apel, C.L.; Kanavarioti, A.; Deamer, D.W. Influence of ionic solutes on self-assembly and polymerization processes related to early forms of life: Implications for a prebiotic aqueous medium. Astrobiology 2002, 2, 139–152. [Google Scholar] [CrossRef] [PubMed]
- Hargreaves, W.R.; Deamer, D.W. Liposomes from ionic, single-chain amphiphiles. Biochemistry 1978, 17, 3759–3768. [Google Scholar] [CrossRef] [PubMed]
- Budin, I.; Szostak, J.W. Physical effects underlying the transition from primitive to modern cell membranes. Proc. Natl. Acad. Sci. USA 2011, 108, 5249–5254. [Google Scholar] [CrossRef] [PubMed]
- Oparin, A.I.; Orlovskii, A.F.; Bukhlaeva, V.Y.; Gladilin, K.L. Influence of the enzymatic synthesis of polyadenylic acid on a coacervate system. Dokl. Akad. Nauk SSSR 1976, 226, 972–994. [Google Scholar] [PubMed]
- Tang, T.Y.D.; Antognozzi, M.; Vicary, J.A.; Perriman, A.W.; Mann, S. Small-molecule uptake in membrane-free peptide/nucleotide protocells. Soft Matter 2013, 9, 7647–7656. [Google Scholar] [CrossRef]
- Albertsen, A.N.; Maurer, S.E.; Nielsen, K.A.; Monnard, P.-A. Transmission of photo-catalytic function in a self-replicating chemical system: In-situ amphiphile production over two protocell generations. Chem. Commun. 2014, 50, 8989–8992. [Google Scholar] [CrossRef]
- Dora Tang, T.Y.; Rohaida Che Hak, C.; Thompson, A.J.; Kuimova, M.K.; Williams, D.S.; Perriman, A.W.; Mann, S. Fatty acid membrane assembly on coacervate microdroplets as a step towards a hybrid protocell model. Nat. Chem. 2014, 6, 527–533. [Google Scholar] [CrossRef] [PubMed]
- Jia, T.; Hentrich, C.; Szostak, J. Rapid RNA Exchange in Aqueous Two-Phase System and Coacervate Droplets. Orig. Life Evol. Biosph. 2014, 44, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Namani, T.; Deamer, D. Stability of Model Membranes in Extreme Environments. Orig. Life Evol. Biosph. 2008, 38, 329–341. [Google Scholar] [CrossRef] [PubMed]
- Groen, J.; Deamer, D.; Kros, A.; Ehrenfreund, P. Polycyclic Aromatic Hydrocarbons as Plausible Prebiotic Membrane Components. Orig. Life Evol. Biosph. 2012, 42, 295–306. [Google Scholar] [CrossRef] [PubMed]
- Arnould, A.; Perez, A.A.; Gaillard, C.; Douliez, J.-P.; Cousin, F.; Santiago, L.G.; Zemb, T.; Anton, M.; Fameau, A.-L. Self-assembly of myristic acid in the presence of choline hydroxide: Effect of molar ratio and temperature. J. Colloid Interf. Sci. 2015, 445, 285–293. [Google Scholar] [CrossRef]
- Douliez, J.-P.; Gaillard, C. Self-assembly of fatty acids: From foams to protocell vesicles. New J. Chem. 2014, 38, 5142–5148. [Google Scholar] [CrossRef]
- Kapoor, S.; Berghaus, M.; Suladze, S.; Prumbaum, D.; Grobelny, S.; Degen, P.; Raunser, S.; Winter, R. Prebiotic Cell Membranes that Survive Extreme Environmental Pressure Conditions. Angew. Chem. Int. Ed. 2014, 53, 8397–8401. [Google Scholar] [CrossRef]
- Cape, J.; Monnard, P.-A.; Boncella, J.M. Prebiotically relevant mixed fatty acid vesicles support anionic solute encapsulation and photochemically catalyzed trans-membrane charge transport. Chem. Sci. 2011, 2, 661–667. [Google Scholar] [CrossRef]
- Hanczyc, M.M.; Fujikawa, S.M.; Szostak, J.W. Experimental Models of Primitive Cellular Compartments: Encapsulation, Growth, and Division. Science 2003, 302, 618–622. [Google Scholar] [CrossRef] [PubMed]
- Budin, I.; Bruckner, R.J.; Szostak, J.W. Formation of Protocell-like Vesicles in a Thermal Diffusion Column. J. Am. Chem. Soc. 2009, 131, 9628–9629. [Google Scholar] [CrossRef] [PubMed]
- Caschera, F.; Bernadino de la Serna, J.; Löffler, P.M.G.; Rasmussen, T.E.; Hanczyc, M.M.; Bagatolli, L.A.; Monnard, P.-A. Stable Vesicles Composed of Mono- or Dicarboxylic Fatty Acids and Trimethylammonium Amphiphiles. Langmuir 2011, 27, 14078–14090. [Google Scholar] [CrossRef]
- Budin, I.; Prywes, N.; Zhang, N.; Szostak, J.W. Chain-Length Heterogeneity Allows for the Assembly of Fatty Acid Vesicles in Dilute Solutions. Biophys. J. 2014, 107, 1582–1590. [Google Scholar] [CrossRef] [PubMed]
- Williams, D.S.; Koga, S.; Hak, C.R.C.; Majrekar, A.; Patil, A.J.; Perriman, A.W.; Mann, S. Polymer/nucleotide droplets as bio-inspired functional micro-compartments. Soft Matter 2012, 8, 6004–6014. [Google Scholar] [CrossRef]
- Yang, D.; Peng, S.; Hartman, M.R.; Gupton-Campolongo, T.; Rice, E.J.; Chang, A.K.; Gu, Z.; Lu, G.Q.; Luo, D. Enhanced transcription and translation in clay hydrogel and implications for early life evolution. Sci. Rep. 2013, 3. [Google Scholar] [CrossRef] [PubMed]
- Ferris, J.P. Montmorillonite-catalysed formation of RNA oligomers: The possible role of catalysis in the origins of life. Phil. Trans. R. Soc. B 2006, 361, 1777–1786. [Google Scholar] [CrossRef] [PubMed]
- Bachmann, P.A.; Walde, P.; Luisi, P.L.; Lang, J. Self-Replicating Reverse Micelles and Chemical Autopoiesis. J. Am. Chem. Soc. 1990, 112, 8200–8201. [Google Scholar] [CrossRef]
- Wächtershäuser, G. Before enzymes and templates: Theory of surface metabolism. Microbiol. Rev. 1988, 52, 452–484. [Google Scholar] [PubMed]
- Blobel, G. Intracellular protein topogenesis. Proc. Natl. Acad. Sci. USA 1980, 77, 1496–1500. [Google Scholar] [CrossRef] [PubMed]
- Rajamani, S.; Vlassov, A.; Coombs, A.; Olasagasti, F.; Deamer, D.W. Lipid-assisted synthesis of RNA-like polymers from mononucleotides. Orig. Life Evol. Biosph. 2008, 38, 57–74. [Google Scholar] [CrossRef] [PubMed]
- Olasagasti, F.; Kim, H.J.; Pourmand, N.; Deamer, D.W. Non-enzymatic transfer of sequence information under plausible prebiotic conditions. Biochimie 2011, 93, 556–561. [Google Scholar] [CrossRef] [PubMed]
- Deamer, D.W. The first living systems: A bioenergetic perspective. Microbiol. Mol. Biol. Rev. 1997, 61, 230–261. [Google Scholar]
- Segre, D.; Ben-Eli, D.; Lancet, D. Compositional genomes: Prebiotic information transfer in mutually catalytic noncovalent assemblies. Proc. Natl. Acad. Sci. USA 2001, 99, 219–230. [Google Scholar]
- Todd, Z.R.; House, C.H. Vesicles Protect Activated Acetic Acid. Astrobiology 2014, 14, 859–865. [Google Scholar] [CrossRef] [PubMed]
- Chen, I.A.; Salehi-Ashtiani, K.; Szostak, J.W. RNA catalysis in model protocell vesicles. J. Am. Chem. Soc. 2005, 127, 13213–13219. [Google Scholar] [CrossRef] [PubMed]
- Mansy, S.S.; Schrum, J.P.; Krishnamurthy, M.; Tobe, S.; Treco, D.A.; Szostak, J.W. Template-directed synthesis of a genetic polymer in a model protocell. Nature 2008, 454, 122–125. [Google Scholar] [CrossRef] [PubMed]
- Deamer, D.W. Polycyclic Aromatic Hydrocarbons: Primitive Pigment Systems in the Prebiotic Environment. Adv. Space Res. 1992, 12, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Summers, D.P.; Noveron, J.; Basa, B.R.C. Energy Transduction Inside of Amphiphilic Vesicles: Encapsulation of Photochemically Active Semiconducting Particles. Orig. Life Evol. Biosph. 2008, 39, 127–140. [Google Scholar] [CrossRef]
- Zhu, T.F.; Adamala, K.; Zhang, N.; Szostak, J.W. Photochemically driven redox chemistry induces protocell membrane pearling and division. Proc. Natl. Acad. Sci. USA 2012, 109, 9828–9832. [Google Scholar] [CrossRef] [PubMed]
- Chen, I.A.; Roberts, R.W.; Szostak, J.W. The emergence of competition between model protocells. Science 2004, 305, 1474–1476. [Google Scholar] [CrossRef] [PubMed]
- Adamala, K.; Szostak, J.W. Competition between model protocells driven by an encapsulated catalyst. Nat. Chem. 2013, 5, 495–501. [Google Scholar] [CrossRef] [PubMed]
- Müller, U.F.; Bartel, D.P. Improved polymerase ribozyme efficiency on hydrophobic assemblies. RNA 2008, 14, 552–562. [Google Scholar] [CrossRef] [PubMed]
- Huber, C.; Wächtershäuser, G. Peptides by Activation of Amino Acids with CO on (Ni,Fe)S Surfaces: Implications for the Origin of Life. Science 1998, 281, 670–672. [Google Scholar] [CrossRef] [PubMed]
- Oberholzer, T.; Nierhaus, K.H.; Luisi, P.L. Protein expression in liposomes. Biochem. Biophys. Res. Commun. 1999, 261, 238–241. [Google Scholar] [CrossRef] [PubMed]
- Kuruma, Y.; Stano, P.; Ueda, T.; Luisi, P.L. A synthetic biology approach to the construction of membrane proteins in semi-synthetic minimal cells. Biochim. Biophys. Acta 2009, 1788, 567–574. [Google Scholar] [CrossRef] [PubMed]
- Kita, H.; Matsuura, T.; Sunami, T.; Hosoda, K.; Ichihashi, N.; Tsukada, K.; Urabe, I.; Yomo, T. Replication of Genetic Information with Self-Encoded Replicase in Liposomes. ChemBioChem 2008, 9, 2403–2410. [Google Scholar] [CrossRef] [PubMed]
- Lentini, R.; Santero, S.P.; Chizzolini, F.; Cecchi, D.; Fontana, J.; Marchioretto, M.; Del Bianco, C.; Terrell, J.L.; Spencer, A.C.; Martini, L.; et al. Integrating artificial with natural cells to translate chemical messages that direct E. coli behaviour. Nat. Commun. 2014, 5. [Google Scholar] [CrossRef] [PubMed]
- Bachmann, P.A.; Luisi, P.A.; Lang, J. Autocatalytic self-replicating micelles as models for prebiotic structures. Nature 1992, 357, 57–59. [Google Scholar] [CrossRef]
- Kurihara, K.; Tamura, M.; Shohda, K.; Toyota, T.; Suzuki, K.; Sugawara, T. Self-reproduction of supramolecular giant vesicles combined with the amplification of encapsulated DNA. Nat. Chem. 2011, 3, 775–781. [Google Scholar] [CrossRef] [PubMed]
- Andes-Koback, M.; Keating, C.D. Complete Budding and Asymmetric Division of Primitive Model Cells To Produce Daughter Vesicles with Different Interior and Membrane Compositions. J. Am. Chem. Soc. 2011, 133, 9545–9555. [Google Scholar] [CrossRef] [PubMed]
- Vasas, V.; Szathmary, E.; Santos, M. Lack of evolvability in self-sustaining autocatalytic networks constraints metabolism-first scenarios for the origin of life. Proc. Natl. Acad. Sci. USA 2010, 107, 1470–1475. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, S.; Chen, L.; Nilsson, M.; Abe, S. Bridging nonliving and living matter. Artif. Life 2003, 9, 269–316. [Google Scholar] [CrossRef] [PubMed]
- Adamala, K.; Szostak, J.W. Nonenzymatic Template-Directed RNA Synthesis inside Model Protocells. Science 2013, 342, 1098–1100. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Monnard, P.-A.; Walde, P. Current Ideas about Prebiological Compartmentalization. Life 2015, 5, 1239-1263. https://doi.org/10.3390/life5021239
Monnard P-A, Walde P. Current Ideas about Prebiological Compartmentalization. Life. 2015; 5(2):1239-1263. https://doi.org/10.3390/life5021239
Chicago/Turabian StyleMonnard, Pierre-Alain, and Peter Walde. 2015. "Current Ideas about Prebiological Compartmentalization" Life 5, no. 2: 1239-1263. https://doi.org/10.3390/life5021239
APA StyleMonnard, P.-A., & Walde, P. (2015). Current Ideas about Prebiological Compartmentalization. Life, 5(2), 1239-1263. https://doi.org/10.3390/life5021239