A Comparative Study of Iron Uptake Rates and Mechanisms amongst Marine and Fresh Water Cyanobacteria: Prevalence of Reductive Iron Uptake
Abstract
:1. Introduction
2. Experimental Section
2.1. Trace Metal Clean Techniques
| Organism # (abbreviation used in figures) | Brief Description | Siderophore Production | Siderophore Transporters | Diameter * (μm) | Growth Temp (°C) | Growth medium § [Fe] | Fe-Stress Indicators |
|---|---|---|---|---|---|---|---|
| Synechococcus WH8102 (WH8102) | Open ocean, unicellular, spherical | No | No | 1.2 | 25 | AMP1 0nM (lim) 300 nM (non-lim) | Changes in intracellular photosynthetic pigment ratios (phycocyanin, phycoerythrin and chlorophyll a) |
| Synechococcus WH7803 (WH7803) | Open ocean, unicellular, grown under dim light | No | No | 1.2 | 25 | AMP1 0 nM (lim) 300 nM (non-lim) | Changes in intracellular photosynthetic pigment ratios (phycocyanin, phycoerythrin and chlorophyll a) |
| Synechococcus CCMP1183 (CCMP1183) | Open ocean, unicellular | Unknown | Unknown | 1.6 | 25 | f/2 0 nM (lim) 300 nM (non-lim) | Decreases in intracellular photosynthetic pigments (chlorophyll a) |
| Prochlorococcus marinus MED4 (MED4) | Open ocean, unicellular | No | No | 0.7 | 25 | AMP1 0 nM (lim) 300 nM (non-lim) | Decreases in intracellular photosynthetic pigments (chlorophyll a) |
| Trichodesmium erythraeum (IMS101) | Open ocean, Filamentousdiazotrophic | No | No | Surface area ~157 μm2 $ | 25 | YBCII 0 nM (lim) 1 μM (non-lim) | Decreased trichome length |
| Synechococcus PCC7002 (PCC7002) | Brackish water (euryhaline) Coastal | Yes | Yes | 1.6 | 30 | A+ 0nM (lim) 1 μM (non-lim) | Decreased growth rate and decreases in intracellular photosynthetic pigments (chlorophyll a) and a blue shift in the absorption spectrum |
| Anabaena UTEX 2576 (UTEX 2576) | Fresh water, Filamentous, diazotrophic | Yes | Yes | Surface area ~60 μm2 $ | 30 | YBG11 0.1 μM (lim) 10 μM (non-lim) | Decreases in intracellular photosynthetic pigments (chlorophyll a) and a blue shift in the absorption spectrum |
| Synechocystis PCC6803 (PCC6803) | Fresh water, unicellular | No | Putative aerobactin transporter | 1 | 30 | YBG11 0.1 μM (lim) 10 μM (non-lim) | Decreases in intracellular photosynthetic pigments (chlorophyll a) and a blue shift in the absorption spectrum |
2.2. Culture Growth and Fe Limitation
2.3. Measuring Fe Uptake Rates
| Organism (abbreviations used in text and figures) | Uptake Medium | * EDTA Concentration (μM) | Substrates Tested |
|---|---|---|---|
| Synechococcus WH8102 (WH8102) | AMP1 salts (Turk’s island salt mix) + 2 mM NaHCO3 | 20 | Fe', FOB, FeAB, FOE |
| Synechococcus WH7803 (WH7803) | AMP1 salts (Turk’s island salt mix) + 2 mM NaHCO3 | 20 | Fe', FOB |
| Synechococcus CCMP1183 (CCMP1183) | Synthetic Ocean water (SOW) | 20 | Fe', FOB, FeAB |
| Prochlorococcus marinus MED4 (MED4) | AMP1 salts (Turk’s island salt mix) + 2 mM NaHCO3 | 20 | Fe', FOB, FeAB |
| Trichodesmium erythraeum (IMS101) | Synthetic Ocean water (SOW) | 20 | Fe' |
| Synechococcus PCC7002 (PCC7002) | A+ salts + 2 mM NaHCO3 | 80 | Fe', FOB, FeAB |
| Anabaena UTEX2576 (UTEX2576) | YBG11 | 16 | Fe', FOB, FeAB, FeSchizokinen |
| Synechocystis PCC6803 (PCC6803) | YBG11 | 16 | FeAB |
2.4. Mechanism of Fe Uptake: the Ferrozine Assay
2.5. Calculation of Uptake Rate Constants—kin
3. Results
3.1. Fe' Uptake
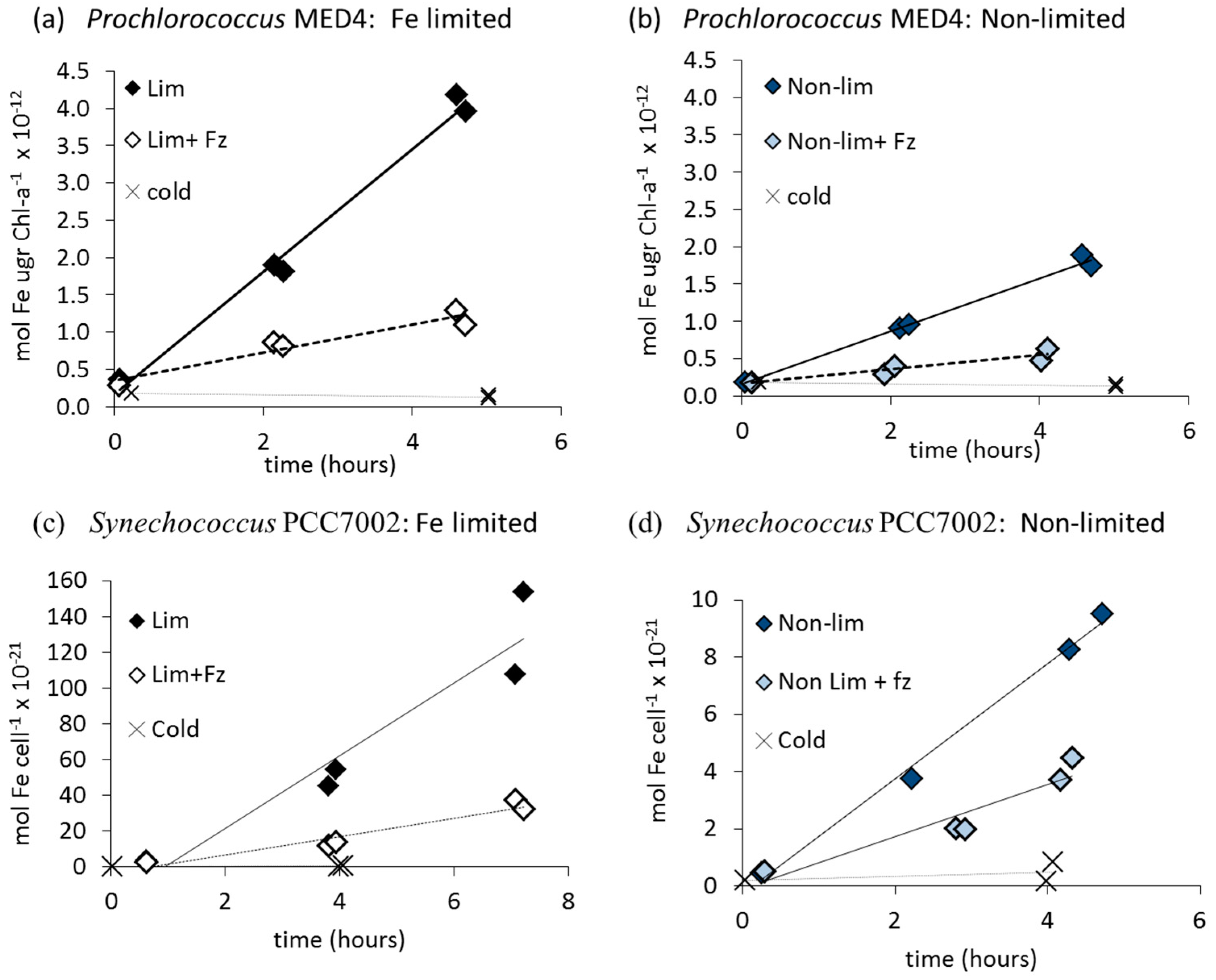
| Organism | Fe' (Free Inorganic Iron) | FOB | FeAB | |
|---|---|---|---|---|
| Not Limited | Fe-Limited | Fe-Limited | Fe-Limited | |
| Synechococcus WH8102 | Yes (+) | Yes (+++) | Yes (++) | n/a |
| Synechococcus WH7803 | Yes (+++) | Yes (+++) | Yes (++) | n/a |
| Synechococcus CCMP1183 | Yes (++) | - | - | - |
| Synechococcus PCC7002 | Yes (++) | Yes (+++) | Yes (+) | No |
| Prochlorococcus MED4 | Yes (++) | Yes (+++) | Yes (+) | n/a |
| Synechocystis PCC6803 | Yes (+++)a | Yes (+++)a | Yes (++)a | Yes (++) |
| Trichodesmium IMS101 | - | Yes (+) | - | - |
| Anabaena UTEX2576 | Yes (+++) | Yes (+++) | No* | No |
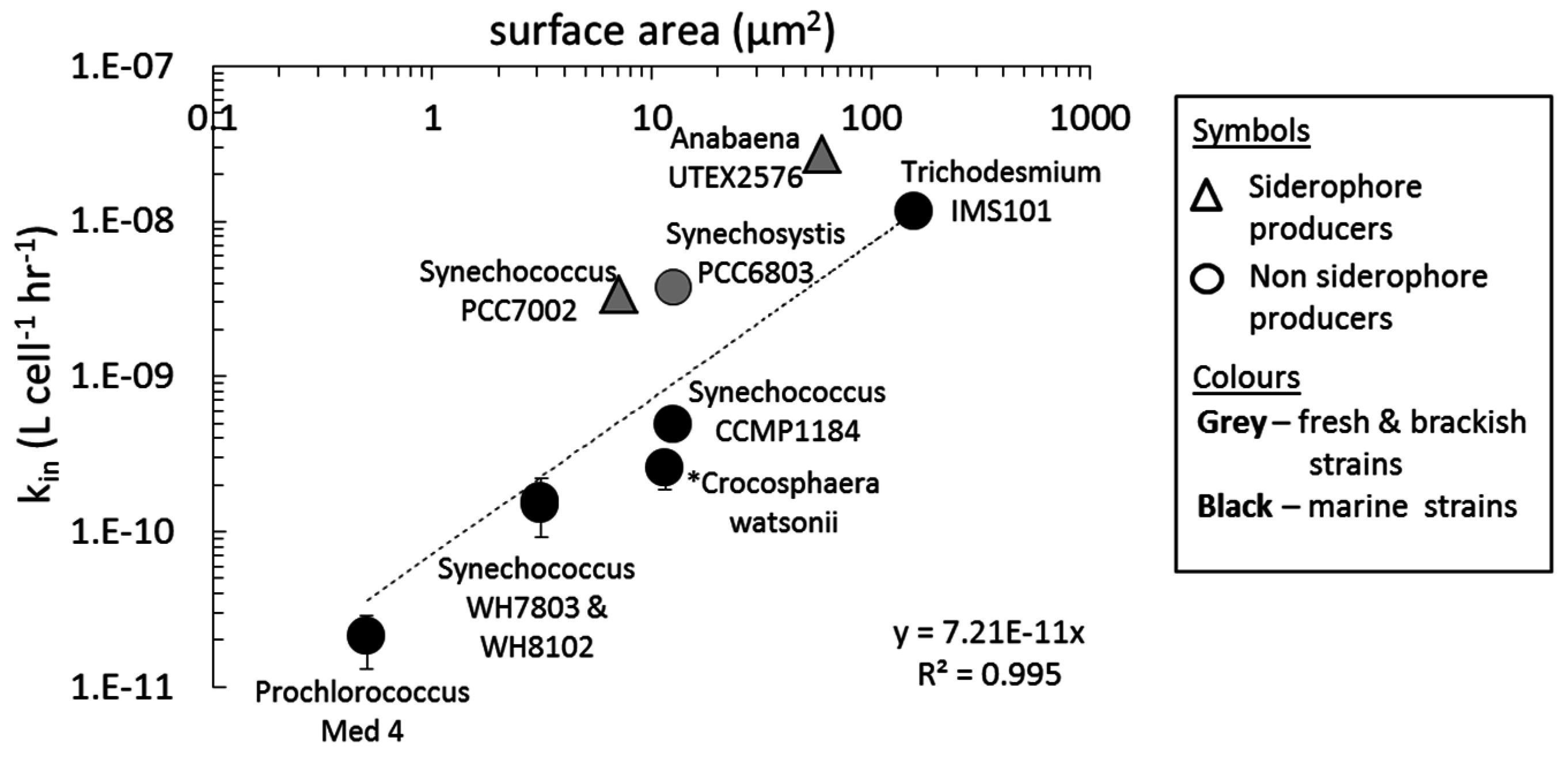
3.2. Ferric Siderophore Uptake
3.2.1. Ferrioxamine B (FOB)
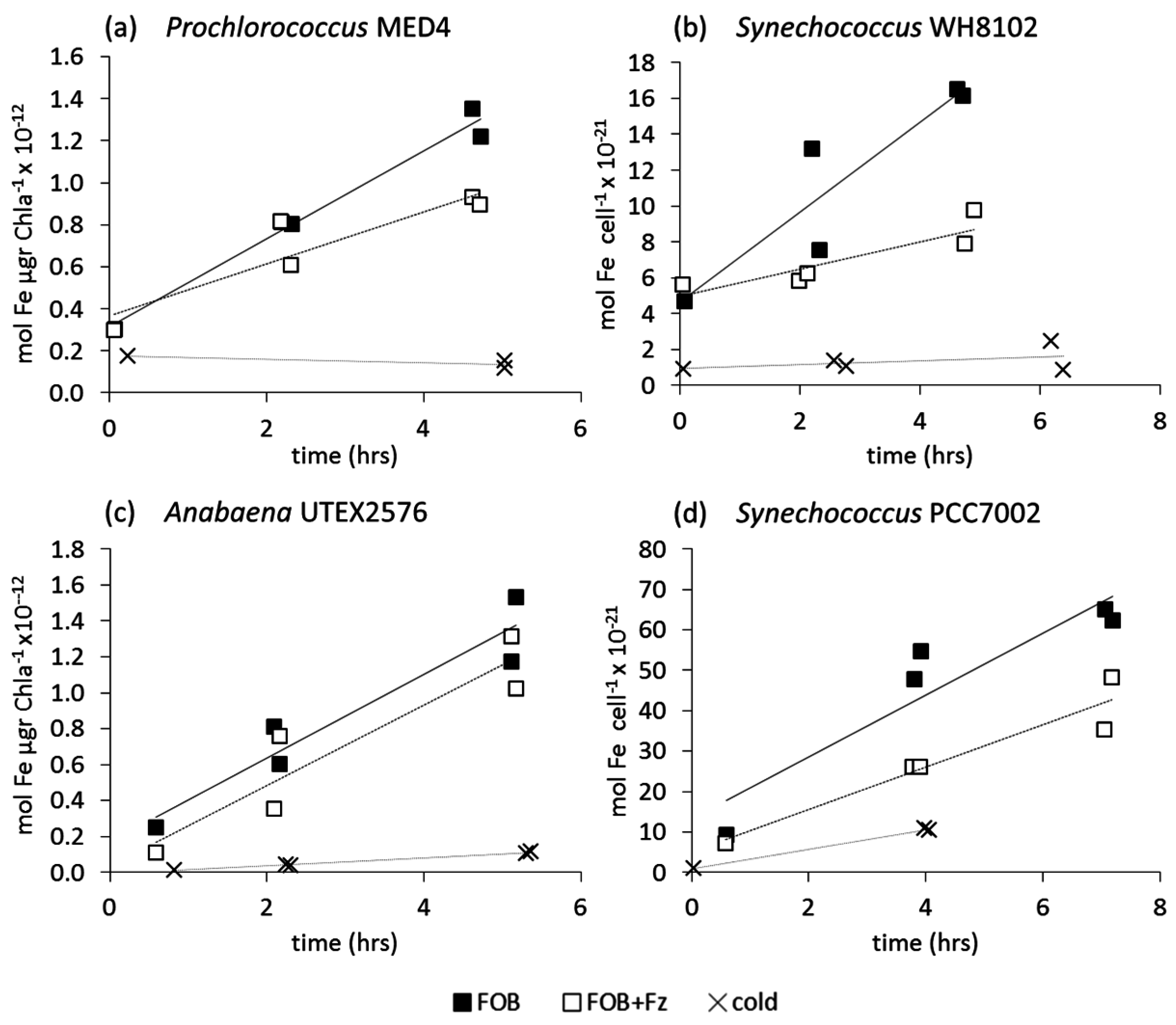
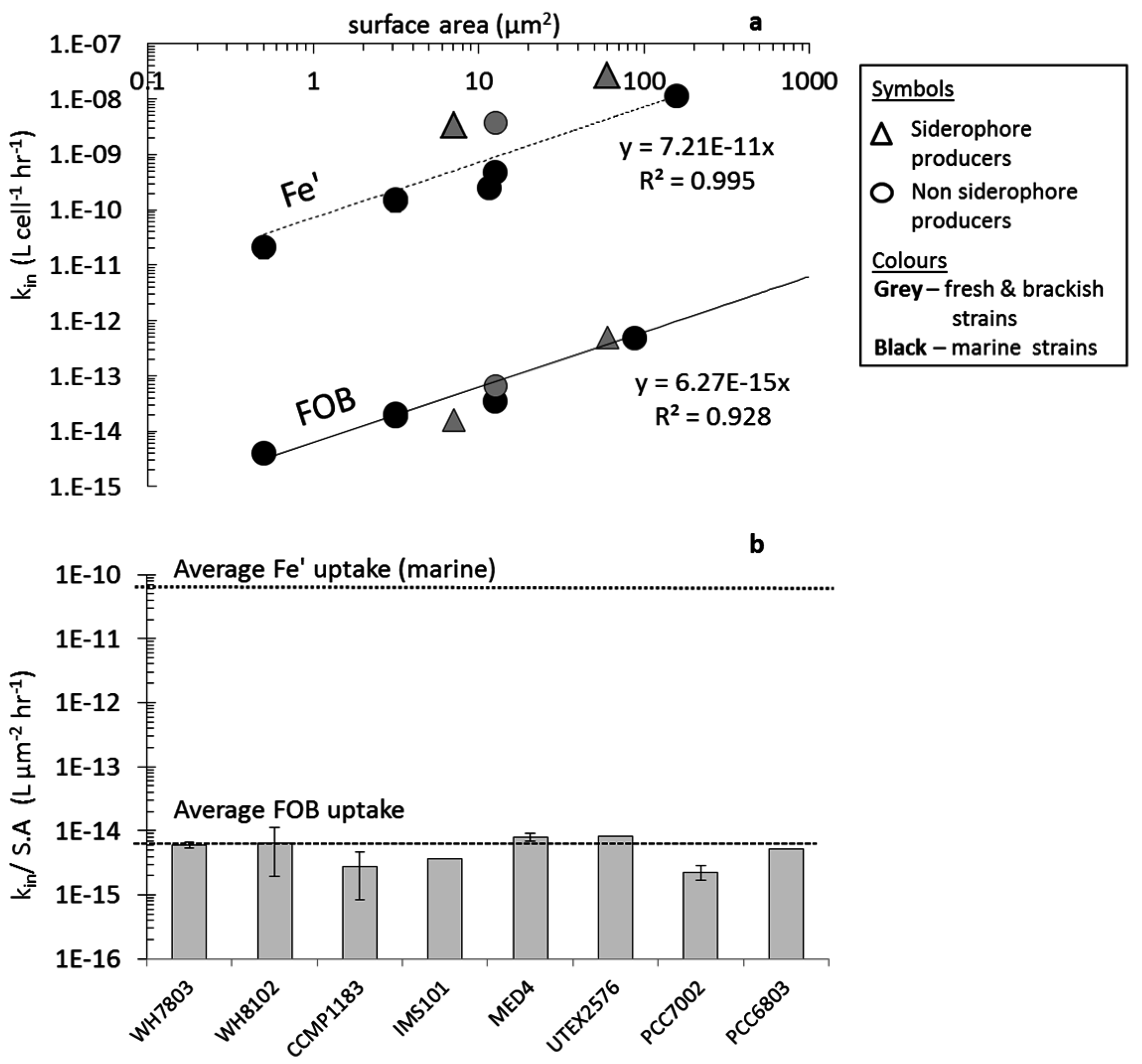
3.2.2. Fe-Aerobactin (FeAB)
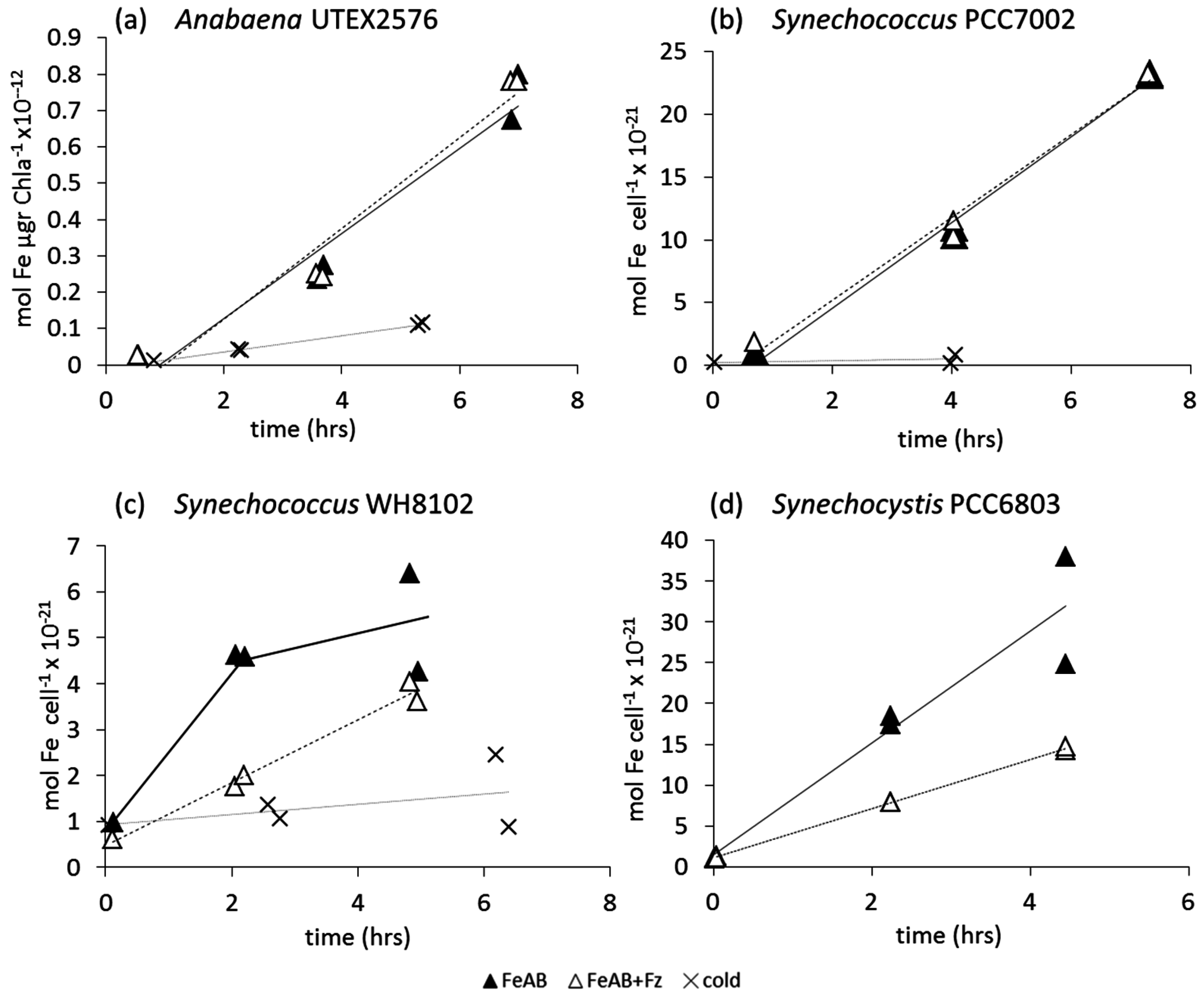
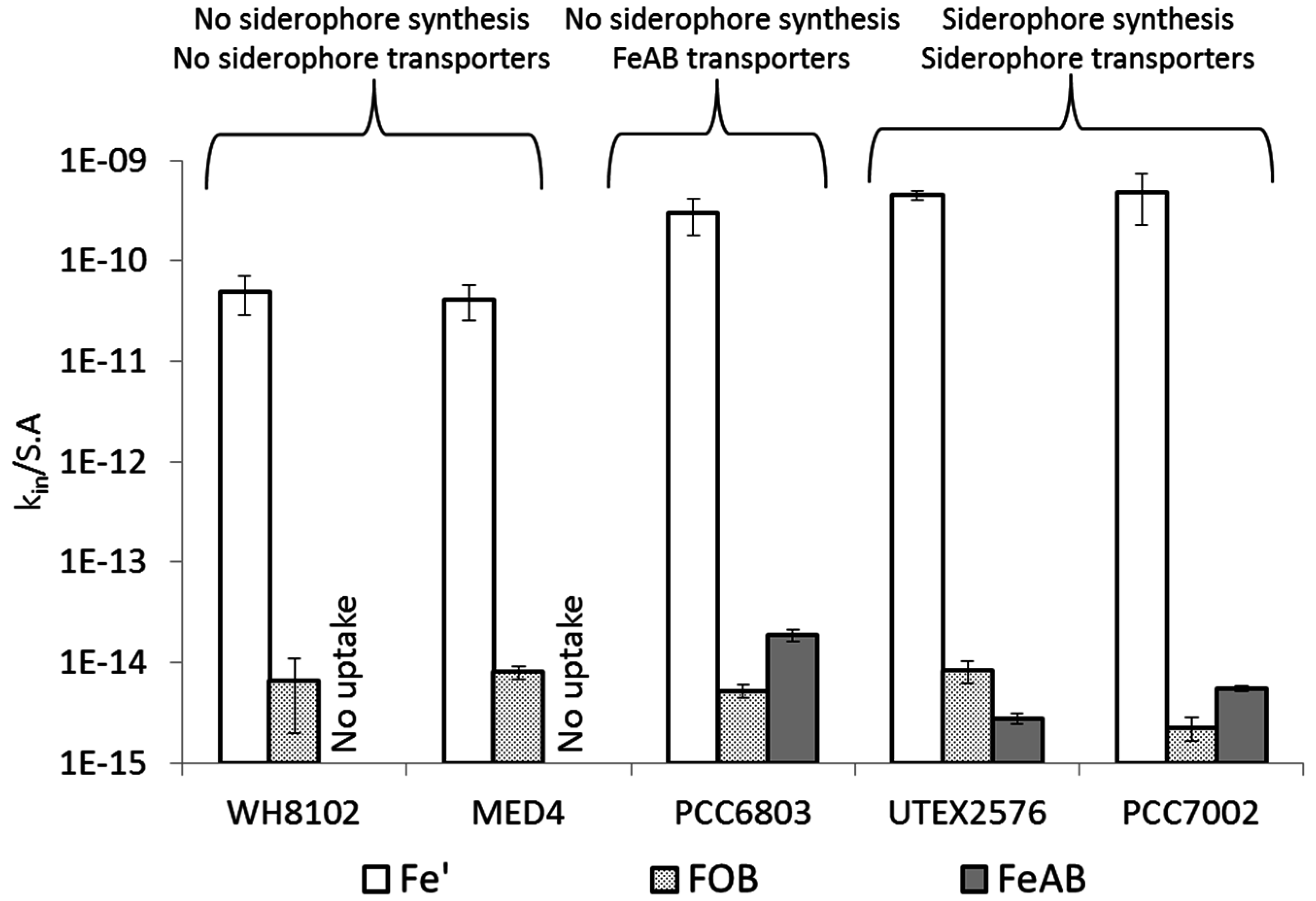
4. Discussion
4.2. Uptake of Fe' and FOB: Similarities amongst Diverse Cyanobacterial Species
4.3. Uptake of FeAB—a Case Study in Differential Bioavailability
4.4. Siderophore vs. Reductive Iron Uptake Pathways: Advantages and Disadvantages
4.5. Cyanobacterial Uptake Rates and Mechanisms: Implications in Natural Environments
5. Conclusions
Supplementary Files
Supplementary File 1Acknowledgments
Author Contributions
Conflicts of Interest
References
- Chisholm, S.W.; Olson, R.J.; Zettler, E.R.; Goericke, R.; Waterbury, J.B.; Welschmeyer, N.A. A novel free-living prochlorophyte abundant in the oceanic euphotic zone. Nature 1988, 34, 340–343. [Google Scholar] [CrossRef]
- Waterbury, J.B.; Watson, S.W.; Guillard, R.R.; Brand, L.E. Widespread occurrence of a unicellular, marine, planktonic, cyanobacterium. Nature 1979, 277, 293–294. [Google Scholar] [CrossRef]
- Gruber, N. The marine nitrogen cycle: Overview and challenges. In Nitrogen in the Marine Environment, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2008; pp. 1–50. [Google Scholar]
- Raven, J.A. Predictions of Mn and Fe use efficiencies of phototrophic growth as a function of light availability for growth and of c assimilation pathway. New Phytol. 1990, 116, 1–18. [Google Scholar] [CrossRef]
- Kustka, A.; Carpenter, E.J.; Sañudo-Wilhelmy, S.A. Iron and marine nitrogen fixation: Progress and future directions. Res. Microbiol. 2002, 153, 255–262. [Google Scholar] [CrossRef] [PubMed]
- Brand, L.E. Minimum iron requirements of marine phytoplankton and the implications for the biogeochemical control of new production. Limnol. Oceanogr. 1991, 36, 1756–1771. [Google Scholar] [CrossRef]
- Keren, N.; Aurora, R.; Pakrasi, H.B. Critical roles of bacterioferritins in iron storage and proliferation of cyanobacteria. Plant Physiol. 2004, 135, 1666–1673. [Google Scholar] [CrossRef] [PubMed]
- Mann, E.L.; Chisholm, S.W. Iron limits the cell division rate of prochlorococcus in the eastern equatorial pacific. Limnol. Oceanogr. 2000, 45, 1067–1076. [Google Scholar] [CrossRef]
- McKay, R.M.L.; Bullerjahn, G.S.; Porta, D.; Brown, E.T.; Sherrell, R.M.; Smutka, T.M.; Sterner, R.W.; Twiss, M.R.; Wilhelm, S.W. Consideration of the bioavailability of iron in the north american great lakes: Development of novel approaches toward understanding iron biogeochemistry. Aquat. Ecosyst. Health Manag. 2004, 7, 475–490. [Google Scholar] [CrossRef]
- Boyd, P.W.; Jickells, T.; Law, C.S.; Blain, S.; Boyle, E.A.; Buesseler, K.O.; Coale, K.H.; Cullen, J.J.; de Baar, H.J.W.; Follows, M.; et al. Mesoscale iron enrichment experiments 1993–2005: Synthesis and future directions. Science 2007, 315, 612–617. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Millero, F.J. The solubility of iron in seawater. Mar. Chem. 2002, 77, 43–54. [Google Scholar] [CrossRef]
- Baker, A.; Croot, P. Atmospheric and marine controls on aerosol iron solubility in seawater. Mar. Chem. 2010, 120, 4–13. [Google Scholar] [CrossRef]
- Johnson, K.S.; Gordon, R.M.; Coale, K.H. What controls dissolved iron concentrations in the world ocean? Mar. Chem. 1997, 57, 137–161. [Google Scholar] [CrossRef]
- Morel, F.M.; Kustka, A.; Shaked, Y. The role of unchelated Fe in the iron nutrition of phytoplankton. Limnol. Oceanogr. 2008, 53, 400–404. [Google Scholar] [CrossRef]
- Shaked, Y.; Lis, H. Disassembling iron availability to phytoplankton. Front. Microbial. 2012, 3. [Google Scholar] [CrossRef]
- Kranzler, C.; Lis, H.; Shaked, Y.; Keren, N. The role of reduction in iron uptake processes in a unicellular, planktonic cyanobacterium. Environ. Microbiol. 2011, 13, 2990–2999. [Google Scholar] [CrossRef] [PubMed]
- Lis, H.; Shaked, Y.; Kranzler, C.; Keren, N.; Morel, F.M.M. Iron bioavailability to phytoplankton: An empirical approach. ISME J. 2014. [Google Scholar] [CrossRef]
- Gledhill, M.; van den Berg, C.M. Determination of complexation of iron (III) with natural organic complexing ligands in seawater using cathodic stripping voltammetry. Mar. Chem. 1994, 47, 41–54. [Google Scholar] [CrossRef]
- Gledhill, M.; Buck, K.N. The organic complexation of iron in the marine environment: A review. Front. Microbial. 2012, 3. [Google Scholar] [CrossRef]
- Rue, E.L.; Bruland, K.W. Complexation of iron (III) by natural organic ligands in the central north pacific as determined by a new competitive ligand equilibration/adsorptive cathodic stripping voltammetric method. Mar. Chem. 1995, 50, 117–138. [Google Scholar] [CrossRef]
- Wu, J.; Luther, G.W., III. Complexation of Fe (III) by natural organic ligands in the northwest Atlantic Ocean by a competitive ligand equilibration method and a kinetic approach. Mar. Chem. 1995, 50, 159–177. [Google Scholar] [CrossRef]
- Braun, V.; Hantke, K. Recent insights into iron import by bacteria. Curr. Opin. Chem. Biol. 2011, 15, 328–334. [Google Scholar] [CrossRef] [PubMed]
- Hopkinson, B.M.; Morel, F.M. The role of siderophores in iron acquisition by photosynthetic marine microorganisms. Biometals 2009, 22, 659–669. [Google Scholar] [CrossRef] [PubMed]
- Hopkinson, B.M.; Barbeau, K.A. Iron transporters in marine prokaryotic genomes and metagenomes. Environ. Microbial. 2012, 14, 114–128. [Google Scholar] [CrossRef]
- Palenik, B.; Brahamsha, B.; Larimer, F.W.; Land, M.; Hauser, L.; Chain, P.; Lamerdin, J.; Regala, W.; Allen, E.E.; McCarren, J.; et al. The genome of a motile marine synechococcus. Nature 2003, 424, 1037–1042. [Google Scholar] [CrossRef] [PubMed]
- Hassler, C.S.; Twiss, M.R. Bioavailability of iron sensed by a phytoplanktonic fe-bioreporter. Environ. Sci. Technol. 2006, 40, 2544–2551. [Google Scholar] [CrossRef] [PubMed]
- Nicolaisen, K.; Moslavac, S.; Samborski, A.; Valdebenito, M.; Hantke, K.; Maldener, I.; Muro-Pastor, A.M.; Flores, E.; Schleiff, E. Alr0397 is an outer membrane transporter for the siderophore schizokinen in anabaena sp. Strain PCC 7120. J. Bacterial. 2008, 190, 7500–7507. [Google Scholar] [CrossRef]
- Jeanjean, R.; Talla, E.; Latifi, A.; Havaux, M.; Janicki, A.; Zhang, C.C. A large gene cluster encoding peptide synthetases and polyketide synthases is involved in production of siderophores and oxidative stress response in the cyanobacterium anabaena sp. Strain PCC 7120. Environ. Microbial. 2008, 10, 2574–2585. [Google Scholar] [CrossRef]
- Wirtz, N.L.; Treble, R.G.; Weger, H.G. Siderophore-independent iron uptake by iron-limited cells of the cyanobacterium anabaena flos-aquae1. J. Phycol. 2010, 46, 947–957. [Google Scholar] [CrossRef]
- Sonier, M.B.; Contreras, D.A.; Treble, R.G.; Weger, H.G. Two distinct pathways for iron acquisition by iron-limited cyanobacterial cells: Evidence from experiments using siderophores and synthetic chelators. Botany 2012, 90, 181–190. [Google Scholar] [CrossRef]
- Salmon, T.P.; Rose, A.L.; Neilan, B.A.; Waite, T.D. The FeL model of iron acquisition: Nondissociative reduction of ferric complexes in the marine environment. Limnol. Oceanogr. 2006, 51, 1744–1754. [Google Scholar] [CrossRef]
- Lis, H.; Shaked, Y. Probing the bioavailability of organically bound iron: A case study in the synechococcus-rich waters of the Gulf of Aqaba. Aquat. Microb. Ecol. 2009, 56, 241–253. [Google Scholar] [CrossRef]
- Maldonado, M.T.; Price, N.M. Reduction and transport of organically bound iron by thalassiosira oceanica (bacillariophyceae). J. Phycol. 2001, 37, 298–310. [Google Scholar] [CrossRef]
- Shaked, Y.; Kustka, A.B.; Morel, F.M. A general kinetic model for iron acquisition by eukaryotic phytoplankton. Limnol. Oceanogr. 2005, 50, 872–882. [Google Scholar] [CrossRef]
- Kranzler, C.; Lis, H.; Finkel, O.M.; Schmetterer, G.; Shaked, Y.; Keren, N. Coordinated transporter activity shapes high-affinity iron acquisition in cyanobacteria. ISME J. 2014, 8, 409–417. [Google Scholar] [CrossRef] [PubMed]
- Sunda, W.G.; Price, N.M.; Morel, F.M. Trace metal ion buffers and their use in culture studies. In Algal Culturing Techniques; Anderson, R., Ed.; Academic Press: Burlington, MA, USA, 2005; pp. 35–63. [Google Scholar]
- Thompson, A.W. Iron and Prochlorococcus. Ph.D. Thesis, Massachusetts Institute of Technology, Cambridge, MA, USA, 2009. [Google Scholar]
- Guikema, J.A.; Sherman, L.A. Organization and function of chlorophyll in membranes of cyanobacteria during iron starvation. Plant Physiol. 1983, 73, 250–256. [Google Scholar] [CrossRef] [PubMed]
- Salomon, E.; Bar-Eyal, L.; Sharon, S.; Keren, N. Balancing photosynthetic electron flow is critical for cyanobacterial acclimation to nitrogen limitation. Biochim. Biophys. Acta (BBA)-Bioenerg. 2013, 1827, 340–347. [Google Scholar] [CrossRef]
- Vaara, M. Agents that increase the permeability of the outer membrane. Microbiol. Rev. 1992, 56, 395–411. [Google Scholar] [PubMed]
- Tang, D.; Morel, F.M.M. Distinguishing between intracellular and extracellular trace metals in phytoplankton. Mar. Chem. 2006, 98, 18–30. [Google Scholar] [CrossRef]
- Shaked, Y.; Kustka, A.B.; Morel, F.M.; Erel, Y. Simultaneous determination of iron reduction and uptake by phytoplankton. Limnol. Oceanogr. Methods 2004, 2, 137–145. [Google Scholar] [CrossRef]
- Gibbs, C.R. Characterization and application of ferrozine iron reagent as a ferrous iron indicator. Anal. Chem. 1976, 48, 1197–1201. [Google Scholar] [CrossRef]
- Petter, G.J. Visual MINTEQ ver. 3.0. Available online: http://www2.lwr.kth.se/English/Oursoftware/vminteq/ (accessed on 4 February 2015).
- Millero, F.J.; Pierrot, D. The activity coefficients of Fe(III) hydroxide complexes in NaCl and NaClO4 solutions. Geochim. Cosmochim. Acta 2007, 71, 4825–4833. [Google Scholar] [CrossRef]
- Jacq, V.; Ridame, C.; L’Helguen, S.; Kaczmar, F.; Saliot, A. Response of the unicellular diazotrophic cyanobacterium crocosphaera watsonii to iron limitation. PLoS One 2014, 9, e86749. [Google Scholar] [CrossRef] [PubMed]
- Mullis, K.B.; Pollack, J.; Neilands, J. Structure of schizokinen, an iron-transport compound from bacillus megaterium. Biochemistry 1971, 10, 4894–4898. [Google Scholar] [CrossRef] [PubMed]
- Ito, Y.; Butler, A. Structure of synechobactins, new siderophores of the marine cyanobacterium synechococcus sp. PCC 7002. Limnol. Oceanogr. 2005, 50, 1918–1923. [Google Scholar] [CrossRef]
- Boukhalfa, H.; Crumbliss, A.L. Chemical aspects of siderophore mediated iron transport. Biometals 2002, 15, 325–339. [Google Scholar] [CrossRef] [PubMed]
- Katoh, H.; Hagino, N.; Grossman, A.R.; Ogawa, T. Genes essential to iron transport in the cyanobacterium synechocystis sp. Strain PCC 6803. J. Bacterial. 2001, 183, 2779–2784. [Google Scholar] [CrossRef]
- Mirus, O.; Strauss, S.; Nicolaisen, K.; von Haeseler, A.; Schleiff, E. Tonb-dependent transporters and their occurrence in cyanobacteria. BMC Biol. 2009, 7. [Google Scholar] [CrossRef] [PubMed]
- Völker, C.; Wolf-Gladrow, D.A. Physical limits on iron uptake mediated by siderophores or surface reductases. Mar. Chem. 1999, 65, 227–244. [Google Scholar] [CrossRef]
- Hutchins, D.A.; Rueter, J.G.; Fish, W. Siderophore production and nitrogen fixation are mutually exclusive strategies in anabaena 7120. Limnol. Oceanogr. 1991, 36. [Google Scholar] [CrossRef]
- Malmstrom, R.R.; Rodrigue, S.; Huang, K.H.; Kelly, L.; Kern, S.E.; Thompson, A.; Roggensack, S.; Berube, P.M.; Henn, M.R.; Chisholm, S.W. Ecology of uncultured prochlorococcus clades revealed through single-cell genomics and biogeographic analysis. ISME J. 2013, 7, 184–198. [Google Scholar] [CrossRef] [PubMed]
- Yooseph, S.; Nealson, K.H.; Rusch, D.B.; McCrow, J.P.; Dupont, C.L.; Kim, M.; Johnson, J.; Montgomery, R.; Ferriera, S.; Beeson, K.; et al. Genomic and functional adaptation in surface ocean planktonic prokaryotes. Nature 2010, 468, 60–66. [Google Scholar] [CrossRef] [PubMed]
- Barbeau, K. Photochemistry of organic iron (III) complexing ligands in oceanic systems. Photochem. Photobiol. 2006, 82, 1505–1516. [Google Scholar] [CrossRef] [PubMed]
- Carrano, C.J.; Kuepper, F.; Green, D. The role of symbiotic bacteria in iron acquisition and algal bloom formation. Available online: https://escholarship.org/uc/item/2kp5c67r (accessed on 4 February 2015).
- Boyd, P.; Ellwood, M. The biogeochemical cycle of iron in the ocean. Nat. Geosci. 2010, 3, 675–682. [Google Scholar] [CrossRef]
- Sunda, W.G.; Huntsman, S.A. Iron uptake and growth limitation in oceanic and coastal phytoplankton. Mar. Chem. 1995, 50, 189–206. [Google Scholar] [CrossRef]
- Beardall, J.; Young, E.; Roberts, S. Approaches for determining phytoplankton nutrient limitation. Aquat. Sci. 2001, 63, 44–69. [Google Scholar] [CrossRef]
- Rubin, M.; Berman-Frank, I.; Shaked, Y. Dust-and mineral-iron utilization by the marine dinitrogen-fixer Trichodesmium. Nat. Geosci. 2011, 4, 529–534. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lis, H.; Kranzler, C.; Keren, N.; Shaked, Y. A Comparative Study of Iron Uptake Rates and Mechanisms amongst Marine and Fresh Water Cyanobacteria: Prevalence of Reductive Iron Uptake. Life 2015, 5, 841-860. https://doi.org/10.3390/life5010841
Lis H, Kranzler C, Keren N, Shaked Y. A Comparative Study of Iron Uptake Rates and Mechanisms amongst Marine and Fresh Water Cyanobacteria: Prevalence of Reductive Iron Uptake. Life. 2015; 5(1):841-860. https://doi.org/10.3390/life5010841
Chicago/Turabian StyleLis, Hagar, Chana Kranzler, Nir Keren, and Yeala Shaked. 2015. "A Comparative Study of Iron Uptake Rates and Mechanisms amongst Marine and Fresh Water Cyanobacteria: Prevalence of Reductive Iron Uptake" Life 5, no. 1: 841-860. https://doi.org/10.3390/life5010841
APA StyleLis, H., Kranzler, C., Keren, N., & Shaked, Y. (2015). A Comparative Study of Iron Uptake Rates and Mechanisms amongst Marine and Fresh Water Cyanobacteria: Prevalence of Reductive Iron Uptake. Life, 5(1), 841-860. https://doi.org/10.3390/life5010841





