New Treatment Options for MASLD Patients with Type 2 Diabetes
Abstract
1. State-of-the-Art
2. Pathogenesis of MASLD and Its Relationship with Type 2 Diabetes Mellitus
3. Assessment of Hepatic Fibrosis in MASLD
4. Focus on Therapy
4.1. Drug Therapy for Type 2 Diabetes Mellitus
- Reduction in blood glucose levels and maintenance of hemoglobin A1c (HbA1c) < 7% (with individualized targets based on age, comorbidities, and hypoglycemia risk).
- Prevention of microvascular and macrovascular complications.
- Body weight control and improvement of the metabolic profile.
- Organ protection (heart, kidney, liver) [31].
4.2. Non-Pharmacological Therapies in MASLD
4.3. Pharmacological Treatment of MASLD
5. Summary of Clinical Evidence on GLP-1RA and SGLT2i in MASLD
5.1. Methods
5.2. Randomized Clinical Trials Investigating GLP-1RA, SGLT2i, and GIP/GLP-1 RA in MASLD
5.3. Future Directions
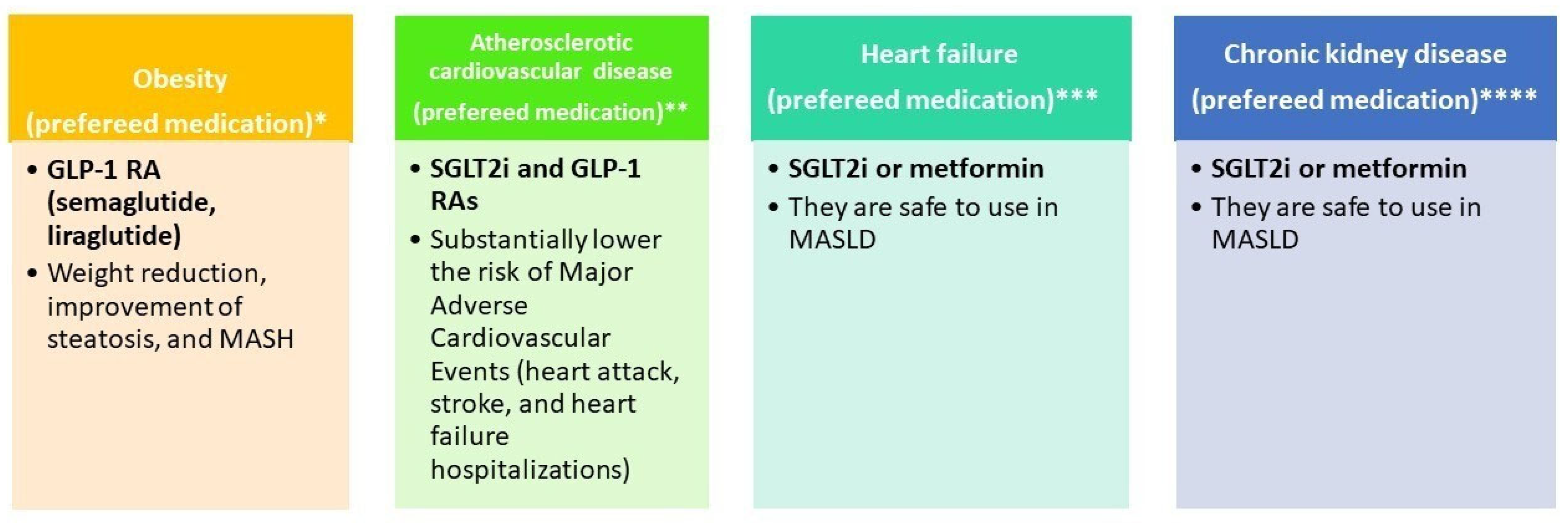
6. Practical Clinical Consideration
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| NASH | Nonalcoholic steatohepatitis |
| NAFLD | Nonalcoholic fatty liver disease |
| MAFLD | Metabolic dysfunction-associated fatty liver disease |
| MASLD | Metabolic dysfunction-associated steatotic liver disease |
| MASH | Metabolic dysfunction-associated steatohepatitis |
| CAP | Controlled Attenuation Parameter |
| MRI-PDFF | Magnetic resonance imaging–proton density fat fraction |
| T2DM | Type 2 diabetes mellitus |
| FLI | Fatty liver index |
| MetALD | Metabolic dysfunction-associated steatotic liver disease and Alcohol-related liver disease |
| EASL–EASD–EASO | European Association for the Study of the Liver, European Association for the Study of Diabetes, European Association for the Study of Obesity |
| GLP-1RAs | Glucagon-like peptide-1 receptor agonists |
| SGLT-2i | Sodium–Glucose Co-transporter Inhibitors |
| ROS | Reactive oxygen species |
| LPS | Lipopolysaccharide |
| TLR | Toll-like receptors |
| HCC | hepatocellular carcinoma |
| MACE | major adverse cardiovascular events |
| ADA/EASD | American Diabetes Association/European Association for the Study of Diabetes |
| FIB-4 | Fibrosis-4 |
| NFS | Nonalcoholic Fatty Liver Disease Fibrosis Score |
| VCTE | vibration-controlled transient elastography |
| MRE | magnetic resonance elastography |
| APRI | AST-to-platelet ratio index |
| LSM | Liver stiffness measurement |
| LSV | Liver stiffness value |
| HbA1c | Hemoglobin A1c |
| MRI | Magnetic resonance imaging |
| DPP-4 | Dipeptidyl peptidase-4 |
| SGLT2 | Sodium glucose co-transporter-2 |
| GLP-1 | Glucagon-like peptide-1 |
References
- Ludwig, J.; Viggiano, T.R.; McGill, D.B.; Oh, B.J. Nonalcoholic steatohepatitis: Mayo Clinic experiences with a hitherto unnamed disease. Mayo Clin. Proc. 1980, 55, 434–438. [Google Scholar] [CrossRef]
- Eslam, M.; Newsome, P.N.; Sarin, S.K.; Anstee, Q.M.; Targher, G.; Romero-Gomez, M.; Zelber-Sagi, S.; Wai-Sun Wong, V.; Dufour, J.F.; Schattenberg, J.M.; et al. A new definition for metabolic dysfunction-associated fatty liver disease: An international expert consensus statement. J. Hepatol. 2020, 73, 202–209. [Google Scholar] [CrossRef]
- Gastaldelli, A.; Newsome, P.N. NAFLD vs MASLD (Metabolic Dysfunction-Associated Steatotic Liver Disease)—Why the Need for a Change of Nomenclature? J. Clin. Endocrinol. Metab. 2025, 110, e2407–e2410. [Google Scholar] [CrossRef]
- Kanwal, F.; Neuschwander-Tetri, B.A.; Loomba, R.; Rinella, M.E. Metabolic dysfunction-associated steatotic liver disease: Update and impact of new nomenclature on the American Association for the Study of Liver Diseases practice guidance on nonalcoholic fatty liver disease. Hepatology 2024, 79, 1212–1219. [Google Scholar] [CrossRef]
- Rinella, M.E.; Lazarus, J.V.; Ratziu, V.; Francque, S.M.; Sanyal, A.J.; Kanwal, F.; Romero, D.; Abdelmalek, M.F.; Anstee, Q.M.; Arab, J.P.; et al. A multisociety Delphi consensus statement on new fatty liver disease nomenclature. J. Hepatol. 2023, 79, 1542–1556. [Google Scholar] [CrossRef]
- Cusi, K.; Abdelmalek, M.F.; Apovian, C.M.; Balapattabi, K.; Bannuru, R.R.; Barb, D.; Bardsley, J.K.; Beverly, E.A.; Corbin, K.D.; ElSayed, N.A.; et al. Metabolic Dysfunction-Associated Steatotic Liver Disease (MASLD) in People with Diabetes: The Need for Screening and Early Intervention. A Consensus Report of the American Diabetes Association. Diabetes Care 2025, 48, 1057–1082. [Google Scholar] [CrossRef]
- Chalasani, N.; Younossi, Z.; Lavine, J.E.; Charlton, M.; Cusi, K.; Rinella, M.; Harrison, S.A.; Brunt, E.M.; Sanyal, A.J. The diagnosis and management of nonalcoholic fatty liver disease: Practice guidance from the American Association for the Study of Liver Diseases. Hepatology 2018, 67, 328–357. [Google Scholar] [CrossRef]
- Hernaez, R.; Lazo, M.; Bonekamp, S.; Kamel, I.; Brancati, F.L.; Guallar, E.; Clark, J.M. Diagnostic accuracy and reliability of ultrasonography for the detection of fatty liver: A meta-analysis. Hepatology 2011, 54, 1082–1090. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- de Lédinghen, V.; Vergniol, J.; Foucher, J.; Merrouche, W.; le Bail, B. Non-invasive diagnosis of liver steatosis using controlled attenuation parameter (CAP) and transient elastography. Liver Int. 2012, 32, 911–918. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Pan, J.; Song, M.; Zhao, Y.C.; Chen, W.; Huang, H.; Zhu, Y.; Chen, F. Performance of Magnetic Resonance Imaging and Ultrasound for Identifying the Different Degrees of Hepatic Steatosis: A Systematic Review and Meta-analysis. Acad. Radiol. 2025, 32, 6528–6540. [Google Scholar] [CrossRef] [PubMed]
- Azizi, N.; Naghibi, H.; Shakiba, M.; Morsali, M.; Zarei, D.; Abbastabar, H.; Ghanaati, H. Evaluation of MRI proton density fat fraction in hepatic steatosis: A systematic review and meta-analysis. Eur. Radiol. 2025, 35, 1794–1807. [Google Scholar] [CrossRef]
- Ahn, S.B. Noninvasive serum biomarkers for liver steatosis in nonalcoholic fatty liver disease: Current and future developments. Clin. Mol. Hepatol. 2023, 29, S150–S156. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Abdelhameed, F.; Kite, C.; Lagojda, L.; Dallaway, A.; Chatha, K.K.; Chaggar, S.S.; Dalamaga, M.; Kassi, E.; Kyrou, I.; Randeva, H.S. Non-invasive Scores and Serum Biomarkers for Fatty Liver in the Era of Metabolic Dysfunction-associated Steatotic Liver Disease (MASLD): A Comprehensive Review From NAFLD to MAFLD and MASLD. Curr. Obes. Rep. 2024, 13, 510–531. [Google Scholar] [CrossRef]
- Younossi, Z.M.; Golabi, P.; Price, J.K.; Owrangi, S.; Gundu-Rao, N.; Satchi, R.; Paik, J.M. The Global Epidemiology of Nonalcoholic Fatty Liver Disease and Nonalcoholic Steatohepatitis Among Patients with Type 2 Diabetes. Clin. Gastroenterol. Hepatol. Off. Clin. Pract. J. Am. Gastroenterol. Assoc. 2024, 22, 1999–2010.e8. [Google Scholar] [CrossRef]
- European Association for the Study of the Liver (EASL); European Association for the Study of Diabetes (EASD); European Association for the Study of Obesity (EASO). EASL-EASD-EASO Clinical Practice Guidelines on the management of metabolic dysfunction-associated steatotic liver disease (MASLD). J. Hepatol. 2024, 81, 492–542. [Google Scholar] [CrossRef]
- American Diabetes Association Professional Practice Committee. 2. Diagnosis and Classification of Diabetes: Standards of Care in Diabetes-2024. Diabetes Care 2024, 47, S20–S42. [Google Scholar] [CrossRef]
- Kalyani, R.R.; Neumiller, J.J.; Maruthur, N.M.; Wexler, D.J. Diagnosis and Treatment of Type 2 Diabetes in Adults: A Review. JAMA 2025, 334, 984–1002. [Google Scholar] [CrossRef]
- American Diabetes Association Professional Practice Committee. 4. Comprehensive Medical Evaluation and Assessment of Comorbidities: Standards of Care in Diabetes-2025. Diabetes Care 2025, 48, S59–S85. [Google Scholar] [CrossRef]
- World Health Organization. Classification of Diabetes Mellitus; World Health Organization: Geneva, Switzerland, 2019. [Google Scholar]
- Ismail-Beigi, F. Clinical practice. Glycemic management of type 2 diabetes mellitus. N. Engl. J. Med. 2012, 366, 1319–1327. [Google Scholar] [CrossRef]
- Stasi, C. Post-COVID-19 Pandemic Sequelae in Liver Diseases. Life 2025, 15, 403. [Google Scholar] [CrossRef]
- Mantovani, A.; Petracca, G.; Beatrice, G.; Tilg, H.; Byrne, C.D.; Targher, G. Non-alcoholic fatty liver disease and risk of incident diabetes mellitus: An updated meta-analysis of 501 022 adult individuals. Gut 2021, 70, 962–969. [Google Scholar] [CrossRef]
- Zheng, Y.; Ley, S.H.; Hu, F.B. Global aetiology and epidemiology of type 2 diabetes mellitus and its complications. Nat. Rev. Endocrinol. 2018, 14, 88–98. [Google Scholar] [CrossRef]
- Cho, Y.; Chang, Y.; Ryu, S.; Wild, S.H.; Byrne, C.D. Nonalcoholic fatty liver disease without overlapping metabolic-associated fatty liver disease and the risk of incident type 2 diabetes. Liver Int. Off. J. Int. Assoc. Study Liver 2023, 43, 2445–2454. [Google Scholar] [CrossRef]
- Buzzetti, E.; Pinzani, M.; Tsochatzis, E.A. The multiple-hit pathogenesis of non-alcoholic fatty liver disease (NAFLD). Metab. Clin. Exp. 2016, 65, 1038–1048. [Google Scholar] [CrossRef]
- Leung, C.; Rivera, L.; Furness, J.B.; Angus, P.W. The role of the gut microbiota in NAFLD. Nat. Rev. Gastroenterol. Hepatol. 2016, 13, 412–425. [Google Scholar] [CrossRef]
- Tilg, H.; Moschen, A.R. Evolution of inflammation in nonalcoholic fatty liver disease: The multiple parallel hits hypothesis. Hepatology 2010, 52, 1836–1846. [Google Scholar] [CrossRef]
- Parthasarathy, G.; Revelo, X.; Malhi, H. Pathogenesis of Nonalcoholic Steatohepatitis: An Overview. Hepatol. Commun. 2020, 4, 478–492. [Google Scholar] [CrossRef]
- Anstee, Q.M.; Darlay, R.; Cockell, S.; Meroni, M.; Govaere, O.; Tiniakos, D.; Burt, A.D.; Bedossa, P.; Palmer, J.; Liu, Y.L.; et al. Genome-wide association study of non-alcoholic fatty liver and steatohepatitis in a histologically characterised cohort. J. Hepatol. 2020, 73, 505–515. [Google Scholar] [CrossRef]
- Eslam, M.; Valenti, L.; Romeo, S. Genetics and epigenetics of NAFLD and NASH: Clinical impact. J. Hepatol. 2018, 68, 268–279. [Google Scholar] [CrossRef]
- European Association for the Study of the Liver. EASL Clinical Practice Guidelines on non-invasive tests for evaluation of liver disease severity and prognosis—2021 update. J. Hepatol. 2021, 75, 659–689. [Google Scholar] [CrossRef]
- Lomonaco, R.; Ortiz-Lopez, C.; Orsak, B.; Webb, A.; Hardies, J.; Darland, C.; Finch, J.; Gastaldelli, A.; Harrison, S.; Tio, F.; et al. Effect of adipose tissue insulin resistance on metabolic parameters and liver histology in obese patients with nonalcoholic fatty liver disease. Hepatology 2012, 55, 1389–1397. [Google Scholar] [CrossRef]
- Smith, G.I.; Shankaran, M.; Yoshino, M.; Schweitzer, G.G.; Chondronikola, M.; Beals, J.W.; Okunade, A.L.; Patterson, B.W.; Nyangau, E.; Field, T.; et al. Insulin resistance drives hepatic de novo lipogenesis in nonalcoholic fatty liver disease. J. Clin. Investig. 2020, 130, 1453–1460. [Google Scholar] [CrossRef]
- Targher, G.; Byrne, C.D.; Lonardo, A.; Zoppini, G.; Barbui, C. Non-alcoholic fatty liver disease and risk of incident cardiovascular disease: A meta-analysis. J. Hepatol. 2016, 65, 589–600. [Google Scholar] [CrossRef]
- Davies, M.J.; Aroda, V.R.; Collins, B.S.; Gabbay, R.A.; Green, J.; Maruthur, N.M.; Rosas, S.E.; Del Prato, S.; Mathieu, C.; Mingrone, G.; et al. Management of Hyperglycemia in Type 2 Diabetes, 2022. A Consensus Report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 2022, 45, 2753–2786. [Google Scholar] [CrossRef]
- Alexander, M.; Loomis, A.K.; Fairburn-Beech, J.; van der Lei, J.; Duarte-Salles, T.; Prieto-Alhambra, D.; Ansell, D.; Pasqua, A.; Lapi, F.; Rijnbeek, P.; et al. Real-world data reveal a diagnostic gap in non-alcoholic fatty liver disease. BMC Med. 2018, 16, 130. [Google Scholar] [CrossRef]
- Bedossa, P.; Patel, K. Biopsy and Noninvasive Methods to Assess Progression of Nonalcoholic Fatty Liver Disease. Gastroenterology 2016, 150, 1811–1822.e4. [Google Scholar] [CrossRef]
- Targher, G.; Valenti, L.; Byrne, C.D. Metabolic Dysfunction-Associated Steatotic Liver Disease. N. Engl. J. Med. 2025, 393, 683–698. [Google Scholar] [CrossRef]
- McPherson, S.; Hardy, T.; Dufour, J.F.; Petta, S.; Romero-Gomez, M.; Allison, M.; Oliveira, C.P.; Francque, S.; Van Gaal, L.; Schattenberg, J.M.; et al. Age as a Confounding Factor for the Accurate Non-Invasive Diagnosis of Advanced NAFLD Fibrosis. Am. J. Gastroenterol. 2017, 112, 740–751. [Google Scholar] [CrossRef]
- Sung, S.; Al-Karaghouli, M.; Tam, M.; Wong, Y.J.; Jayakumar, S.; Davyduke, T.; Ma, M.; Abraldes, J.G. Age-dependent differences in FIB-4 predictions of fibrosis in patients with MASLD referred from primary care. Hepatol. Commun. 2024, 9, e0609. [Google Scholar] [CrossRef]
- Wu, Y.L.; Kumar, R.; Wang, M.F.; Singh, M.; Huang, J.F.; Zhu, Y.Y.; Lin, S. Validation of conventional non-invasive fibrosis scoring systems in patients with metabolic associated fatty liver disease. World J. Gastroenterol. 2021, 27, 5753–5763. [Google Scholar] [CrossRef]
- Jang, S.Y.; Yoon, K.T.; Cho, Y.Y.; Jo, H.G.; Baek, Y.H.; Moon, S.Y.; Jo, A.J.; Kweon, Y.O.; Park, S.Y.; Lee, Y.R.; et al. Aspartate aminotransferase-to-platelet ratio index outperforms Fibrosis-4 in 2843 Korean patients with metabolic dysfunction-associated steatotic liver disease. Hepatol. Res. Off. J. Jpn. Soc. Hepatol. 2025, 55, 479–491. [Google Scholar] [CrossRef] [PubMed]
- Yi, H.; Zhang, Y.; Zhou, Z.; Sun, W.; Wang, Y.; Tao, W.; Yu, H.; Yao, L.; Li, J.; Li, L. Diagnostic Performance of Noninvasive Tests for Identifying Advanced Fibrosis in Metabolic Dysfunction-Associated Fatty Liver Disease with Mixed Etiologies. Endocr. Pract. Off. J. Am. Coll. Endocrinol. Am. Assoc. Clin. Endocrinol. 2025, 31, 995–1001. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.Y.; Noh, E.S.; Jeong, H.; Hwang, I.I.T. Prediction of hepatic fibrosis using the aspartate transaminase-to-platelet ratio index in children and adolescents with metabolic dysfunction-associated steatotic liver disease. BMC Pediatr. 2024, 24, 788. [Google Scholar] [CrossRef] [PubMed]
- Altamirano, J.; Qi, Q.; Choudhry, S.; Abdallah, M.; Singal, A.K.; Humar, A.; Bataller, R.; Borhani, A.A.; Duarte-Rojo, A. Non-invasive diagnosis: Non-alcoholic fatty liver disease and alcoholic liver disease. Transl. Gastroenterol. Hepatol. 2020, 5, 31. [Google Scholar] [CrossRef]
- Vallet-Pichard, A.; Mallet, V.; Nalpas, B.; Verkarre, V.; Nalpas, A.; Dhalluin-Venier, V.; Fontaine, H.; Pol, S. FIB-4: An inexpensive and accurate marker of fibrosis in HCV infection. comparison with liver biopsy and fibrotest. Hepatology 2007, 46, 32–36. [Google Scholar] [CrossRef]
- Roh, Y.H.; Kang, B.K.; Jun, D.W.; Lee, C.M.; Kim, M. Role of FIB-4 for reassessment of hepatic fibrosis burden in referral center. Sci. Rep. 2021, 11, 13616. [Google Scholar] [CrossRef]
- Wai, C.T.; Greenson, J.K.; Fontana, R.J.; Kalbfleisch, J.D.; Marrero, J.A.; Conjeevaram, H.S.; Lok, A.S. A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology 2003, 38, 518–526. [Google Scholar] [CrossRef]
- Jayakumar, S.; Harrison, S.A.; Loomba, R. Noninvasive Markers of Fibrosis and Inflammation in Nonalcoholic Fatty Liver Disease. Curr. Hepatol. Rep. 2016, 15, 86–95. [Google Scholar] [CrossRef]
- Harrison, S.A.; Oliver, D.; Arnold, H.L.; Gogia, S.; Neuschwander-Tetri, B.A. Development and validation of a simple NAFLD clinical scoring system for identifying patients without advanced disease. Gut 2008, 57, 1441–1447. [Google Scholar] [CrossRef]
- Angulo, P.; Hui, J.M.; Marchesini, G.; Bugianesi, E.; George, J.; Farrell, G.C.; Enders, F.; Saksena, S.; Burt, A.D.; Bida, J.P.; et al. The NAFLD fibrosis score: A noninvasive system that identifies liver fibrosis in patients with NAFLD. Hepatology 2007, 45, 846–854. [Google Scholar] [CrossRef]
- Rinella, M.E.; Neuschwander-Tetri, B.A.; Siddiqui, M.S.; Abdelmalek, M.F.; Caldwell, S.; Barb, D.; Kleiner, D.E.; Loomba, R. AASLD Practice Guidance on the clinical assessment and management of nonalcoholic fatty liver disease. Hepatology 2023, 77, 1797–1835. [Google Scholar] [CrossRef]
- European Association for the Study of the Liver. EASL Clinical Practice Guidelines on the management of hepatitis B virus infection. J. Hepatol. 2025, 83, 502–583. [Google Scholar] [CrossRef] [PubMed]
- Castera, L.; Forns, X.; Alberti, A. Non-invasive evaluation of liver fibrosis using transient elastography. J. Hepatol. 2008, 48, 835–847. [Google Scholar] [CrossRef] [PubMed]
- Nguyen-Khac, E.; Thiele, M.; Voican, C.; Nahon, P.; Moreno, C.; Boursier, J.; Mueller, S.; de Ledinghen, V.; Stärkel, P.; Gyune Kim, S.; et al. Non-invasive diagnosis of liver fibrosis in patients with alcohol-related liver disease by transient elastography: An individual patient data meta-analysis. The lancet. Gastroenterol. Hepatol. 2018, 3, 614–625. [Google Scholar] [CrossRef] [PubMed]
- Manzo-Francisco, L.A.; Aquino-Matus, J.; Vidaña-Pérez, D.; Uribe, M.; Chavez-Tapia, N. Systematic review and meta-analysis: Transient elastography compared to liver biopsy for staging of liver fibrosis in primary biliary cholangitis. Ann. Hepatol. 2023, 28, 101107. [Google Scholar] [CrossRef]
- Oeda, S.; Takahashi, H.; Imajo, K.; Seko, Y.; Ogawa, Y.; Moriguchi, M.; Yoneda, M.; Anzai, K.; Aishima, S.; Kage, M.; et al. Accuracy of liver stiffness measurement and controlled attenuation parameter using FibroScan® M/XL probes to diagnose liver fibrosis and steatosis in patients with nonalcoholic fatty liver disease: A multicenter prospective study. J. Gastroenterol. 2020, 55, 428–440. [Google Scholar] [CrossRef]
- Stasi, C.; Milani, S. Evolving strategies for liver fibrosis staging: Non-invasive assessment. World J. Gastroenterol. 2017, 23, 191–196. [Google Scholar] [CrossRef]
- Arena, U.; Vizzutti, F.; Corti, G.; Ambu, S.; Stasi, C.; Bresci, S.; Moscarella, S.; Boddi, V.; Petrarca, A.; Laffi, G.; et al. Acute viral hepatitis increases liver stiffness values measured by transient elastography. Hepatology 2008, 47, 380–384. [Google Scholar] [CrossRef]
- Lemmer, A.; VanWagner, L.; Ganger, D. Congestive hepatopathy: Differentiating congestion from fibrosis. Clin. Liver Dis. 2018, 10, 139–143. [Google Scholar] [CrossRef]
- Berger, A.; Shili, S.; Zuberbuhler, F.; Hiriart, J.B.; Lannes, A.; Chermak, F.; Hunault, G.; Foucher, J.; Oberti, F.; Fouchard-Hubert, I.; et al. Liver Stiffness Measurement with FibroScan: Use the Right Probe in the Right Conditions! Clin. Transl. Gastroenterol. 2019, 10, e00023. [Google Scholar] [CrossRef]
- The French Metavir Cooperative Study Group. Intraobserver and inter-observer variations in liver biopsy interpretation in patients with chronic hepatitis C. Hepatology 1994, 20, 15–20. [Google Scholar] [CrossRef]
- Kleiner, D.E.; Brunt, E.M.; Van Natta, M.; Behling, C.; Contos, M.J.; Cummings, O.W.; Ferrell, L.D.; Liu, Y.C.; Torbenson, M.S.; Unalp-Arida, A.; et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology 2005, 41, 1313–1321. [Google Scholar] [CrossRef]
- Chowdhury, A.B.; Mehta, K.J. Liver biopsy for assessment of chronic liver diseases: A synopsis. Clin. Exp. Med. 2023, 23, 273–285. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira Terzi, F.V.; Parente, D.B.; Camargo, G.C.; Pittella, A.M.; Silva-Junior, G.; de Novaes, G.G.; Oliveira Neto, J.A.; Barroso, J.M.; Pinheiro, M.V.T.; Xavier-de-Brito, A.S.; et al. MRI-derived extracellular volume to assess liver fibrosis in patients with metabolic-associated steatotic liver disease. Abdom. Radiol. 2025, 50, 5223–5231. [Google Scholar] [CrossRef] [PubMed]
- Jalali, M.; Rahimlou, M.; Mahmoodi, M.; Moosavian, S.P.; Symonds, M.E.; Jalali, R.; Zare, M.; Imanieh, M.H.; Stasi, C. The effects of metformin administration on liver enzymes and body composition in non-diabetic patients with non-alcoholic fatty liver disease and/or non-alcoholic steatohepatitis: An up-to date systematic review and meta-analysis of randomized controlled trials. Pharmacol. Res. 2020, 159, 104799. [Google Scholar] [CrossRef] [PubMed]
- Drake, T.; Landsteiner, A.; Langsetmo, L.; MacDonald, R.; Anthony, M.; Kalinowski, C.; Ullman, K.; Billington, C.J.; Kaka, A.; Sultan, S.; et al. Newer Pharmacologic Treatments in Adults with Type 2 Diabetes: A Systematic Review and Network Meta-analysis for the American College of Physicians. Ann. Intern. Med. 2024, 177, 618–632. [Google Scholar] [CrossRef] [PubMed]
- Boussageon, R.; Maynié-François, C. Glucose-Lowering Drugs to Reduce Cardiovascular Risk in Type 2 Diabetes. N. Engl. J. Med. 2021, 385, 671–672. [Google Scholar] [CrossRef]
- Vaughan, E.M.; Santiago-Delgado, Z.M. Management of Type 2 Diabetes Mellitus with Noninsulin Pharmacotherapy. Am. Fam. Physician 2024, 109, 333–342. [Google Scholar]
- FDA Orange Book: Approved Drug Products with Therapeutic Equivalence Evaluations. U.S. Food and Drug Administration. Available online: https://www.fda.gov/drugs/drug-approvals-and-databases/approved-drug-products-therapeutic-equivalence-evaluations-orange-book (accessed on 15 January 2026).
- Marso, S.P.; Daniels, G.H.; Brown-Frandsen, K.; Kristensen, P.; Mann, J.F.; Nauck, M.A.; Nissen, S.E.; Pocock, S.; Poulter, N.R.; Ravn, L.S.; et al. Liraglutide and Cardiovascular Outcomes in Type 2 Diabetes. N. Engl. J. Med. 2016, 375, 311–322. [Google Scholar] [CrossRef]
- Frías, J.P.; Davies, M.J.; Rosenstock, J.; Pérez Manghi, F.C.; Fernández Landó, L.; Bergman, B.K.; Liu, B.; Cui, X.; Brown, K.; SURPASS-2 Investigators. Tirzepatide versus Semaglutide Once Weekly in Patients with Type 2 Diabetes. N. Engl. J. Med. 2021, 385, 503–515. [Google Scholar] [CrossRef]
- Cusi, K.; Isaacs, S.; Barb, D.; Basu, R.; Caprio, S.; Garvey, W.T.; Kashyap, S.; Mechanick, J.I.; Mouzaki, M.; Nadolsky, K.; et al. American Association of Clinical Endocrinology Clinical Practice Guideline for the Diagnosis and Management of Nonalcoholic Fatty Liver Disease in Primary Care and Endocrinology Clinical Settings: Co-Sponsored by the American Association for the Study of Liver Diseases (AASLD). Endocr. Pract. Off. J. Am. Coll. Endocrinol. Am. Assoc. Clin. Endocrinol. 2022, 28, 528–562. [Google Scholar] [CrossRef]
- Estruch, R.; Ros, E.; Salas-Salvadó, J.; Covas, M.I.; Corella, D.; Arós, F.; Gómez-Gracia, E.; Ruiz-Gutiérrez, V.; Fiol, M.; Lapetra, J.; et al. Primary Prevention of Cardiovascular Disease with a Mediterranean Diet Supplemented with Extra-Virgin Olive Oil or Nuts. N. Engl. J. Med. 2018, 378, e34. [Google Scholar] [CrossRef] [PubMed]
- Keating, S.E.; Hackett, D.A.; George, J.; Johnson, N.A. Exercise and non-alcoholic fatty liver disease: A systematic review and meta-analysis. J. Hepatol. 2012, 57, 157–166. [Google Scholar] [CrossRef] [PubMed]
- Cusi, K. Role of obesity and lipotoxicity in the development of nonalcoholic steatohepatitis: Pathophysiology and clinical implications. Gastroenterology 2012, 142, 711–725.e6. [Google Scholar] [CrossRef]
- Vilar-Gomez, E.; Martinez-Perez, Y.; Calzadilla-Bertot, L.; Torres-Gonzalez, A.; Gra-Oramas, B.; Gonzalez-Fabian, L.; Friedman, S.L.; Diago, M.; Romero-Gomez, M. Weight Loss Through Lifestyle Modification Significantly Reduces Features of Nonalcoholic Steatohepatitis. Gastroenterology 2015, 149, 367–378.e5. [Google Scholar] [CrossRef]
- Lassailly, G.; Caiazzo, R.; Buob, D.; Pigeyre, M.; Verkindt, H.; Labreuche, J.; Raverdy, V.; Leteurtre, E.; Dharancy, S.; Louvet, A.; et al. Bariatric Surgery Reduces Features of Nonalcoholic Steatohepatitis in Morbidly Obese Patients. Gastroenterology 2015, 149, 379-e16. [Google Scholar] [CrossRef]
- Promrat, K.; Kleiner, D.E.; Niemeier, H.M.; Jackvony, E.; Kearns, M.; Wands, J.R.; Fava, J.L.; Wing, R.R. Randomized controlled trial testing the effects of weight loss on nonalcoholic steatohepatitis. Hepatology 2010, 51, 121–129. [Google Scholar] [CrossRef]
- Younossi, Z.M.; Loomba, R.; Anstee, Q.M.; Rinella, M.E.; Bugianesi, E.; Marchesini, G.; Neuschwander-Tetri, B.A.; Serfaty, L.; Negro, F.; Caldwell, S.H.; et al. Diagnostic modalities for nonalcoholic fatty liver disease, nonalcoholic steatohepatitis, and associated fibrosis. Hepatology 2018, 68, 349–360. [Google Scholar] [CrossRef]
- Sanyal, A.J.; Brunt, E.M.; Kleiner, D.E.; Kowdley, K.V.; Chalasani, N.; Lavine, J.E.; Ratziu, V.; McCullough, A. Endpoints and clinical trial design for nonalcoholic steatohepatitis. Hepatology 2011, 54, 344–353. [Google Scholar] [CrossRef]
- Stasi, C.; Mega, A. Improvement of Liver Fibrosis in Patients with MASLD Undergoing Pioglitazone Treatment: An Update. Life 2025, 15, 1682. [Google Scholar] [CrossRef]
- American Diabetes Association Professional Practice Committee. 9. Pharmacologic Approaches to Glycemic Treatment: Standards of Care in Diabetes-2025. Diabetes Care 2025, 48, S181–S206. [Google Scholar] [CrossRef] [PubMed]
- DeFronzo, R.A.; Inzucchi, S.; Abdul-Ghani, M.; Nissen, S.E. Pioglitazone: The forgotten, cost-effective cardioprotective drug for type 2 diabetes. Diabetes Vasc. Dis. Res. 2019, 16, 133–143. [Google Scholar] [CrossRef] [PubMed]
- Harrison, S.A.; Bedossa, P.; Guy, C.D.; Schattenberg, J.M.; Loomba, R.; Taub, R.; Labriola, D.; Moussa, S.E.; Neff, G.W.; Rinella, M.E.; et al. A Phase 3, Randomized, Controlled Trial of Resmetirom in NASH with Liver Fibrosis. N. Engl. J. Med. 2024, 390, 497–509. [Google Scholar] [CrossRef] [PubMed]
- FDA. FDA Approves Treatment for Serious Liver Disease Known as ‘MASH’. Available online: https://www.fda.gov/drugs/news-events-human-drugs/fda-approves-treatment-serious-liver-disease-known-mash (accessed on 15 August 2025).
- EMA. Rezdiffra. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/rezdiffra (accessed on 10 November 2025).
- Armstrong, M.J.; Gaunt, P.; Aithal, G.P.; Barton, D.; Hull, D.; Parker, R.; Hazlehurst, J.M.; Guo, K.; LEAN Trial Team; Abouda, G.; et al. Liraglutide safety and efficacy in patients with non-alcoholic steatohepatitis (LEAN): A multicentre, double-blind, randomised, placebo-controlled phase 2 study. Lancet 2016, 387, 679–690. [Google Scholar] [CrossRef]
- Newsome, P.N.; Buchholtz, K.; Cusi, K.; Linder, M.; Okanoue, T.; Ratziu, V.; Sanyal, A.J.; Sejling, A.S.; Harrison, S.A.; NN9931-4296 Investigators. A Placebo-Controlled Trial of Subcutaneous Semaglutide in Nonalcoholic Steatohepatitis. N. Engl. J. Med. 2021, 384, 1113–1124. [Google Scholar] [CrossRef]
- FDA. Highlights of Prescribing Information. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2025/209637s025lbl.pdf (accessed on 17 January 2026).
- Kuchay, M.S.; Krishan, S.; Mishra, S.K.; Farooqui, K.J.; Singh, M.K.; Wasir, J.S.; Bansal, B.; Kaur, P.; Jevalikar, G.; Gill, H.K.; et al. Effect of Empagliflozin on Liver Fat in Patients with Type 2 Diabetes and Nonalcoholic Fatty Liver Disease (E-LIFT Trial): A Randomized Clinical Trial. Diabetes Care 2018, 41, 1801–1808. [Google Scholar] [CrossRef]
- FDA Drug Safety Communication: FDA Strengthens Kidney Warnings for Diabetes Medicines Canagliflozin (Invokana, Invokamet) and Dapagliflozin (Farxiga, Xigduo XR). Available online: https://www.fda.gov/drugs/drug-safety-and-availability/fda-drug-safety-communication-fda-strengthens-kidney-warnings-diabetes-medicines-canagliflozin (accessed on 14 June 2016).
- Sanyal, A.J.; Chalasani, N.; Kowdley, K.V.; McCullough, A.; Diehl, A.M.; Bass, N.M.; Neuschwander-Tetri, B.A.; Lavine, J.E.; Tonascia, J.; Unalp, A.; et al. Pioglitazone, vitamin E, or placebo for nonalcoholic steatohepatitis. N. Engl. J. Med. 2010, 362, 1675–1685. [Google Scholar] [CrossRef]
- Cusi, K.; Orsak, B.; Bril, F.; Lomonaco, R.; Hecht, J.; Ortiz-Lopez, C.; Tio, F.; Hardies, J.; Darland, C.; Musi, N.; et al. Long-Term Pioglitazone Treatment for Patients with Nonalcoholic Steatohepatitis and Prediabetes or Type 2 Diabetes Mellitus: A Randomized Trial. Ann. Intern. Med. 2016, 165, 305–315. [Google Scholar] [CrossRef]
- FDA. Pioglitazone and Metformin- Pioglitazone and Metformin Tablet, Film Coated. Available online: https://dailymed.nlm.nih.gov/dailymed/fda/fdaDrugXsl.cfm?setid=29145eca-d3de-475d-9171-3803966d6ba7&type=display (accessed on 17 January 2026).
- Kazemi, R.; Aduli, M.; Sotoudeh, M.; Malekzadeh, R.; Seddighi, N.; Sepanlou, S.G.; Merat, S. Metformin in nonalcoholic steatohepatitis: A randomized controlled trial. Middle East J. Dig. Dis. 2012, 4, 16–22. [Google Scholar]
- Lavine, J.E.; Schwimmer, J.B.; Van Natta, M.L.; Molleston, J.P.; Murray, K.F.; Rosenthal, P.; Abrams, S.H.; Scheimann, A.O.; Sanyal, A.J.; Chalasani, N.; et al. Effect of vitamin E or metformin for treatment of nonalcoholic fatty liver disease in children and adolescents: The TONIC randomized controlled trial. JAMA 2011, 305, 1659–1668. [Google Scholar] [CrossRef]
- Dahl, D.; Onishi, Y.; Norwood, P.; Huh, R.; Bray, R.; Patel, H.; Rodríguez, Á. Effect of Subcutaneous Tirzepatide vs Placebo Added to Titrated Insulin Glargine on Glycemic Control in Patients with Type 2 Diabetes: The SURPASS-5 Randomized Clinical Trial. JAMA 2022, 327, 534–545. [Google Scholar] [CrossRef] [PubMed]
- FDA Approves New Medication for Chronic Weight Management. News Release. FDA. Available online: https://www.fda.gov/news-events/press-announcements/fda-approves-new-medication-chronic-weight-management (accessed on 8 November 2023).
- FDA Approves First Medication for Obstructive Sleep Apnea. News Release. FDA. Available online: https://www.fda.gov/news-events/press-announcements/fda-approves-first-medication-obstructive-sleep-apnea (accessed on 20 December 2024).
- Francque, S.M.; Bedossa, P.; Ratziu, V.; Anstee, Q.M.; Bugianesi, E.; Sanyal, A.J.; Loomba, R.; Harrison, S.A.; Balabanska, R.; Mateva, L.; et al. A Randomized, Controlled Trial of the Pan-PPAR Agonist Lanifibranor in NASH. N. Engl. J. Med. 2021, 385, 1547–1558. [Google Scholar] [CrossRef] [PubMed]
- Boursier, J.; Hervé, H.; Roux, M.; Abdelmalek, M.F.; Francque, S.M.; Broqua, P.; Junien, J.L.; Abitbol, J.L.; Huot-Marchand, P.; Dzen, L.; et al. Biomarkers of Histological Response in Lanifibranor-treated Patients with Metabolic Dysfunction-associated Steatohepatitis. Clin. Gastroenterol. Hepatol. Off. Clin. Pract. J. Am. Gastroenterol. Assoc. 2025, 23, 2499–2508.e8. [Google Scholar] [CrossRef] [PubMed]
- Inventiva. Inventiva Receives FDA Breakthrough Therapy Designation for Lead Drug Candidate Lanifibranor in NASH. Available online: https://www.globenewswire.com/news-release/2020/10/12/2107044/0/en/Inventiva-receives-FDA-Breakthrough-Therapy-designation-for-lead-drug-candidate-lanifibranor-in-NASH.html (accessed on 12 October 2020).
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Sanyal, A.J.; Newsome, P.N.; Kliers, I.; Østergaard, L.H.; Long, M.T.; Kjær, M.S.; Cali, A.M.G.; Bugianesi, E.; Rinella, M.E.; Roden, M.; et al. Phase 3 Trial of Semaglutide in Metabolic Dysfunction-Associated Steatohepatitis. N. Engl. J. Med. 2025, 392, 2089–2099. [Google Scholar] [CrossRef]
- Ratziu, V.; Francque, S.; Behling, C.A.; Cejvanovic, V.; Cortez-Pinto, H.; Iyer, J.S.; Krarup, N.; Le, Q.; Sejling, A.S.; Tiniakos, D.; et al. Artificial intelligence scoring of liver biopsies in a phase II trial of semaglutide in nonalcoholic steatohepatitis. Hepatology 2024, 80, 173–185. [Google Scholar] [CrossRef]
- Loomba, R.; Abdelmalek, M.F.; Armstrong, M.J.; Jara, M.; Kjær, M.S.; Krarup, N.; Lawitz, E.; Ratziu, V.; Sanyal, A.J.; Schattenberg, J.M.; et al. Semaglutide 2·4 mg once weekly in patients with non-alcoholic steatohepatitis-related cirrhosis: A randomised, placebo-controlled phase 2 trial. Lancet Gastroenterol. Hepatol. 2023, 8, 511–522. [Google Scholar] [CrossRef]
- Caussy, C.; Cusi, K.; Rosenstock, J.; Bugianesi, E.; Thomas, M.K.; Tang, Y.; Mather, K.J.; Loomba, R.; Sanyal, A.J.; Hartman, M.L. Relationship Between Metabolic and Histological Responses in People With Metabolic Dysfunction- Associated Steatohepatitis with and Without Type 2 Diabetes: Participant-Level Exploratory Analysis of the SYNERGY-NASH Trial with Tirzepatide. Diabetes Care 2025, 48, 2074–2083. [Google Scholar] [CrossRef]
- Hartman, M.L.; Sanyal, A.J.; Loomba, R.; Wilson, J.M.; Nikooienejad, A.; Bray, R.; Karanikas, C.A.; Duffin, K.L.; Robins, D.A.; Haupt, A. Effects of Novel Dual GIP and GLP-1 Receptor Agonist Tirzepatide on Biomarkers of Nonalcoholic Steatohepatitis in Patients with Type 2 Diabetes. Diabetes Care 2020, 43, 1352–1355. [Google Scholar] [CrossRef]
- Taheri, H.; Malek, M.; Ismail-Beigi, F.; Zamani, F.; Sohrabi, M.; Reza Babaei, M.; Khamseh, M.E. Effect of Empagliflozin on Liver Steatosis and Fibrosis in Patients with Non-Alcoholic Fatty Liver Disease Without Diabetes: A Randomized, Double-Blind, Placebo-Controlled Trial. Adv. Ther. 2020, 37, 4697–4708. [Google Scholar] [CrossRef]
- Shimizu, M.; Suzuki, K.; Kato, K.; Jojima, T.; Iijima, T.; Murohisa, T.; Iijima, M.; Takekawa, H.; Usui, I.; Hiraishi, H.; et al. Evaluation of the effects of dapagliflozin, a sodium-glucose co-transporter-2 inhibitor, on hepatic steatosis and fibrosis using transient elastography in patients with type 2 diabetes and non-alcoholic fatty liver disease. Diabetes Obes. Metab. 2019, 21, 285–292. [Google Scholar] [CrossRef]
- Lin, J.; Huang, Y.; Xu, B.; Gu, X.; Huang, J.; Sun, J.; Jia, L.; He, J.; Huang, C.; Wei, X.; et al. Effect of dapagliflozin on metabolic dysfunction-associated steatohepatitis: Multicentre, double blind, randomised, placebo controlled trial. BMJ 2025, 389, e083735. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Abdelgani, S.; Khattab, A.; Adams, J.; Baskoy, G.; Brown, M.; Clarke, G.; Larvenenko, O.; DeFronzo, R.A.; Abdul-Ghani, M. Empagliflozin Reduces Liver Fat in Individuals with and Without Diabetes. Diabetes Care 2024, 47, 668–675. [Google Scholar] [CrossRef] [PubMed]
- Souza, M.; Al-Sharif, L.; Antunes, V.L.J.; Huang, D.Q.; Loomba, R. Comparison of pharmacological therapies in metabolic dysfunction-associated steatohepatitis for fibrosis regression and MASH resolution: Systematic review and network meta-analysis. Hepatology 2025, 82, 1523–1533. [Google Scholar] [CrossRef] [PubMed]
- Amin, M.A.; Sadik, N.A.; Saad, H.A.; Fawzy, M.; Elsheimy, H.A. The effect of SGLT2 inhibitors on hepatic steatosis detected by MRI-PDFF in patients with type 2 Diabetes mellitus and metabolic-associated steatotic liver disease. Intern. Emerg. Med. 2025, 20, 1025–1033. [Google Scholar] [CrossRef]
- Dawed, A.Y.; Mari, A.; Brown, A.; McDonald, T.J.; Li, L.; Wang, S.; Hong, M.G.; Sharma, S.; Robertson, N.R.; Mahajan, A.; et al. Pharmacogenomics of GLP-1 receptor agonists: A genome-wide analysis of observational data and large randomised controlled trials. Lancet Diabetes Endocrinol. 2023, 11, 33–41. [Google Scholar] [CrossRef]
- Tonin, G.; Goričar, K.; Blagus, T.; Janež, A.; Dolžan, V.; Klen, J. Genetic variability in sodium-glucose cotransporter 2 and glucagon-like peptide 1 receptor effect on glycemic and pressure control in type 2 diabetes patients treated with SGLT2 inhibitors and GLP-1RA in the everyday clinical practice. Front. Endocrinol. 2025, 16, 1547920. [Google Scholar] [CrossRef]
- Guan, Z.; Du, Y.; Li, R.; Zhang, S.; Xu, Y.; Zhang, X.; Zhang, F.; Yin, Y.; Wu, K.; Li, X.; et al. Association between glucagon-like peptide-1 receptor gene polymorphism and treatment response to GLP1R agonists in Chinese patients with type 2 diabetes: A prospective cohort study. Eur. J. Clin. Pharmacol. 2022, 78, 793–799. [Google Scholar] [CrossRef]
- Rathmann, W.; Bongaerts, B. Pharmacogenetics of novel glucose-lowering drugs. Diabetologia 2021, 64, 1201–1212. [Google Scholar] [CrossRef]
- Klen, J.; Dolžan, V. Treatment Response to SGLT2 Inhibitors: From Clinical Characteristics to Genetic Variations. Int. J. Mol. Sci. 2021, 22, 9800. [Google Scholar] [CrossRef]
- Kyriakidou, A.; Koufakis, T.; Goulis, D.G.; Vasilopoulos, Y.; Zebekakis, P.; Kotsa, K. Pharmacogenetics of the Glucagon-like Peptide-1 Receptor Agonist Liraglutide: A Step Towards Personalized Type 2 Diabetes Management. Curr. Pharm. Des. 2021, 27, 1025–1034. [Google Scholar] [CrossRef]
- Xu, B.; Li, S.; Kang, B.; Fan, S.; Chen, C.; Li, W.; Chen, J.; He, Z.; Tang, F.; Zhou, J. Role of SLC5A2 polymorphisms and effects of genetic polymorphism on sodium glucose cotransporter 2 inhibitorsinhibitor response. Mol. Biol. Rep. 2023, 50, 9637–9647. [Google Scholar] [CrossRef] [PubMed]
- Naagaard, M.D.; Chang, R.; Någård, M.; Tang, W.; Boulton, D.W. Common UGT1A9 polymorphisms do not have a clinically meaningful impact on the apparent oral clearance of dapagliflozin in type 2 diabetes mellitus. Br. J. Clin. Pharmacol. 2022, 88, 1942–1946. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Stefan, N.; Yki-Järvinen, H.; Neuschwander-Tetri, B.A. Metabolic dysfunction-associated steatotic liver disease: Heterogeneous pathomechanisms and effectiveness of metabolism-based treatment. Lancet Diabetes Endocrinol. 2025, 13, 134–148. [Google Scholar] [CrossRef] [PubMed]
- Nowak, K.; Łupina, K.; Romac, A.; Kalisz, A.; Ilkiewicz, Ł.; Janczura, J. Therapeutic Potential of GLP-1 Receptor Agonists in Metabolic Associated Steatotic Liver Disease. Ann. Pharmacother. 2025, 59, 928–936. [Google Scholar] [CrossRef] [PubMed]
- Dusilová, T.; Kovář, J.; Laňková, I.; Thieme, L.; Hubáčková, M.; Šedivý, P.; Pajuelo, D.; Burian, M.; Dezortová, M.; Miklánková, D.; et al. Semaglutide Treatment Effects on Liver Fat Content in Obese Subjects with Metabolic-Associated Steatotic Liver Disease (MASLD). J. Clin. Med. 2024, 13, 6100. [Google Scholar] [CrossRef]
- Xie, W.; Hong, Z.; Li, B.; Huang, B.; Dong, S.; Cai, Y.; Ruan, L.; Xu, Q.; Mou, L.; Zhang, Y. Influence of glucagon-like peptide-1 receptor agonists on fat accumulation in patients with diabetes mellitus and non-alcoholic fatty liver disease or obesity: A sys-tematic review and meta-analysis of randomized control trials. J. Diabetes Its Complicat. 2024, 38, 108743. [Google Scholar] [CrossRef]
- Zelniker, T.A.; Wiviott, S.D.; Raz, I.; Im, K.; Goodrich, E.L.; Furtado, R.H.M.; Bonaca, M.P.; Mosenzon, O.; Kato, E.T.; Cahn, A.; et al. Comparison of the Effects of Glucagon-Like Peptide Receptor Agonists and Sodium-Glucose Cotransporter 2 Inhibitors for Prevention of Major Adverse Cardiovascular and Renal Outcomes in Type 2 Diabetes Mellitus. Circulation 2019, 139, 2022–2031. [Google Scholar] [CrossRef]
- Bea, S.; Ko, H.Y.; Bae, J.H.; Cho, Y.M.; Chang, Y.; Ryu, S.; Byrne, C.D.; Shin, J.Y. Risk of hepatic events associated with use of sodium-glucose cotransporter-2 inhibitors versus glucagon-like peptide-1 receptor agonists, and thiazolidinediones among patients with metabolic dysfunction-associated steatotic liver disease. Gut 2025, 74, 284–294. [Google Scholar] [CrossRef]
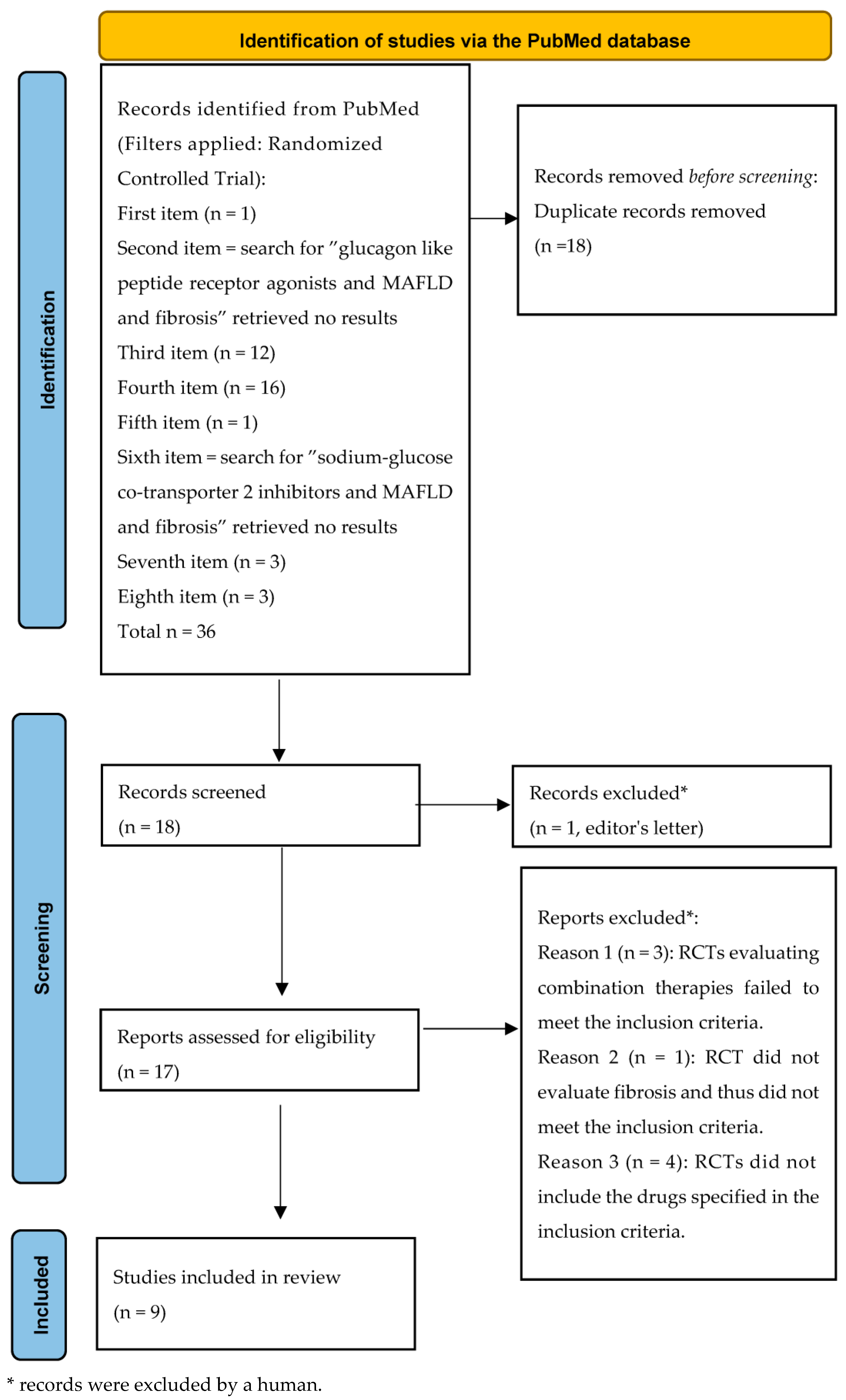
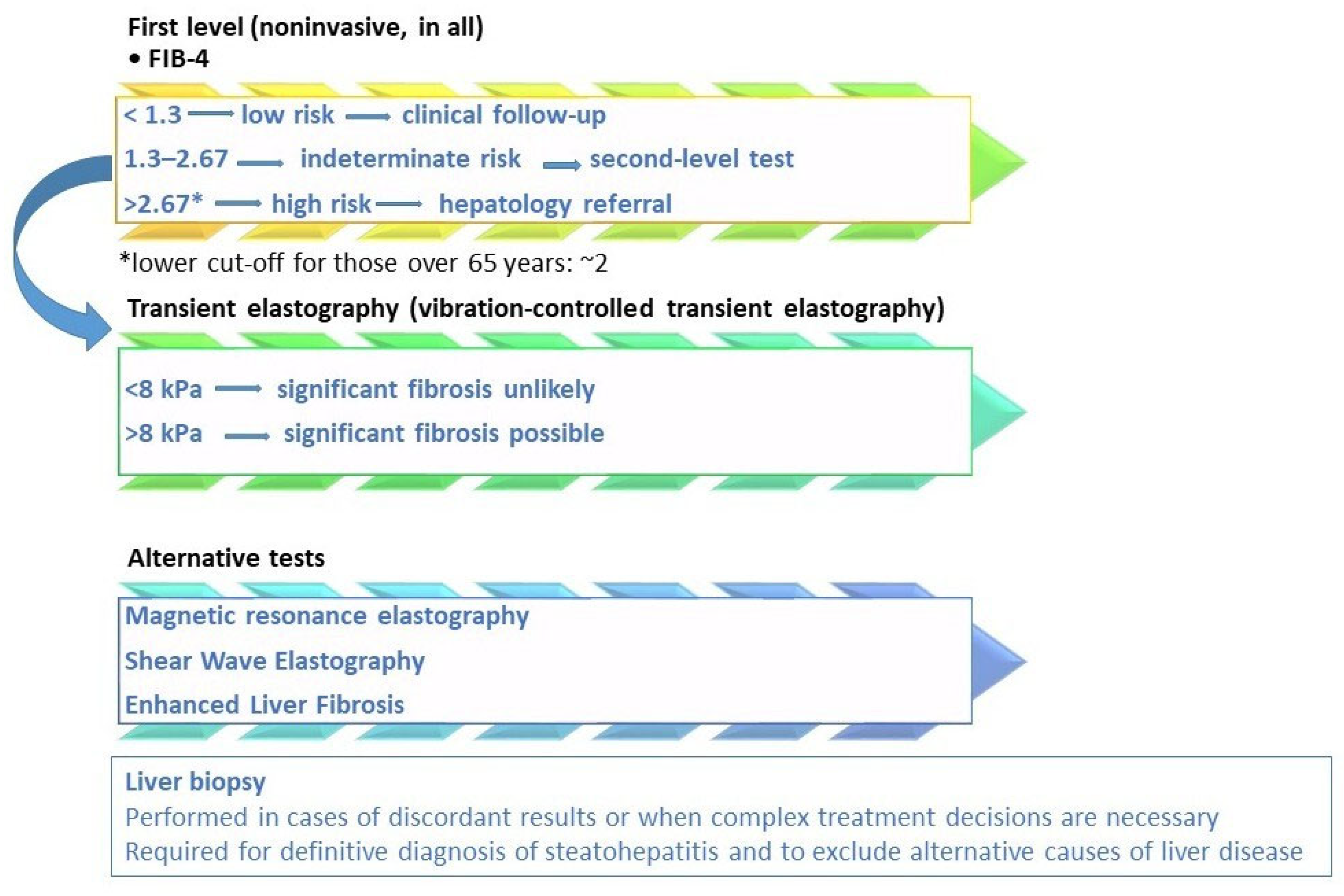
| Score | Formula | Cut-Offs | Sensitivity | Specificity | Predictive Values |
|---|---|---|---|---|---|
| FIB-4 [31,46,47] | <1.45 → low risk | ≈70% (rule-out), >80% (rule-in, high cut-off) | ≈65–80% (depending on cut-off) | NPV: ~90% (<1.45) PPV: ~65–75% (>3.25) | |
| 1.45–3.25 → indeterminate | |||||
| >3.25 → high risk | |||||
| APRI [48,49] | <0.5 → low risk | ≈91 for low risk and 41 for advanced fibrosis | ≈47 for low risk and 95% for advanced fibrosis | NPV high (86%) at a low cut-off (<0.5) PPV ~88% at >1.5 | |
| 0.5–1.5 → indeterminate | |||||
| >1.5 → advanced fibrosis/cirrhosis | |||||
| NFS [50,51] | −1.675 + (0.037 × age) + (0.094 × BMI) + (1.13 × IFG/diabetes [yes = 1,no = 0]) + (0.99 × AST/ALT) − (0.013 × platelets) − (0.66 × albumin) | <−1.455 → low risk | ≈77% (low cut-off, rule-out) ≈43% (high cut-off, rule-in) | ≈71% (low cut-off) ≈96% (high cut-off) | NPV: ~88% (<−1.455) PPV: ~82% (>0.676) |
| −1.455 to 0.676 → indeterminate | |||||
| >0.676 → advanced fibrosis |
| Optimal Cut-Off for Significant Fibrosis (Stage ≥ F2) | Optimal Cut-Off for Advanced Fibrosis (Stage F3 or F4) | Optimal Cut-Off for Cirrhosis (Stage F4) | |
|---|---|---|---|
| MASLD/MASH [52] | ≥12 kPa | ≥20 kPa | |
| HBV [53] | >7 kPa | >8 kPa | >11 kPa |
| HCV [54] | 7.1 kPa | 9.5 kPa | 12.5 kPa |
| Alcohol-related liver disease [55] | 9 kPa | 12.1 kPa | 18.6 kPa |
| Primary biliary cholangitis [56] | 9.28 kPa | 15.2 kPa |
| Fibrosis | M Probe (kPa) | XL Probe (kPa) |
|---|---|---|
| Significant fibrosis (F2 or F3 or F4) | 7 | 6.7 |
| Advanced fibrosis (F3 or F4) | 10.8 | 8.2 |
| Cirrhosis (F4) | 16.8 | 14.3 |
| Agent | Mechanism of Action | Histologic/Hepatic Evidence | Additional Benefits | Main Adverse Effects | Indications/Restrictions |
|---|---|---|---|---|---|
| Resmetirom [85,86] | THR-β agonist: ↑ hepatic lipid metabolism | Phase 3 data: ↓ steatosis ↓ MASH in F2–F3 fibrosis | Targeted hepatic effect | Diarrhea, nausea | ✓ F2–F3 MASH ✕ Cirrhosis [86,87] |
| GLP-1RA [88,89] | Incretin mimetics: ↑ satiety ↓ weight ↓ steatosis | LEAN: MASH resolution | Weight loss, ↓ CV, and renal risk | GI symptoms ↑ gallstones | ✓ GLP-1RAs are safe to use in MASH (including compensated cirrhosis) and should be used for their respective indications (e.g., T2DM, obesity) [15] ✕ Personal or family history of Multiple Endocrine Neoplasia syndrome type 2 or medullary thyroid carcinoma [90] |
| SGLT2 inhibitors [15,91] | ↓ renal glucose reabsorption: ↓ glucose, ↓ hepatic fat, osmotic diuresis | E-LIFT trial: ↓ hepatic fat (MRI-PDFF) | ↓ CV and renal risk ↓ weight | Genitourinary infections, volume depletion | ✓ SGLT2 can be used safely in MASLD and should be used for its respective indications (e.g., T2DM) [15] ✕ Renal impairment [92] |
| Pioglitazone [93,94] | PPAR-γ agonist: ↑ insulin sensitivity | PIVENS, other trials: ↑MASH and fibrosis | ↑ glycemic control | ↑ Weight heart failure bone fractures | ✓ Pioglitazone is safe in biopsy-proven MASH + T2DM; ✕ Pioglitazone cannot be recommended as a MASH-targeted therapy [15] ✕ CHF ✕ Impaired kidney function [95] |
| Vitamin E (α-tocopherol) [93] | Antioxidant: ↓ oxidative stress | PIVENS: ↓ steatosis and inflammation | Oral intake | long-term CV risk, prostate cancer | Given the lack of robust histological efficacy, vitamin E cannot be recommended as a MASH-targeted therapy [15] |
| Metformin [96,97] | ↑ insulin sensitivity via AMPK activation | No proven histologic benefit in MASH | Strong evidence of T2DM, ↓ metabolic risk | GI upset, lactic acidosis (rare) | ✓ Metformin can be used safely in MASLD and should be used for its respective indications (e.g., T2DM) [15] |
| Tirzepatide [98] | Dual incretin agonists (GIP, GLP-1) | Phase 3: ↓weight, ↓ liver fat | In patients with T2D and inadequate glycemic control despite treatment with insulin glargine, the addition of subcutaneous tirzepatide improves glycemic control after 40 weeks | GI symptoms | ✓ T2DM ✓ Obesity or overweight with at least one weight-related condition [99] ✓ Obstructive sleep apnea in adults with obesity [100] |
| Lanifibranor [101,102] | Pan-PPAR agonist | Phase 2b: ↓ MASH, ↓ fibrosis | Insulin sensitization | Weight gain, edema | Breakthrough therapy designation [103] |
| Clinical Trials Registry Number | Drug | Patients (n) | Primary Endpoints | Methods | Key Results |
|---|---|---|---|---|---|
| NCT01237119 (LEAN phase II) 2016 [88] | Liraglutide | 52; liraglutide group (n = 26) and placebo group (n = 26) | MASH resolution without worsening of fibrosis after 48 weeks | Histologically proven | MASH resolution 39% in the liraglutide group vs. 9% the placebo group |
| NCT02970942 (phase II) 2021 [89] | Semaglutide | 320; semaglutide 0.1 mg (n = 80), 0.2 mg (n = 78), or 0.4 mg (n = 82) or to receive placebo (n = 80) | MASH resolution without worsening of fibrosis after 72 weeks | Histologically proven | MASH resolution up to 59% in the semaglutide 0.4 mg vs. the placebo group, without worsening of fibrosis. |
| NCT04822181 (phase III) 2025 [105] | Semaglutide | 800; semaglutide group (n = 534) and placebo group (n = 266) | MASH resolution without worsening of fibrosis after 72 weeks | Histologically proven | MASH resolution without worsening of fibrosis in 62.9% of patients in the semaglutide group vs. 34.3% in the placebo group. |
| NCT02970942 (phase II) 2024 [106] | Semaglutide | 251 randomly assigned to receive once-daily s.c. semaglutide 0.1, 0.2, or 0.4 mg | MASH resolution without worsening of fibrosis after 72 weeks | Histologically proven | MASH resolution without worsening of fibrosis was significantly higher in patients receiving semaglutide 0.4 mg (58.5%) than in those receiving placebo (22.0%). |
| NCT03987451 (phase II) 2023 [107] | Semaglutide | 71 randomly assigned (2:1) to receive either once-weekly subcutaneous semaglutide 2.4 mg or a visually matching placebo | Improvement in liver fibrosis of one stage or more without worsening of MASH after 48 weeks | Histologically proven | No significant improvement in liver fibrosis of one stage or more without worsening of MASH. |
| NCT04166773 (phase II) 2025 [108] | Tirzepatide | 190 randomly assigned to receive tirzepatide (5, 10, or 15 mg) or placebo once weekly | MASH resolution without worsening of fibrosis | Histologically proven | MASH resolution and fibrosis improvement were associated with body weight reduction, improved glycemic control, and normalization of liver fat. |
| NCT03131687 (phase II) 2020 [109] | Tirzepatide | 316 received either once weekly tirzepatide (1, 5, 10, or 15 mg), dulaglutide (1.5 mg), or placebo for 26 weeks | Effect of tirzepatide on biomarkers of MASH and fibrosis | Biomarkers | Significant improvement of MASH-related biomarkers in a T2DM population. |
| IRCT20190122042450N1 2020 [110] | Empagliflozin | 90 randomly assigned to empagliflozin 10 mg/day (n = 43) or placebo (n = 47) | Change in controlled attenuation parameter from baseline to 24 weeks of treatment. The secondary endpoint was the change in liver stiffness values from baseline to 24 weeks | Transient elastography with controlled attenuation parameter | No significant difference in controlled attenuation parameter score was observed between the two groups. Liver stiffness values significantly decreased after 24 weeks in the empagliflozin group. |
| UMIN000022155 2019 [111] | Dapaglifozin | 57 randomly assigned to a dapagliflozin group with a dose of 5 mg/d (n = 33) or a control group (n = 24) | Change in controlled attenuation parameter from baseline to 24 weeks of treatment. The key secondary endpoint was the change in LSV from baseline to 24 weeks of treatment, | Transient elastography with controlled attenuation parameter | Significantly decreased controlled attenuation parameter score and improvement of liver fibrosis only in patients with significant liver fibrosis. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2026 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Mega, A., on behalf of the A.I.G.O. (Italian Association of Hospital Gastroenterologists) and C.L.E.O. (Italian Association of Hospital Hepatologists); Turri, C.; Marzi, L.; Dauriz, M.; Sacco, R.; Floreani, A.; Stasi, C. New Treatment Options for MASLD Patients with Type 2 Diabetes. Life 2026, 16, 254. https://doi.org/10.3390/life16020254
Mega A on behalf of the A.I.G.O. (Italian Association of Hospital Gastroenterologists) and C.L.E.O. (Italian Association of Hospital Hepatologists), Turri C, Marzi L, Dauriz M, Sacco R, Floreani A, Stasi C. New Treatment Options for MASLD Patients with Type 2 Diabetes. Life. 2026; 16(2):254. https://doi.org/10.3390/life16020254
Chicago/Turabian StyleMega, Andrea on behalf of the A.I.G.O. (Italian Association of Hospital Gastroenterologists) and C.L.E.O. (Italian Association of Hospital Hepatologists), Chiara Turri, Luca Marzi, Marco Dauriz, Rodolfo Sacco, Annarosa Floreani, and Cristina Stasi. 2026. "New Treatment Options for MASLD Patients with Type 2 Diabetes" Life 16, no. 2: 254. https://doi.org/10.3390/life16020254
APA StyleMega, A., on behalf of the A.I.G.O. (Italian Association of Hospital Gastroenterologists) and C.L.E.O. (Italian Association of Hospital Hepatologists), Turri, C., Marzi, L., Dauriz, M., Sacco, R., Floreani, A., & Stasi, C. (2026). New Treatment Options for MASLD Patients with Type 2 Diabetes. Life, 16(2), 254. https://doi.org/10.3390/life16020254








