The Human Penile Fibro-Vascular Assembly Requires the Integrity of Ten Fibro-Ligaments
Abstract
1. Introduction
Practitioner Points
- 1.
- As a male potency reconstructive practitioner, maintaining the integrity of the penile fibro-vascular assembly is second to none. The human penis is the ultimate coevolutionary nature as an intromittent organ. The penile fibro-vascular assembly relies on a combination to ensure its physiological integrity.
- 2.
- The penile fibrous, anatomical ten and functionally six, play a structural role in supporting and stabilizing the various components of the penis; these ligaments include the arcuate pubic ligament, cavernosal ligaments, distal ligament, fundiform ligament, spongiosal ligament, and suspensory ligament.
- 3.
- Given the importance of these ligaments in maintaining penile sexual health, caution should be taken when considering procedures such as ligamentolysis, which involves the release or modification of these ligaments; preserving the integrity and functionality of the ligaments is essential for ensuring optimal physiological and functional outcomes in the penile fibro-vascular assembly.
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hosken, D.J.; Stockley, P. Sexual selection and genital evolution. Trends Ecol. Evol. 2004, 82, 87–93. [Google Scholar] [CrossRef]
- Hublin, J.J.; Ben-Ncer, A.; Bailey, S.E.; Freidline, S.E.; Neubauer, S.; Skinner, M.M.; Bergmann, I.; Le Cabec, A.; Benazzi, S.; Harvati-Papatheodorou, K.; et al. New fossils from Jebel Irhoud, Morocco and the pan-African origin of Homo sapiens. Nature 2017, 546, 289–292. [Google Scholar] [CrossRef]
- Brennan, P.L. Studying Genital Coevolution to Understand Intromittent Organ Morphology. Integr. Comp. Biol. 2016, 56, 669–681. [Google Scholar] [CrossRef] [PubMed]
- Menken, M.F.; Rock, J. In Vitro Fertilization and Cleavage of Human Ovarian Eggs. Am. J. Obstet. Gynecol. 1948, 55, 440–452. [Google Scholar] [CrossRef]
- Simmons, L.W. Sexual selection and genital evolution. Austral Entomol. 2014, 53, 1–17. [Google Scholar] [CrossRef]
- Kelly, D.A. Intromittent Organ Morphology and Biomechanics: Defining the Physical Challenges of Copulation. Integr. Comp. Biol. 2016, 56, 705–714. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Dean, R.C.; Lue, T.F. Physiology of penile erection and pathophysiology of erectile dysfunction. Urol. Clin. N. Am. 2005, 32, 379–395. [Google Scholar] [CrossRef]
- Wierzbicki, A.S.; Jackson, G. NO problem: Arterial and venous endothelial function and erectile dysfunction. Eur. Urol. 2011, 59, 956–958. [Google Scholar] [CrossRef] [PubMed]
- Bettany-Saltikov, J.; Turnbull, D.; Ng, S.Y.; Webb, R. Management of Spinal Deformities and Evidence of Treatment Effectiveness. Open Orthop. J. 2017, 11, 1521–1547. [Google Scholar] [CrossRef]
- Emico, T.J.; Lonner, B.S.; Moulton, A.W. Clinically Relevant Spinal anatomy. In Surgical Management of Spinal Deformities E-Book; Elsevier: Amsterdam, The Netherlands, 2009; pp. 13–43. [Google Scholar]
- Hsu, G.L.; Chang, H.C.; Molodysky, E.; Hsu, C.Y.; Tsai, M.H.; Yin, J.H.; Chen, M.T. A detailed analysis of the penile fibro-vascular assembly. J. Sex. Med. 2025, 22, 225–234. [Google Scholar] [CrossRef]
- Gratzke, C.; Angulo, J.; Chitaley, K.; Dai, Y.-T.; Kim, N.N.; Paick, J.-S.; Simonsen, U.; Ückert, S.; Wespes, E.; Andersson, K.E.; et al. Anatomy, physiology, and pathophysiology of erectile dysfunction. J. Sex. Med. 2010, 7, 445–475. [Google Scholar] [CrossRef]
- Hsu, G.L. The hypothesis of human penile anatomy, erection hemodynamic and their clinical applications. Asian J. Androl. 2006, 8, 225–234. [Google Scholar] [CrossRef] [PubMed]
- Halliday, D. Pascal’s principle, fluids. In Fundamentals of Physics; Halliday, D., Resnick, R., Walker, J., Eds.; Wiley: New York, NY, USA, 1997; pp. 355–356. [Google Scholar]
- Hsu, G.L.; Brock, G.; von Heyden, B.; Lue, T.F. The three-dimensional structure of the human tunica albuginea: Anatomical and ultrastructural level. Int. J. Impot. Res. 1992, 4, 117–129. [Google Scholar]
- Bitsch, M.; Kromann-Andersen, B.; Schou, J.; Sjøntoft, E. The elasticity and the tensile strength of tunica albuginea of the corpora cavernosa. J. Urol. 1990, 143, 642–645. [Google Scholar] [CrossRef] [PubMed]
- Hsu, G.L.; Brock, G.; Martinez-Pineiro, L.; von Heyden, B.; Lue, T.F.; Tanagho, E.A. Anatomy and strength of the tunica albuginea: Its relevance to penile prosthesis extrusion. J. Urol. 1994, 151, 1205–1208. [Google Scholar] [CrossRef]
- Cunningham, D.J. Cunningham’s Text-Book of Anatomy, 5th ed.; Oxford Press: Oxford, UK, 1918; p. 1593. [Google Scholar]
- Hsu, G.L.; Brock, G.; von Heyden, B.; Nunes, L.; Lue, T.; Tanagho, E. The distribution of elastic fibrous elements within the human penis. Br. J. Urol. 1994, 73, 566–571. [Google Scholar] [CrossRef]
- Hinman, F., Jr. Penis and male urethra. In Atlas of Urological Anatomy; WB Saunders: Philadelphia, PA, USA, 1993. [Google Scholar]
- Smith, T.C.; Hechtel, L. Erectile dysfunction and the baculum. Evol. Med. Public Health 2019, 2019, 147–148. [Google Scholar] [CrossRef]
- Hsu, G.L.; Chang, H.C.; Molodysky, E.; Hsu, C.Y.; Tsai, M.H.; Yin, J.H.; Chen, M.T. The penile fibro-vascular assembly is the last remaining independent vascular compartment to be elucidated in the entire human body. J. Sex. Med. 2022, in press. [Google Scholar]
- Loreto, C.; Garaffa, G.; Dijnovic, R.; Barbagli, G.; Villa, M.; Sansalone, S. Penile disassembly: Anatomical surgical steps. BJU Int. 2013, 112, 1035–1045. [Google Scholar] [CrossRef][Green Version]
- Toledo-Pereyra, L.H. De Humani Corporis Fabrica surgical revolution. J. Investig. Surg. 2008, 21, 232–236. [Google Scholar] [CrossRef] [PubMed]
- Hsu, G.L.; Hill, J.W.; Chen, H.S.; Huang, S.-J. Novel pilot films providing indispensable information in pharmaco-cavernosography. Transl. Androl. Urol. 2014, 4, 398–405. [Google Scholar]
- Schultz, N.G.; Lough-Stevens, M.; Abreu, E.; Orr, T.; Dean, M.D. The baculum was gained and lost multiple times during mammalian evolution. Integr. Comp. Biol. 2016, 56, 644–656. [Google Scholar] [CrossRef] [PubMed]
- Hsu, G.L.; Lin, C.W.; Hsieh, C.H.; Hsieh, J.; Chen, S.; Kuo, T.; Ling, P.; Huang, H.; Wang, C.; Tseng, G. Distal ligament in human glans: A comparative study of penile architecture. J. Androl. 2005, 26, 624–628. [Google Scholar] [CrossRef] [PubMed]
- Hsu, G.L.; Hung, Y.P.; Tsai, M.H.; Hsieh, C.; Chen, H.; Molodysky, E.; Huynh, C.C.; Yu, H. Penile veins are the principal component in erectile rigidity: A study of penile venous stripping on defrosted human cadavers. J. Androl. 2012, 33, 1176–1185. [Google Scholar] [CrossRef]
- André, G.I.; Firman, R.C.; Simmons, L.W. The coevolution of male and female genitalia in a mammal: A quantitative genetic insight. Evolution 2020, 74, 1558–1567. [Google Scholar] [CrossRef] [PubMed]
- Hsu, G.L.; Chiang, I.N. Penile Vascular Surgery for Erectile Dysfunction. In Encyclopedia of Reproduction, 3rd ed.; Skinn: Los Angeles, CA, USA, 2026; accepted, in press. [Google Scholar]
- Eardley, I.; Sethia, K. Anatomy and Physiology of Erection. In Erectile Dysfunction, Current Investigation and Management; Mosby: London, UK, 2003; pp. 7–23. [Google Scholar]
- Ducasse, E.; Speziale, F.; Baste, J.C.; Midy, D. Vascular knowledge in medieval times was the turning point for the humanistic trend. Eur. J. Vasc. Endovasc. Surg. 2006, 31, 600–608. [Google Scholar] [CrossRef][Green Version]
- Keele, K.D. Leonardo da Vinci’s Influence on Renaissance Anatomy. Med. Hist. 1964, 8, 360–370. [Google Scholar] [CrossRef]
- Sam, P.; LaGrange, C.A. Anatomy, Abdomen and Pelvis, Penis; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Hsieh, C.H.; Huang, Y.P.; Tsai, M.H.; Chen, H.S.; Huang, P.C.; Lin, C.W.; Hsu, G.L. Tunical Outer Layer Plays an Essential Role in Penile veno-occlusive Mechanism Evidenced from Electrocautery Effects to the Corpora Cavernosa in Defrosted Human Cadavers. Urology 2015, 86, 1129–1136. [Google Scholar] [CrossRef]
- Hsu, G.L.; Chen, H.S.; Huang, S.J. Does tunica anatomy matter in penile implants? Transl. Androl. Urol. 2014, 4, 406–412. [Google Scholar]
- Hsieh, C.H.; Liu, S.P.; Hsu, G.L.; Chen, H.S.; Molodysky, E.; Chen, Y.H.; Yu, H.J. Advances in our understanding of mammalian penile evolution, human penile anatomy and human erection physiology: Clinical implications for physicians and surgeons. Med. Sci. Monit. 2012, 18, RA118–RA125. [Google Scholar]
- Hsu, G.L.; Liu, S.P. Penis Structure. In Encyclopedia of Reproduction; Skinner, M.K., Ed.; Elsevier: Amsterdam, The Netherlands, 2018; Volume 1, pp. 357–366. [Google Scholar] [CrossRef]
- Chen, X.; Wu, Y.; Tao, L.; Yan, Y.; Pang, J.; Zhang, S.; Li, S. Visualization of Penile Suspensory Ligamentous System Based on Visible Human Data Sets. Med. Sci. Monit. 2017, 23, 2436–2444. [Google Scholar] [CrossRef]
- Ghafoori, M. CT Cavernosography: A New Method for Evaluating venous Incompetence in Impotent Patients. Indian J. Radiol. 2010, 7, e78769. [Google Scholar]
- Kurbatov, D.G.; Kuznetsky, Y.Y.; Kitaev, S.V.; Brusensky, V.A. Magnetic resonance imaging as a potential tool for objective visualization of venous leakage in patients with veno-occlusive erectile dysfunction. Int. J. Impot. Res. 2008, 20, 192–198. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hoznek, A.; Rahmouni, A.; Abbou, C.; Delmas, V.; Colombel, M. The suspensory ligament of the penis: An anatomic and radiologic description. Surg. Radiol. Anat. 1998, 20, 413–417. [Google Scholar] [CrossRef] [PubMed]
- Li, C.Y.; Agrawal, V.; Minhas, S.; Ralph, D.J. The penile suspensory ligament: Abnormalities and repair. BJU Int. 2007, 99, 117–120. [Google Scholar] [CrossRef] [PubMed]
- Spyropoulos, E.; Christoforidis, C.; Borousas, D.; Mavrikos, S.; Bourounis, M.; Athanasiadis, S. Augmentation phalloplasty surgery for penile dysmorphophobia in young adults: Considerations regarding patient selection, outcome evaluation and techniques applied. Eur. Urol. 2005, 48, 121–128. [Google Scholar] [CrossRef]
- Hsu, G.L.; Molodysky, E.; Liu, S.P.; Chang, H.C.; Hsieh, C.H.; Hsu, C.Y. Reconstructive surgery for idealizing penile shape and erectile functional restoration on patients with penile dysmorphology and erectile dysfunction. Arab J. Urol. 2013, 11, 375–383. [Google Scholar] [CrossRef]
- Brant, W.O.; Bella, A.I.; Lue, T.F. Anatomy of erectile function. In Textbook of Erectile Dysfunction, 2nd ed.; Carson, C.C., III, Kirby, R.S., Goldstein, I., Wyllie, M.G., Eds.; Informa Health Care: St Heler, UK, 2008; pp. 25–27. [Google Scholar]
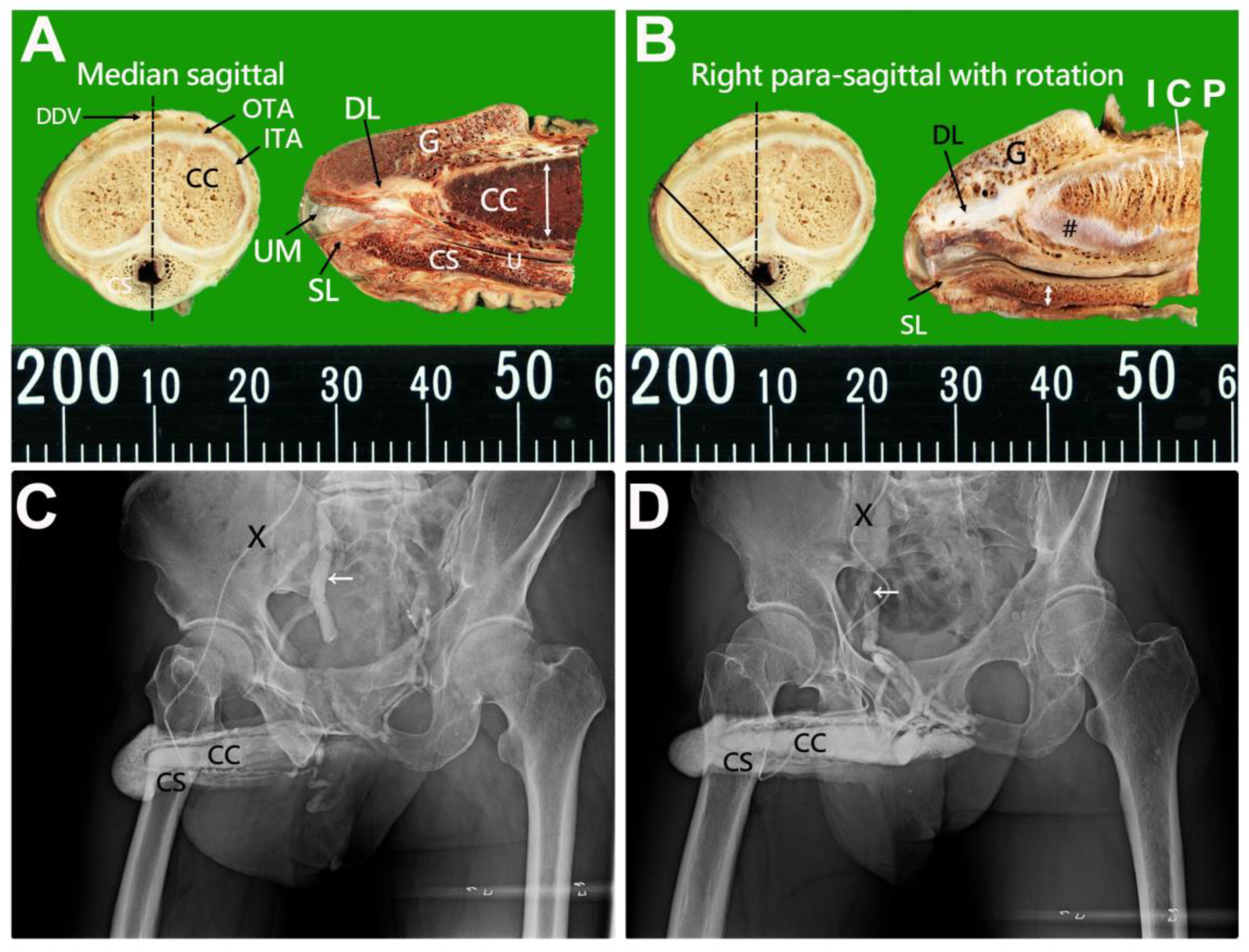
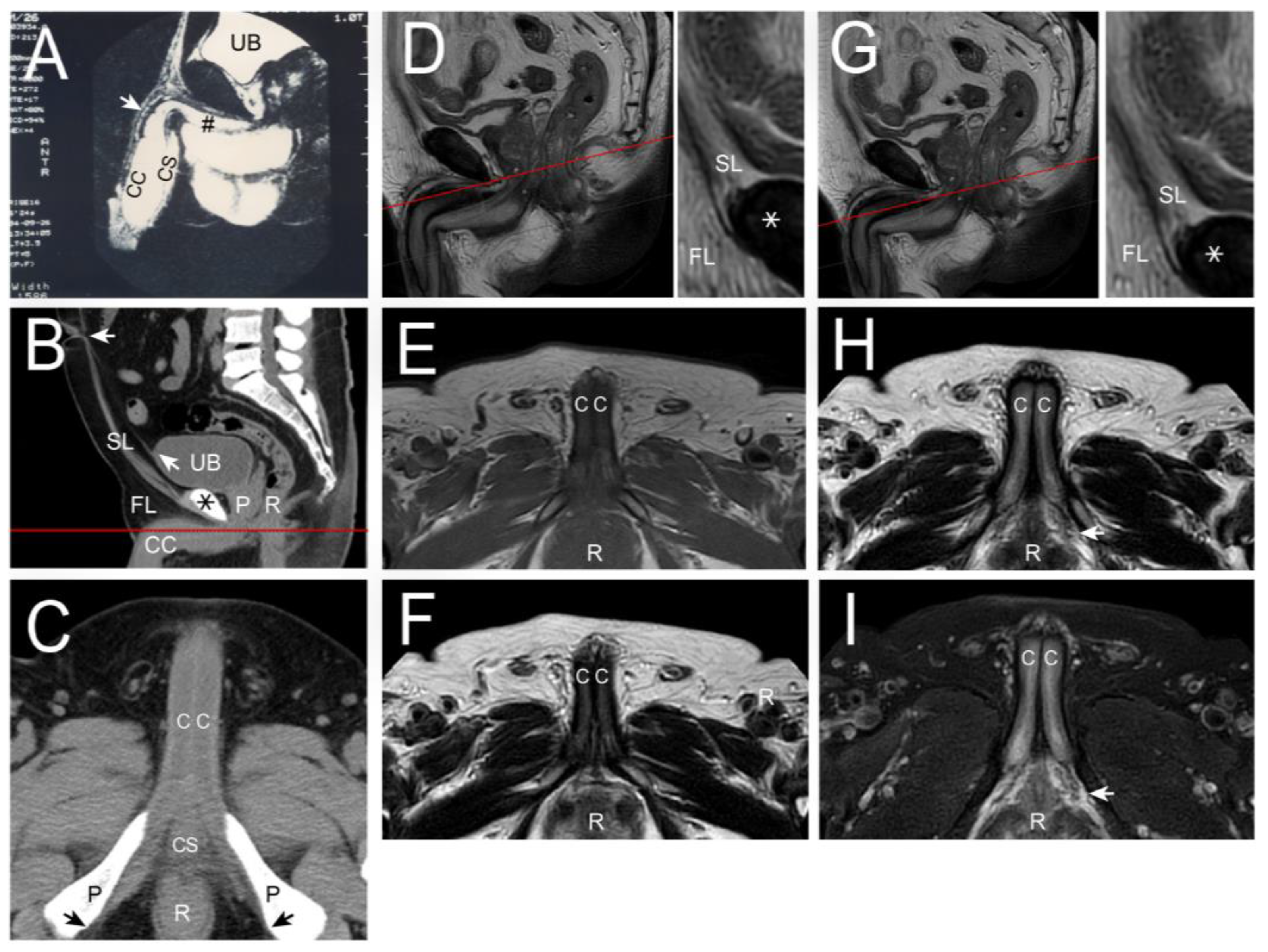
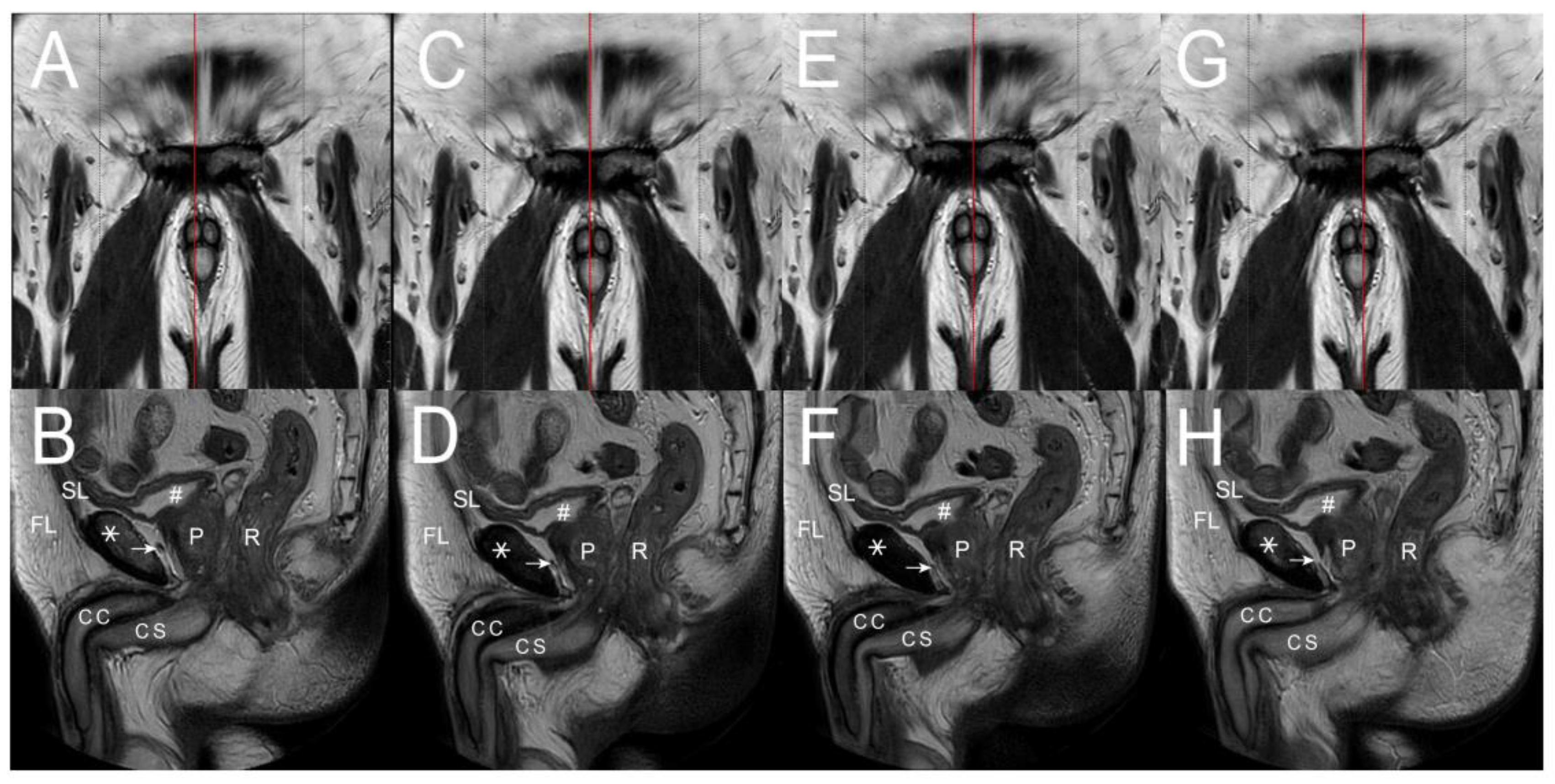
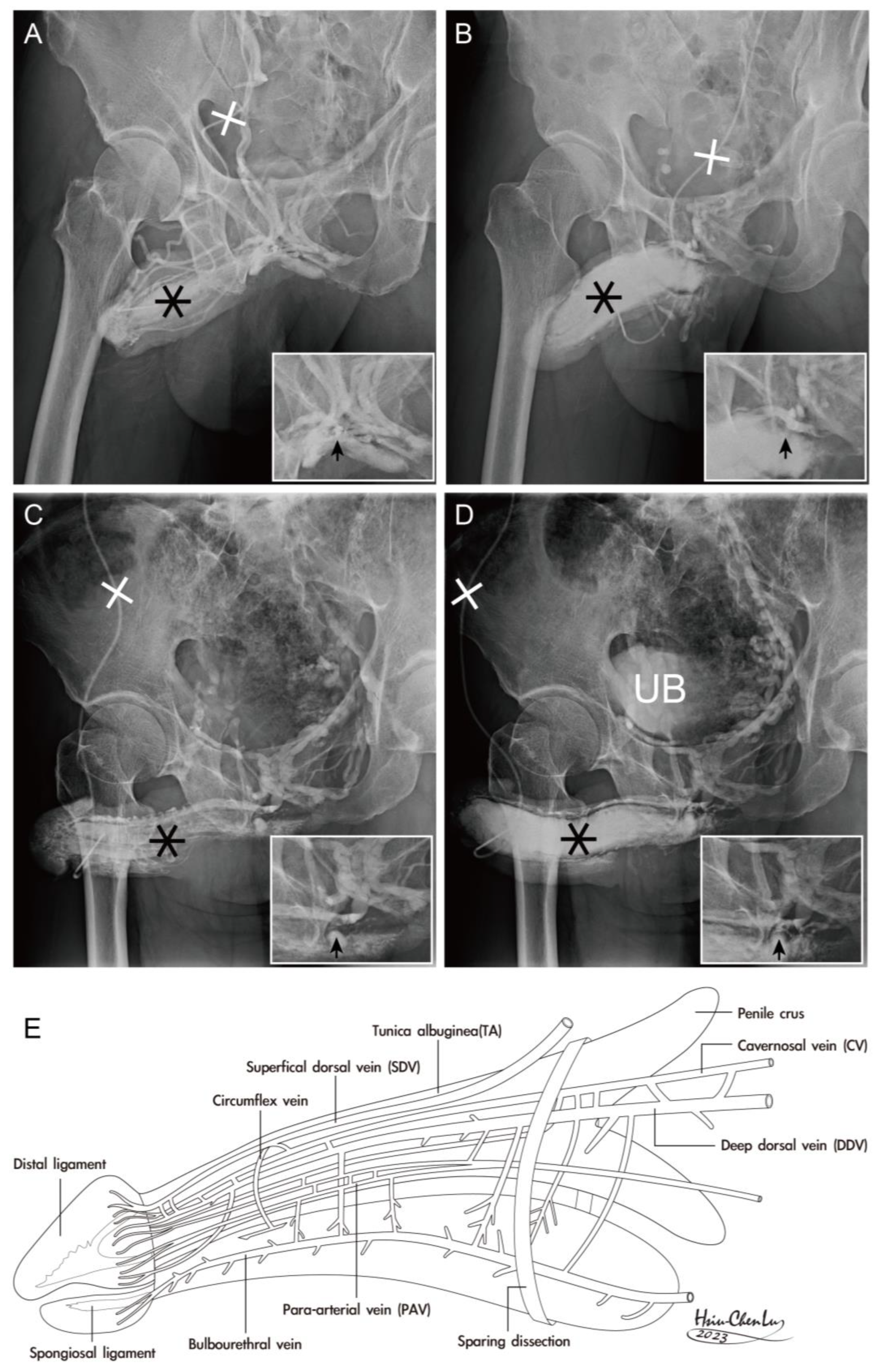

| No | Terminology | Origin | Insertion | Number of Ligaments | Connecting Structures | |
|---|---|---|---|---|---|---|
| Ligament/T Ligament/Tendon | Anatomical Functional | |||||
| 1 | Cavernosal | Ischial tuberosity | Dorsal aspect of outer Tunica | 2 | 1 | Ischium and corpora cavernosa |
| 2 | Suspensory | Buck’s fascia | Pubic symphysis | 2 | 1 | Buck’s fascia∼Pubic symphysis |
| 3 | Fundiform | Lateral pubic bone | Lateral pubic bone | 2 | 1 | Lateral pubic bone∼Penile base |
| 4 | Arcuate pubic ligament Distal (os analog) | Buck’s fascia Corpora cavernosa | Medial surface of pubic ramus Glans penis | 2 1 | 1 1 | Glans penis and corpora cavernosa Gans penis and corpora cavernosa |
| 5 | Spongiosal | Corpus spongiosum | Frenulum | 1 | 1 | Frenulum and corpus spongiosum |
| Total | 10 | 6 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, H.-S.; Fang, C.-W.; Tsai, R.W.M.; Hsu, C.-Y.; Hsu, G.-L.; Lu, H.-C.; Tsai, M.-H.; Chueh, J.S.C. The Human Penile Fibro-Vascular Assembly Requires the Integrity of Ten Fibro-Ligaments. Life 2025, 15, 1492. https://doi.org/10.3390/life15091492
Chen H-S, Fang C-W, Tsai RWM, Hsu C-Y, Hsu G-L, Lu H-C, Tsai M-H, Chueh JSC. The Human Penile Fibro-Vascular Assembly Requires the Integrity of Ten Fibro-Ligaments. Life. 2025; 15(9):1492. https://doi.org/10.3390/life15091492
Chicago/Turabian StyleChen, Heng-Shuen, Chu-Wen Fang, Raymond W. M. Tsai, Chih-Yuan Hsu, Geng-Long Hsu, Hsiu-Chen Lu, Mang-Hung Tsai, and Jeff S. C. Chueh. 2025. "The Human Penile Fibro-Vascular Assembly Requires the Integrity of Ten Fibro-Ligaments" Life 15, no. 9: 1492. https://doi.org/10.3390/life15091492
APA StyleChen, H.-S., Fang, C.-W., Tsai, R. W. M., Hsu, C.-Y., Hsu, G.-L., Lu, H.-C., Tsai, M.-H., & Chueh, J. S. C. (2025). The Human Penile Fibro-Vascular Assembly Requires the Integrity of Ten Fibro-Ligaments. Life, 15(9), 1492. https://doi.org/10.3390/life15091492







