Pancreatic Injury in Severe SARS-CoV-2 Infection: A Retrospective Study Across Three Pandemic Waves
Abstract
1. Introduction
2. Materials and Methods
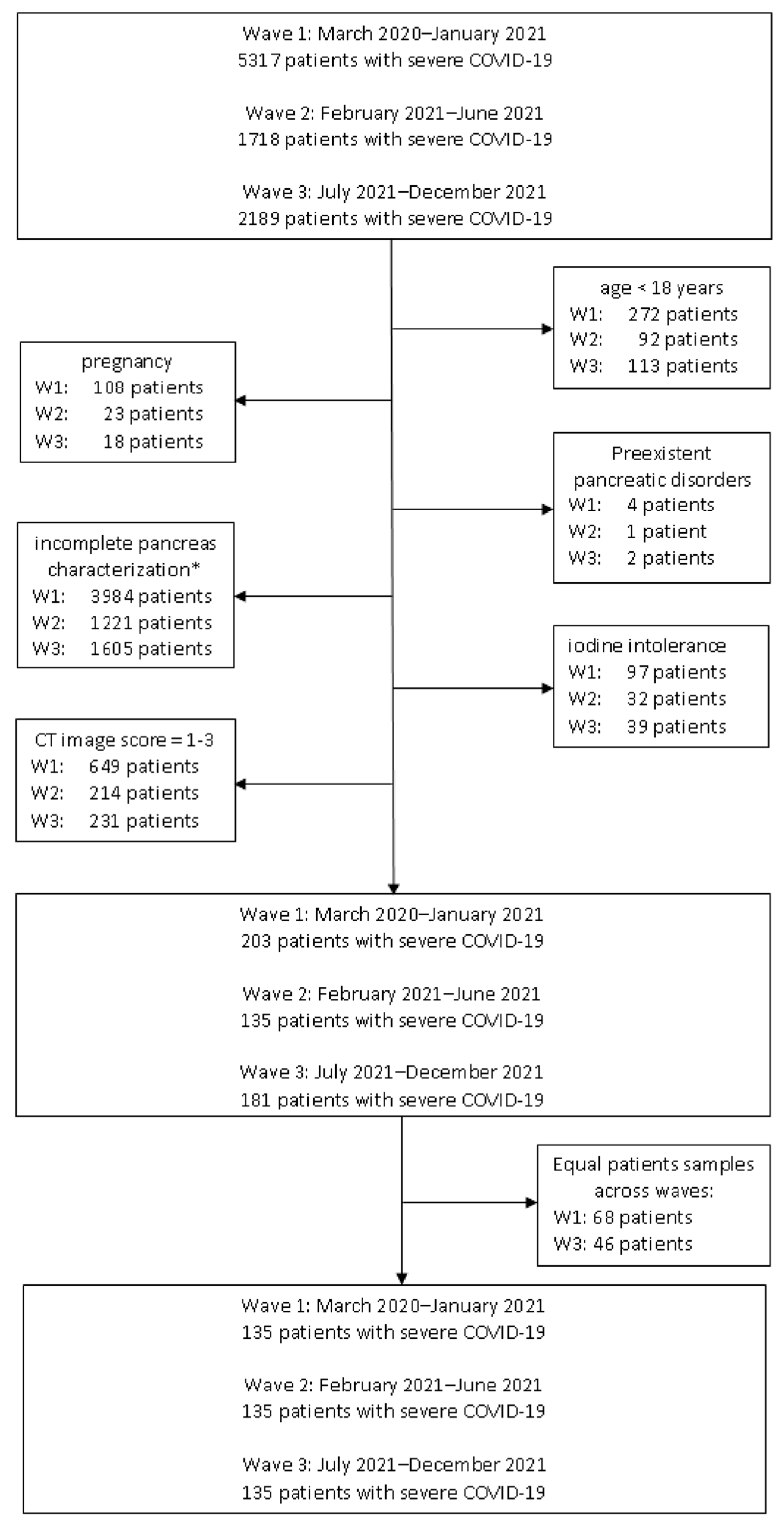
2.1. Study Population
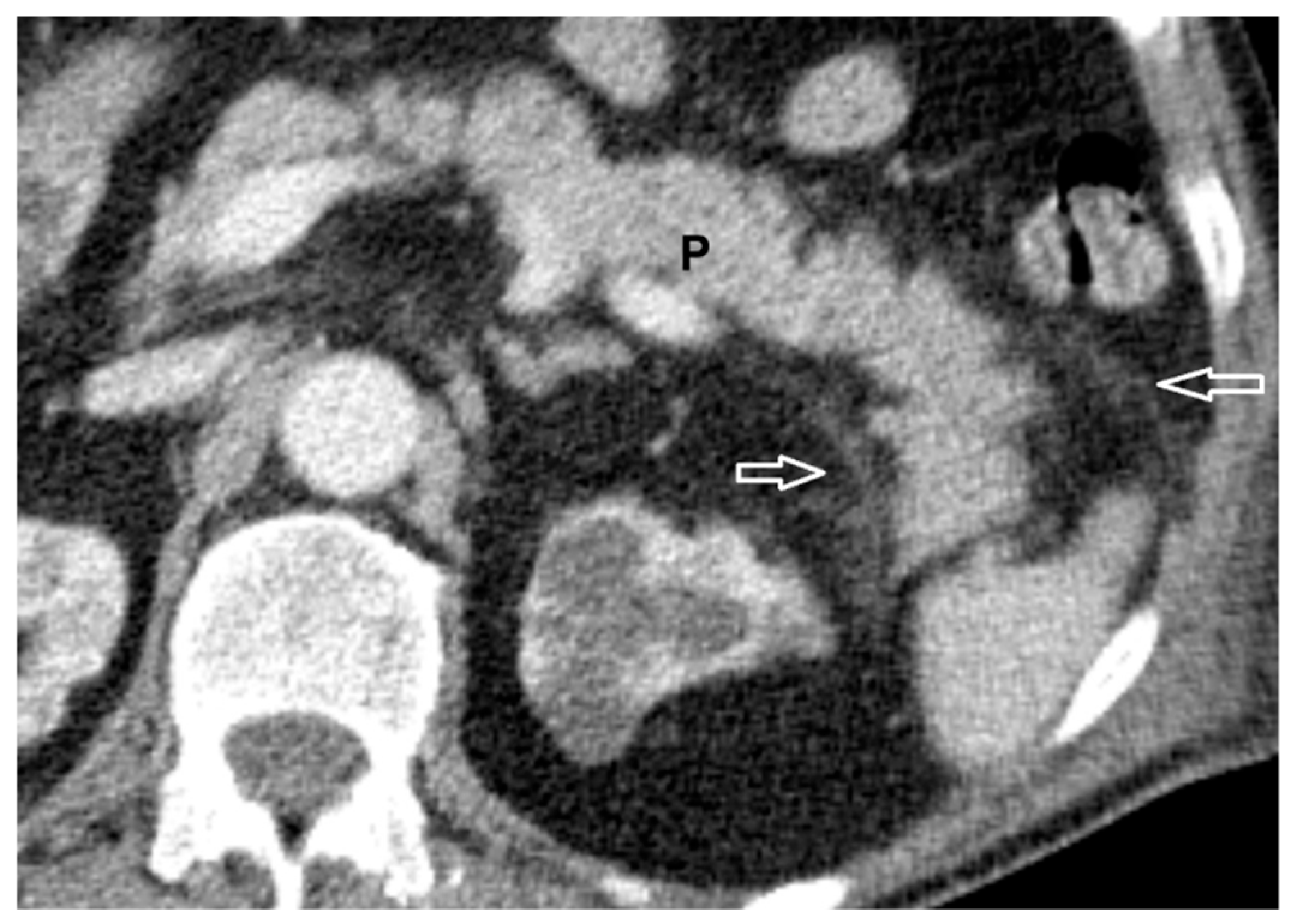
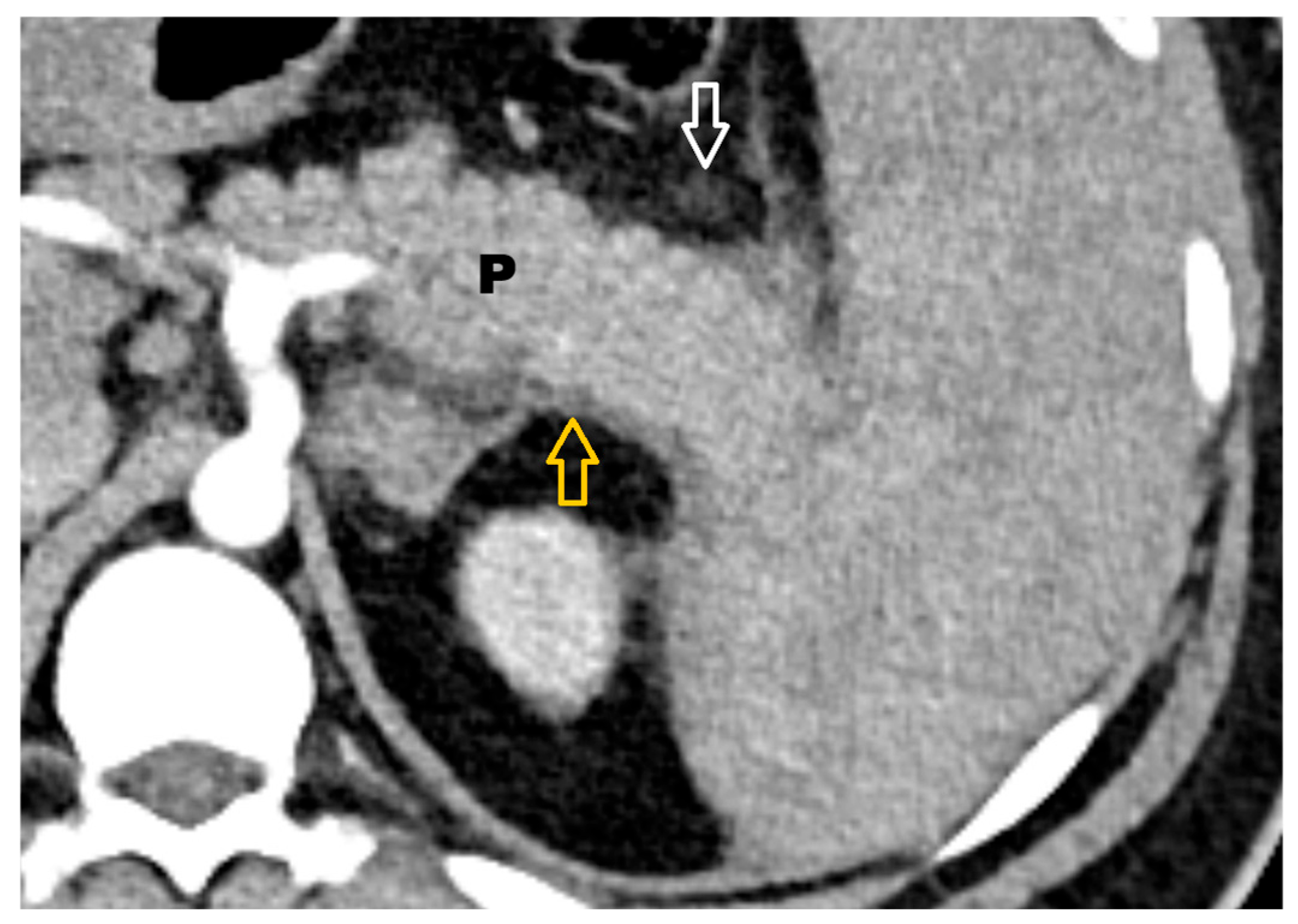
2.2. Definitions
- Poor—indistinct parenchymal detail, poorly defined contours, and pronounced motion artifacts.
- Fair—acceptable parenchymal clarity and contour definition, with moderate motion artifacts.
- Adequate—acceptable clarity and contour definition, with occasional motion artifacts.
- Good—well-defined parenchymal structures and contours, with minimal motion artifacts.
- Excellent—sharply defined parenchymal and vascular anatomy, with no motion artifacts [22].
- -
- clinical
- -
- sudden onset of epigastric pain, often radiating to the back;
- -
- nausea, vomiting;
- -
- serum amylase or lipase ≥ 3× upper limit of normal (ULN)—we used the pancreatic lipase in our study because it is more specific and remains elevated longer;
- -
- contrastenhanced CT (CECT)—for assessing severity and complications [23].
- -
- normal pancreas → 0;
- -
- diffuse or focal enlargement of the pancreas → 1;
- -
- minimal peripancreatic changes → 2;
- -
- single peripancreatic fluid collection → 3;
- -
- ≥2 fluid collections or presence of peripancreatic gas → 4.
- -
- 0%→ 0;
- -
- ≤30%→ 2;
- -
- 30–50%→ 4;
- -
- 50%→ 6.
- -
- 0–3: mild AP;
- -
- 4–6: moderate;
- -
- 7–10: severe, high risk of complications/mortality.
2.3. Demographic and Biological Parameters
2.4. CT Examination Protocol
2.5. Identification of the Risk Factors/Predictors for AP in Patients with SARS-CoV-2 Infection
2.6. Statistical Analysis
3. Results
[1 + EXP (−5.552 + 0.002 × D-Dimers + 0.045 × Lung interstitial lesions + 0.011 × CRP)]
4. Discussion
4.1. Epidemiology and Demographics
4.2. Pathophysiological Mechanisms
4.2.1. DirectViral Injury Can Induce AP
4.2.2. Systemic COVID-19 Inflammation as a Trigger for Pancreatic Injury
4.2.3. Drug-Induced Pancreatic Injury
- (i)
- Seeding: virus reaches the pancreas via haematogenous spread or retrograde ductal flow. Ductal epithelial cells and capillary endothelium expressing ACE2 (with TMPRSS2) are initial targets.
- (ii)
- Entry and ACE2 depletion: virus spikes–ACE2 engagement and TMPRSS2 priming permit fusion; receptor internalization and sheddase activity deplete surface ACE2, locally increasing angiotensin (Ang) II tone and reducing Ang-(1–7).
- (iii)
- Cytopathic stress: infected ductal cells reduce bicarbonate secretion and alter luminal rheology; infected acinar cells experience ER stress and impaired autophagic flux, leading to trypsinogen activation, vacuolization, and mitochondria-dependent cell death.
- (iv)
- Microvascular injury: endothelial infection and Ang II–biased signaling drive microthrombi and ischemia (“thrombofibrosis”), compounding acinar necrosis.
- (v)
- Lesion propagation: DAMPs, activated proteases, and cytokines amplify lobular injury and recruit inflammatory cells, closing a feed-forward loop of AP.
- (vi)
- NSAIDs and corticosteroids: may aggravate existing pancreatic inflammation or even induce it via prostaglandin depletion, leading to ductal changes, direct oxidative injury, secretory alteration, and hyperlipidemia.
4.3. Risk Factors in AP
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Whitcomb, D.C. Clinical practice.Acute pancreatitis. N. Engl. J. Med. 2006, 354, 2142–2150. [Google Scholar] [CrossRef]
- Crockett, S.; Falck-Ytter, Y.; Wani, S.; Gardner, T.B. Acute Pancreatitis Guideline. Gastroenterology 2018, 154, 1102. [Google Scholar] [CrossRef]
- Yu, X.; Wang, M.; Kong, Q. Viral pancreatitis: Research advances and mechanisms. N. Engl. J. Med. 2024, 14, 1326837. [Google Scholar] [CrossRef]
- Imam, Z.; Simons-Linares, C.R.; Chahal, P. Infectious causes of acute pancreatitis: A systematic review. Pancreatology 2020, 20, 1312–1322. [Google Scholar] [CrossRef]
- Panic, N.; Mihajlovic, S.; Vujasinovic, M.; Bulajic, M.; Löhr, J.-M. Pancreatitis Associated with Viral Hepatitis: Systematic Review. J. Clin. Med. 2020, 9, 3309. [Google Scholar] [CrossRef]
- Simons-Linares, C.R.; Imam, Z.; Chahal, P. Viral-Attributed Acute Pancreatitis: A Systematic Review. Dig. Dis. Sci. 2021, 66, 2162–2172. [Google Scholar] [CrossRef] [PubMed]
- Sarshari, B.; Zareh-Khoshchehreh, R.; Keshavarz, M.; Dehghan Manshadi, S.A.; Seyed Alinaghi, S.; Asadzadeh Aghdaei, H.; Mohebbi, S.R. The possible role of viral infections in acute pancreatitis: A review of literature. Gastroenterol. Hepatol. Bed Bench 2023, 16, 270–281. [Google Scholar]
- Alves, A.M.; Yvamoto, E.Y.; Marzinotto, M.A.N.; Teixeira, A.C.S.; Carrilho, F.J. SARS-CoV-2 leading to acute pancreatitis: An unusual presentation. Braz. J. Infect. Dis. 2020, 24, 561–564. [Google Scholar] [CrossRef] [PubMed]
- Inamdar, S.; Benias, P.C.; Liu, Y.; Sejpal, D.V.; Satapathy, S.K.; Trindade, A.J.; Northwell COVID-19 Research Consortium. Prevalence, Risk Factors, and Outcomes of Hospitalized Patients with Coronavirus Disease 2019 Presenting as Acute Pancreatitis. Gastroenterology 2020, 159, 2226–2228.e2. [Google Scholar] [CrossRef]
- Cheung, K.S.; Hung, I.F.N.; Chan, P.P.Y.; Lung, K.C.; Tso, E.; Liu, R.; Ng, Y.Y.; Chu, M.Y.; Chung, T.W.H.; Tam, A.R.; et al. Gastrointestinal Manifestations of SARS-CoV-2 Infection and Virus Load in Fecal Samples From a Hong Kong Cohort: Systematic Review and Meta-analysis. Gastroenterology 2020, 159, 81–95. [Google Scholar] [CrossRef] [PubMed]
- Sultan, S.; Altayar, O.; Siddique, S.M.; Davitkov, P.; Feuerstein, J.D.; Lim, J.K.; Falck-Ytter, Y.; El-Serag, H.B.; AGA Institute. AGA Institute Rapid Review of the Gastrointestinal and Liver Manifestations of COVID-19; Meta-Analysis of International Data, and Recommendations for the Consultative Management of Patients with COVID-19. Gastroenterology 2020, 159, 320–334.e27. [Google Scholar] [CrossRef] [PubMed]
- Dumea, E.; Barbu, E.C.; Chiţu, C.E.; Lazăr, M.; Ion, D.A. Clinical, biochemical and pulmonary CT imaging features for hepatobiliary involvement in COVID-19. Germs 2023, 13, 121–129. [Google Scholar] [CrossRef]
- Lazar, M.; Barbu, E.C.; Chitu, C.E.; Anghel, A.M.; Niculae, C.M.; Manea, E.D.; Damalan, A.C.; Bel, A.A.; Patrascu, R.E.; Hristea, A.; et al. Pericardial Involvement in Severe COVID-19 Patients. Medicina 2022, 58, 1093. [Google Scholar] [CrossRef]
- Liu, F.; Long, X.; Zhang, B.; Zhang, W.; Chen, X.; Zhang, Z. ACE2 Expression in Pancreas May Cause Pancreatic Damage After SARS-CoV-2 Infection. Clin. Gastroenterol. Hepatol. 2020, 18, 2128–2130.e2. [Google Scholar] [CrossRef]
- Coate, K.C.; Cha, J.; Shrestha, S.; Wang, W.; Gonçalves, L.M.; Almaça, J.; Kapp, M.E.; Fasolino, M.; Morgan, A.; Dai, C.; et al. SARS-CoV-2 Cell Entry Factors ACE2 and TMPRSS2 Are Expressed in the Microvasculature and Ducts of Human Pancreas but Are Not Enriched in β Cells. Cell Metab. 2020, 32, 1028–1040.e4. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Wang, H.; Fan, J.; Zhang, Y.; Wang, H.; Zhao, Q. Pancreatic Injury Patterns in Patients with Coronavirus Disease 19 Pneumonia. Gastroenterology 2020, 159, 367–370. [Google Scholar] [CrossRef] [PubMed]
- Aloysius, M.M.; Thatti, A.; Gupta, A.; Sharma, N.; Bansal, P.; Goyal, H. COVID-19 presenting as acute pancreatitis. Pancreatology 2020, 20, 1026–1027. [Google Scholar] [CrossRef]
- Hadi, A.; Werge, M.; Kristiansen, K.T.; Pedersen, U.G.; Karstensen, J.G.; Novovic, S.; Gluud, L.L. Coronavirus Disease-19 (COVID-19) associated with severe acute pancreatitis: Case report on three family members. Pancreatology 2020, 20, 665–667. [Google Scholar] [CrossRef]
- Szatmary, P.; Arora, A.; Thomas Raraty, M.G.; Joseph Dunne, D.F.; Baron, R.D.; Halloran, C.M. Emerging Phenotype of Severe Acute Respiratory Syndrome-Coronavirus 2-associated Pancreatitis. Gastroenterology 2020, 159, 1551–1554. [Google Scholar] [CrossRef]
- Akarsu, C.; Karabulut, M.; Aydin, H.; Sahbaz, N.A.; Dural, A.C.; Yegul, D.; Peker, K.D.; Ferahman, S.; Bulut, S.; Dönmez, T.; et al. Association between Acute Pancreatitis and COVID-19: Could Pancreatitis Be the Missing Piece of the Puzzle about Increased Mortality Rates? J. Investig. Surg. 2022, 35, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Anghel, A.M.; Niculae, C.M.; Manea, E.D.; Lazar, M.; Popescu, M.; Damalan, A.C.; Bel, A.A.; Nedelcu, I.M.; Patrascu, R.E.; Hristea, A. The Impact of Tocilizumab on Radiological Changes Assessed by Quantitative Chest CT in Severe COVID-19 Patients. J. Clin. Med. 2022, 11, 1247. [Google Scholar] [CrossRef] [PubMed]
- Lazar, M.; Barbu, E.C.; Chitu, C.E.; Buzoianu, M.; Petre, A.C.; Tiliscan, C.; Arama, S.S.; Arama, V.; Ion, D.A.; Olariu, M.C. Surviving COVID-19 and Battling Fibrosis: A Retrospective Cohort Study Across Three Pandemic Waves. Diagnostics 2024, 14, 2811. [Google Scholar] [CrossRef] [PubMed]
- Banks, P.A.; Bollen, T.L.; Dervenis, C.; Gooszen, H.G.; Johnson, C.D.; Sarr, M.G.; Tsiotos, G.G.; Vege, S.S. Acute Pancreatitis Classification Working Group. Classification of acute pancreatitis--2012: Revision of the Atlanta classification and definitions by international consensus. Gut 2013, 62, 102–111. [Google Scholar] [CrossRef] [PubMed]
- Balthazar, E.J. Acute pancreatitis: Assessment of severity with clinical and CT evaluation. Radiology 2002, 223, 603–613. [Google Scholar] [CrossRef]
- Silva, J.T.C.; Fonseca Neto, O.C.L.D. Acute pancreatitis and COVID-19: An integrative review of the literature. Rev. Col. Bras. Cir. 2023, 50, e20233559. [Google Scholar] [CrossRef]
- Kang, D.; Park, S.H.; Oh, C.; Kim, Y.J.; Kim, J.B.; Park, S.H.; Lee, M.S.; Park, J.K. Prevalence and prognosis of acute pancreatitis in critically ill patients with COVID-19. Hepatobiliary Pancreat. Dis. Int. 2023, 22, 399–402. [Google Scholar] [CrossRef]
- Chaudhry, H.; Sohal, A.; Kohli, I.; Dukovic, D.; Sharma, R.; Singla, P.; Hu, B.; Prajapati, D.; Yang, J. The burden of acute pancreatitis on COVID-19 in the United States. Ann. Gastroenterol. 2023, 36, 208–215. [Google Scholar] [CrossRef]
- Barlass, U.; Wiliams, B.; Dhana, K.; Adnan, D.; Khan, S.R.; Mahdavinia, M.; Bishehsari, F. Marked elevation of lipase in COVID-19 disease: A cohort study. Clin. Transl. Gastroenterol. 2020, 11, e00215. [Google Scholar] [CrossRef]
- Pandanaboyana, S.; Moir, J.; Leeds, J.S.; Oppong, K.; Kanwar, A.; Marzouk, A.; Belgaumkar, A.; Gupta, A.; Siriwardena, A.K.; Haque, A.R.; et al. SARS-CoV-2 infection in acute pancreatitis increases disease severity and 30-day mortality: COVID PAN collaborative study. Gut 2021, 70, 1061–1069. [Google Scholar] [CrossRef]
- Bulthuis, M.C.; Boxhoorn, L.; Beudel, M.; Elbers, P.W.G.; Kop, M.P.M.; van Wanrooij, R.L.J.; Besselink, M.G.; Voermans, R.P. Acute pancreatitis in COVID-19 patients: True risk? Scand. J. Gastroenterol. 2021, 56, 585–587. [Google Scholar] [CrossRef]
- Vatansev, H.; Yıldırım, M.A.; Kuccukturk, S.; Karaselek, M.A.; Kadiyoran, C. Clinical Evaluation of Acute Pancreatitis Caused by SARS-CoV-2 Virus Infection. Gastroenterol. Res. Pract. 2021, 2021, 5579795. [Google Scholar] [CrossRef]
- de Sá, T.C.; Soares, C.; Rocha, M. Acute pancreatitis and COVID-19: A literature review. World J. Gastrointest. Surg. 2021, 13, 574–584. [Google Scholar] [CrossRef]
- Goyal, H.; Kopel, J.; Ristić, B.; Perisetti, A.; Anastasiou, J.; Chandan, S.; Tharian, B.; Inamdar, S. The pancreas and COVID-19: A clinical conundrum. Am. J. Transl. Res. 2021, 13, 11004–11013. [Google Scholar]
- Müller, J.A.; Groß, R.; Conzelmann, C.; Krüger, J.; Merle, U.; Steinhart, J.; Weil, T.; Koepke, L.; Bozzo, C.P.; Read, C.; et al. SARS-CoV-2 infects and replicates in cells of the human endocrine and exocrine pancreas. Nat. Metab. 2021, 3, 149–165. [Google Scholar] [CrossRef] [PubMed]
- van der Heide, V.; Jangra, S.; Cohen, P.; Rathnasinghe, R.; Aslam, S.; Aydillo, T.; Geanon, D.; Handler, D.; Kelley, G.; Lee, B.; et al. Limited extent and consequences of pancreatic SARS-CoV-2 infection. Cell Rep. 2022, 38, 110508. [Google Scholar] [CrossRef] [PubMed]
- Gheblawi, M.; Wang, K.; Viveiros, A.; Nguyen, Q.; Zhong, J.C.; Turner, A.J.; Raizada, M.K.; Grant, M.B.; Oudit, G.Y. Angiotensin-Converting Enzyme 2: SARS-CoV-2 Receptor and Regulator of the Renin-Angiotensin System: Celebrating the 20th Anniversary of the Discovery of ACE2. Circ. Res. 2020, 126, 1456–1474. [Google Scholar] [CrossRef] [PubMed]
- Habtezion, A.; Gukovskaya, A.S.; Pandol, S.J. Acute Pancreatitis: A Multifaceted Set of Organelle and Cellular Interactions. Gastroenterology 2019, 156, 1941–1950. [Google Scholar] [CrossRef]
- Qadir, M.M.F.; Bhondeley, M.; Beatty, W.; Gaupp, D.D.; Doyle-Meyers, L.A.; Fischer, T.; Bandyopadhyay, I.; Blair, R.V.; Bohm, R.; Rappaport, J.; et al. SARS-CoV-2 infection of the pancreas promotes thrombofibrosis and is associated with new-onset diabetes. JCI Insight 2021, 6, e151551. [Google Scholar] [CrossRef]
- Wu, C.T.; Lidsky, P.V.; Xiao, Y.; Lee, I.T.; Cheng, R.; Nakayama, T.; Jiang, S.; Demeter, J.; Bevacqua, R.J.; Chang, C.A.; et al. SARS-CoV-2 infects human pancreatic β cells and elicits β cell impairment. Cell Metab. 2021, 33, 1565–1576.e5. [Google Scholar] [CrossRef]
- Ben Nasr, M.; D’Addio, F.; Montefusco, L.; Usuelli, V.; Loretelli, C.; Rossi, A.; Pastore, I.; Abdelsalam, A.; Maestroni, A.; Dell’Acqua, M.; et al. Indirect and Direct Effects of SARS-CoV-2 on Human Pancreatic Islets. Diabetes 2022, 71, 1579–1590. [Google Scholar] [CrossRef]
- Fajgenbaum, D.C.; June, C.H. Cytokine Storm. N. Engl. J. Med. 2020, 383, 2255–2273. [Google Scholar] [CrossRef]
- Merad, M.; Martin, J.C. Pathological inflammation in patients with COVID-19: A key role for monocytes and macrophages. Nat. Rev. Immunol. 2020, 20, 355–362, Erratum in: Nat. Rev. Immunol. 2020, 20, 448. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Xie, X.; Tu, Z.; Fu, J.; Xu, D.; Zhou, Y. The signal pathways and treatment of cytokine storm in COVID-19. Signal Transduct. Target. Ther. 2021, 6, 255, Erratum in: Signal Transduct. Target. Ther. 2021, 6, 326. [Google Scholar] [CrossRef] [PubMed]
- Freeman, T.L.; Swartz, T.H. Targeting the NLRP3 Inflammasome in Severe COVID-19. Front. Immunol. 2020, 11, 1518. [Google Scholar] [CrossRef]
- Mehta, P.; McAuley, D.F.; Brown, M.; Sanchez, E.; Tattersall, R.S.; Manson, J.J.; HLH Across Speciality Collaboration, U.K. COVID-19: Consider cytokine storm syndromes and immunosuppression. Lancet 2020, 395, 1033–1034. [Google Scholar] [CrossRef]
- Papantoniou, K.; Aggeletopoulou, I.; Michailides, C.; Pastras, P.; Triantos, C. Understanding the Role of NLRP3 Inflammasome in Acute Pancreatitis. Biology 2024, 13, 945. [Google Scholar] [CrossRef]
- Hu, B.; Huang, S.; Yin, L. The cytokine storm and COVID-19. J. Med. Virol. 2021, 93, 250–256. [Google Scholar] [CrossRef] [PubMed]
- Ferrero-Andrés, A.; Panisello-Roselló, A.; Roselló-Catafau, J.; Folch-Puy, E. NLRP3 Inflammasome-Mediated Inflammation in Acute Pancreatitis. Int. J. Mol. Sci. 2020, 21, 5386. [Google Scholar] [CrossRef]
- Zhao, N.; Di, B.; Xu, L.L. The NLRP3 inflammasome and COVID-19: Activation, pathogenesis and therapeutic strategies. Cytokine Growth Factor Rev. 2021, 61, 2–15. [Google Scholar] [CrossRef]
- Shah, A. Novel Coronavirus-Induced NLRP3 Inflammasome Activation: A Potential Drug Target in the Treatment of COVID-19. Front. Immunol. 2020, 11, 1021. [Google Scholar] [CrossRef]
- Declercq, J.; De Leeuw, E.; Lambrecht, B.N. Inflammasomes and IL-1 family cytokines in SARS-CoV-2 infection: From prognostic marker to therapeutic agent. Cytokine 2022, 157, 155934. [Google Scholar] [CrossRef]
- Gao, D.; Madi, M.; Ding, C.; Fok, M.; Steele, T.; Ford, C.; Hunter, L.; Bing, C. Interleukin-1β mediates macrophage-induced impairment of insulin signaling in human primary adipocytes. Am. J. Physiol. Endocrinol. Metab. 2014, 307, E289–E304. [Google Scholar] [CrossRef]
- Khreefa, Z.; Barbier, M.T.; Koksal, A.R.; Love, G.; Del Valle, L. Pathogenesis and Mechanisms of SARS-CoV-2 Infection in the Intestine, Liver, and Pancreas. Cells 2023, 12, 262. [Google Scholar] [CrossRef]
- Rafaqat, S.; Patoulias, D.; Behnoush, A.H.; Sharif, S.; Klisic, A. Interleukins: Pathophysiological role in acute pancreatitis. Arch Med. Sci. 2024, 20, 138–156. [Google Scholar] [CrossRef]
- Grendell, J.H. Editorial: Drug-induced acute pancreatitis: Uncommon or commonplace? Am. J. Gastroenterol. 2011, 106, 2189–2191. [Google Scholar] [CrossRef] [PubMed]
- Khan, I.; Edward, N. Pancreatitis associated with diclofenac. Postgrad. Med. J. 1993, 69, 486–487. [Google Scholar] [CrossRef] [PubMed]
- Kaufman, M. Drug-induced pancreatitis: A potentially serious and underreported problem. Pharm. Ther. 2013, 38, 349–351. [Google Scholar]
- Jones, M.; Hall, M.; Kaye, M.; Kaye, A.D. Drug-induced acute pancreatitis: A review. Ochsner J. 2015, 15, 45–51. [Google Scholar]
- Reyes, J.V.; Patel, B.M.; Malik, F.; Gonzalez, M.O. Non-steroidal Anti-inflammatory Drug-induced Acute Pancreatitis: A Case Report. Cureus 2019, 11, e5926. [Google Scholar] [CrossRef] [PubMed]
- Weissman, S.; Aziz, M.; Perumpail, R.B.; Mehta, T.I.; Patel, R.; Tabibian, J.H. Ever-increasing diversity of drug-induced pancreatitis. World J. Gastroenterol. 2020, 26, 2902–2915. [Google Scholar] [CrossRef]
- Lin, W.; Zheng, Q.; Wang, X.; Lin, X.; Ni, X.; Pan, J.; Zippi, M.; Fiorino, S.; Hong, W. The causality between use of glucocorticoids and risk of pancreatitis: A Mendelian randomization study. Front. Immunol. 2024, 15, 1420840. [Google Scholar] [CrossRef]
- Nango, D.; Hirose, Y.; Goto, M.; Echizen, H. Analysis of the Association of Administration of various glucocorticoids with development of acute pancreatitis using US Food and Drug Administration adverse event reporting system (FAERS). J. Pharm. Health Care Sci. 2019, 5, 5. [Google Scholar] [CrossRef] [PubMed]
- Sadr-Azodi, O.; Mattsson, F.; Bexlius, T.S.; Lindblad, M.; Lagergren, J.; Ljung, R. Association of oral glucocorticoid use with an increased risk of acute pancreatitis: A population-based nested case-control study. JAMA Intern. Med. 2013, 173, 444–449. [Google Scholar] [CrossRef]
- Kandil, E.; Lin, Y.Y.; Bluth, M.H.; Zhang, H.; Levi, G.; Zenilman, M.E. Dexamethasone mediates protection against acute pancreatitis via upregulation of pancreatitis-associated proteins. World J. Gastroenterol. 2006, 12, 6806–6811. [Google Scholar] [CrossRef]
- RECOVERY Collaborative Group; Horby, P.; Lim, W.S.; Emberson, J.R.; Mafham, M.; Bell, J.L.; Linsell, L.; Staplin, N.; Brightling, C.; Ustianowski, A.; et al. Dexamethasone in Hospitalized Patients with COVID-19. N. Engl. J. Med. 2021, 384, 693–704. [Google Scholar]
- Chitu-Tisu, C.E.; Barbu, E.C.; Lazar, M.; Ionescu, R.; Bojinca, M.; Ion, D.A.; Badarau, I.A. An overview of bone disease in HIV-infected patients. Acta Medica Mediterr. 2015, 31, 1139–1151. [Google Scholar]
- Munteanu, D.; Negru, A.; Mihăilescu, R.; Tilișcan, C.; Tudor, A.M.; Lazăr, M.; Aramă, Ș.S.; Ion, D.; Popescu, C.; Aramă, V. Evaluation of bone mineral density and correlations with inflammation markers in romanianhiv-positive patients undergoing combined antiretroviral therapy. Farmacia 2017, 65, 114–119. [Google Scholar]
- Barbu, E.C.; Chiţu-Tișu, C.E.; Lazăr, M.; Olariu, C.M.; Olteanu, D.; Bojincă, M.; Abagiu, A.O.; Aramă, V.; Ion, D.A.; Bădărău, I.A. Body Composition Changes in Patients with Chronic Hepatitis C. J. Gastrointest. Liver Dis. JGLD 2016, 25, 323–329. [Google Scholar] [CrossRef]
- Qian, F.H.; Cao, Y.; Liu, Y.X.; Huang, J.; Zhu, R.H. A predictive model to explore risk factors for severe COVID-19. Sci. Rep. 2024, 14, 18197. [Google Scholar] [CrossRef] [PubMed]
- Ding, P.; Song, B.; Liu, X.; Fang, X.; Cai, H.; Zhang, D.; Zheng, X. Elevated Pancreatic Enzymes in ICU Patients with COVID-19 in Wuhan, China: A Retrospective Study. Front. Med. 2021, 8, 663646. [Google Scholar] [CrossRef] [PubMed]
- Pawlowski, C.; Wagner, T.; Puranik, A.; Murugadoss, K.; Loscalzo, L.; Venkatakrishnan, A.J.; Pruthi, R.K.; Houghton, D.E.; O’Horo, J.C.; Morice, W.G., 2nd; et al. Inference from longitudinal laboratory tests characterizes temporal evolution of COVID-19-associated coagulopathy (CAC). eLife 2020, 9, e59209. [Google Scholar] [CrossRef]
- Huang, Y.; Guo, L.; Chen, J.; Wu, M.; Zhang, C.; Liu, Z.; Li, J.; Li, K.; Xiong, Z.; Wu, Q.; et al. Serum Lactate Dehydrogenase Level as a Prognostic Factor for COVID-19: A Retrospective Study Based on a Large Sample Size. Front. Med. 2022, 8, 671667. [Google Scholar] [CrossRef]
- Ahmed, A.; Fisher, J.C.; Pochapin, M.B.; Freedman, S.D.; Kothari, D.J.; Shah, P.C.; Sheth, S.G. Hyperlipasemia in absence of acute pancreatitis is associated with elevated D-dimer and adverse outcomes in COVID 19 disease. Pancreatology 2021, 21, 698–703. [Google Scholar] [CrossRef]
- Yadav, R.; Yadav, V.; Pokhriyal, S.; Zahid, U.; Gandhi, A. Retrospective Observational Study of Complete Blood Count (CBC) Parameters and ICU Mortality of COVID-19 Disease in Delta Variant and Omicron Variant in a Community-Based Hospital in New York City. Cureus 2023, 15, e34894. [Google Scholar] [CrossRef]
- Wan, J.; Yang, X.; He, W.; Zhu, Y.; Zhu, Y.; Zeng, H.; Liu, P.; Xia, L.; Lu, N. Serum D-dimer levels at admission for prediction of outcomes in acute pancreatitis. BMC Gastroenterol. 2019, 19, 67. [Google Scholar] [CrossRef]
- Salomone, T.; Tosi, P.; Palareti, G.; Tomassetti, P.; Migliori, M.; Guariento, A.; Saieva, C.; Raiti, C.; Romboli, M.; Gullo, L. Coagulative disorders in human acute pancreatitis: Role for the D-dimer. Pancreas 2003, 26, 111–116. [Google Scholar] [CrossRef]
- Li, L.; Tan, Q.; Wu, X.; Mou, X.; Lin, Z.; Liu, T.; Huang, W.; Deng, L.; Jin, T.; Xia, Q. Coagulopathy and acute pancreatitis:Pathophysiology and clinical treatment. Front. Immunol. 2024, 15, 1477160. [Google Scholar]
- McFadyen, J.D.; Stevens, H.; Peter, K. The Emerging Threat of (Micro)Thrombosis in COVID-19 and Its Therapeutic Implications. Circ. Res. 2020, 127, 571–587. [Google Scholar] [CrossRef]
- Poudel, A.; Poudel, Y.; Adhikari, A.; Aryal, B.B.; Dangol, D.; Bajracharya, T.; Maharjan, A.; Gautam, R. D-dimer as a biomarker for assessment of COVID-19 prognosis: D-dimer levels on admission and its role in predicting disease outcome in hospitalized patients with COVID-19. PLoS ONE 2021, 16, e0256744. [Google Scholar] [CrossRef]
- Bikdeli, B.; Madhavan, M.V.; Jimenez, D.; Chuich, T.; Dreyfus, I.; Driggin, E.; Nigoghossian, C.; Ageno, W.; Madjid, M.; Guo, Y.; et al. COVID-19 and Thrombotic or Thromboembolic Disease: Implications for Prevention, Antithrombotic Therapy, and Follow-Up: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2020, 75, 2950–2973. [Google Scholar] [CrossRef]
- Bunch, C.M.; Moore, E.E.; Moore, H.B.; Neal, M.D.; Thomas, A.V.; Zackariya, N.; Zhao, J.; Zackariya, S.; Brenner, T.J.; Berquist, M.; et al. Immuno-Thrombotic Complications of COVID-19: Implications for Timing of Surgery and Anticoagulation. Front. Surg. 2022, 9, 889999. [Google Scholar] [CrossRef]
- Merza, M.; Hartman, H.; Rahman, M.; Hwaiz, R.; Zhang, E.; Renström, E.; Luo, L.; Mörgelin, M.; Regner, S.; Thorlacius, H. Neutrophil Extracellular Traps Induce Trypsin Activation, Inflammation, and Tissue Damage in Mice with Severe Acute Pancreatitis. Gastroenterology 2015, 149, 1920–1931.e8. [Google Scholar] [CrossRef] [PubMed]
- Zuo, Y.; Yalavarthi, S.; Shi, H.; Gockman, K.; Zuo, M.; Madison, J.A.; Blair, C.; Weber, A.; Barnes, B.J.; Egeblad, M.; et al. Neutrophil extracellular traps in COVID-19. JCI Insight 2020, 5, e138999. [Google Scholar] [CrossRef]
- Tuculeanu, G.; Barbu, E.C.; Lazar, M.; Chitu-Tisu, C.E.; Moisa, E.; Negoita, S.I.; Ion, D.A. Coagulation Disorders in Sepsis and COVID-19—Two Sides of the Same Coin? A Review of Inflammation—Coagulation Crosstalk in Bacterial Sepsis and COVID-19. J. Clin. Med. 2023, 12, 601. [Google Scholar] [CrossRef]
- Murthy, P.; Singhi, A.D.; Ross, M.A.; Loughran, P.; Paragomi, P.; Papachristou, G.I.; Whitcomb, D.C.; Zureikat, A.H.; Lotze, M.T.; Zeh Iii, H.J.; et al. Enhanced Neutrophil Extracellular Trap Formation in Acute Pancreatitis Contributes to Disease Severity and Is Reduced by Chloroquine. Front. Immunol. 2019, 10, 28. [Google Scholar] [CrossRef]
- Cho, S.K.; Jung, S.; Lee, K.J.; Kim, J.W. Neutrophil to lymphocyte ratio and platelet to lymphocyte ratio can predict the severity of gallstone pancreatitis. BMC Gastroenterol. 2018, 18, 18. [Google Scholar] [CrossRef] [PubMed]
- Alberca, G.G.F.; Cardoso, N.S.S.; Alberca, R.W. Could immune activation cause pancreatitis in COVID-19 patients? Transl. Gastroenterol. Hepatol. 2022, 7, 45. [Google Scholar] [CrossRef]
- Hegyi, P.; Szakács, Z.; Sahin-Tóth, M. Lipotoxicity and Cytokine Storm in Severe Acute Pancreatitis and COVID-19. Gastroenterology 2020, 159, 824–827. [Google Scholar] [CrossRef]
- Hackert, T.; Hartwig, W.; Fritz, S.; Schneider, L.; Strobel, O.; Werner, J. Ischemic acute pancreatitis: Clinical features of 11 patients and review of the literature. Am. J. Surg. 2009, 197, 450–454. [Google Scholar] [CrossRef]
- Alfano, G.; Ferrari, A.; Fontana, F.; Perrone, R.; Mori, G.; Ascione, E.; Magistroni, R.; Venturi, G.; Pederzoli, S.; Margiotta, G.; et al. Hypokalemia in Patients with COVID-19. Clin. Exp. Nephrol. 2021, 25, 401–409. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Li, X.; Song, Q.; Hu, C.; Su, F.; Dai, J.; Ye, Y.; Huang, J.; Zhang, X. Assessment of Hypokalemia and Clinical Characteristics in Patients with Coronavirus Disease 2019 in Wenzhou, China. JAMA Netw. Open 2020, 3, e2011122. [Google Scholar] [CrossRef] [PubMed]
- Leppäniemi, A.; Tolonen, M.; Tarasconi, A.; Segovia-Lohse, H.; Gamberini, E.; Kirkpatrick, A.W.; Ball, C.G.; Parry, N.; Sartelli, M.; Wolbrink, D.; et al. 2019 WSES guidelines for the management of severe acute pancreatitis. World J. Emerg. Surg. 2019, 14, 27. [Google Scholar] [CrossRef] [PubMed]
- de-Madaria, E.; Capurso, G. COVID-19 and acute pancreatitis: Examining the causality. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 3–4. [Google Scholar] [CrossRef]
- Reiterer, M.; Rajan, M.; Gómez-Banoy, N.; Lau, J.D.; Gomez-Escobar, L.G.; Ma, L.; Gilani, A.; Alvarez-Mulett, S.; Sholle, E.T.; Chandar, V.; et al. Hyperglycemia in acute COVID-19 is characterized by insulin resistance and adipose tissue infectivity by SARS-CoV-2. Cell Metab. 2021, 33, 2174–2188.e5, Erratum in: Cell Metab. 2021, 33, 2484. [Google Scholar] [CrossRef]
- Zhu, P.; Wang, X.; Hu, C.; Zhang, X.; Lin, Z.; Jin, T.; Li, L.; Shi, N.; Yang, X.; Huang, W.; et al. The Stress Hyperglycemia Ratio as a Predictor of Clinical Outcomes in Acute Pancreatitis: A Retrospective Cohort Study. J. Clin. Med. 2025, 14, 4970. [Google Scholar] [CrossRef]
- Zahedi, M.; Kordrostami, S.; Kalantarhormozi, M.; Bagheri, M. A Review of Hyperglycemia in COVID-19. Cureus 2023, 15, e37487. [Google Scholar] [CrossRef]
- Balaban, M.; Balaban, D.V.; Enache, I.; Nedelcu, I.C.; Jinga, M.; Gheorghe, C. Impact of Serum Glucose Levels on Outcomes in Acute Pancreatitis: A Retrospective Analysis. Medicina 2024, 60, 856. [Google Scholar] [CrossRef]
- Ackermann, M.; Verleden, S.E.; Kuehnel, M.; Haverich, A.; Welte, T.; Laenger, F.; Vanstapel, A.; Werlein, C.; Stark, H.; Tzankov, A.; et al. Pulmonary Vascular Endothelialitis, Thrombosis, and Angiogenesis in COVID-19. N. Engl. J. Med. 2020, 383, 120–128. [Google Scholar] [CrossRef]
- Cuthbertson, C.M.; Christophi, C. Disturbances of the microcirculation in acute pancreatitis. Br. J. Surg. 2006, 93, 518–530. [Google Scholar] [CrossRef]
- Kotan, R.; Peto, K.; Deak, A.; Szentkereszty, Z.; Nemeth, N. Hemorheological and Microcirculatory Relations of Acute Pancreatitis. Metabolites 2023, 13, 4. [Google Scholar] [CrossRef] [PubMed]
- Plötz, F.B.; Slutsky, A.S.; van Vught, A.J.; Heijnen, C.J. Ventilator-induced lung injury and multiple system organ failure: A critical review of facts and hypotheses. Intensive Care Med. 2004, 30, 1865–1872. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.Y.; Chiang, C.H.; Chuang, C.H.; Liu, S.L.; Jheng, Y.H.; Ryu, J.H. Spillover of cytokines and reactive oxygen species in ventilator-induced lung injury associated with inflammation and apoptosis in distal organs. Respir. Care 2014, 59, 1422–1432. [Google Scholar] [CrossRef] [PubMed]
| Parameters | CT Scan Values |
|---|---|
| Slice thickness (mm) | 3 |
| Reconstruction thickness (mm) | 1.5 |
| Colimmation | 1.2 |
| Reference mAs | 250 |
| Reference kV | 120 |
| Rotation time (s) | 0.5 |
| Pitch | 0.35 |
| FOV | Both lungs/thorax and superior abdomen included |
| Reconstruction kernels | H31f for mediastinum and superior abdomen; H60f for lung |
| Comorbidity | Group A (n, %) | Group B (n, %) |
|---|---|---|
| Obesity | 14 (43.7%) | 126 (33.7%) |
| Diabetes mellitus type-2 | 7 (21.8%) | 36 (9.6%) |
| Arterial hypertension | 17 (53.1%) | 115 (30.8%) |
| Congestive heart failure | 2 (6.2%) | 7 (1.8%) |
| Peripheral vascular disease | 1 (3.1%) | 4 (1.1%) |
| Chronic obstructive pulmonary disorder | 2 (6.2%) | 25 (6.7%) |
| Chronic viral hepatitis | 1 (3.1%) | 11 (2.9%) |
| History of neoplasia | 2 (6.2%) | 14 (3.7%) |
| History of ischemic stroke | 1 (3.1%) | 18 (4.8%) |
| Dementia | 1 (3.1%) | 5 (1.3%) |
| History of peptic ulcer | 1 (3.1%) | 4 (1.1%) |
| Clinical, Biological, and Imaging Characteristics | Group A (Median, Q1, Q3) | Group B (Median, Q1, Q3) | p-Value |
|---|---|---|---|
| Heart rate (beats/min) | 96.5 [82.5; 111.2] | 93 [81; 106] | 0.174 |
| Respiratory rate (breaths/minute) | 17 [16; 21] | 18 [16; 20] | 0.776 |
| Saturation (O2) % | 92 [88.2; 96.7] | 92 [86; 96] | 0.694 |
| Hospital stay (days) | 15 [11; 23] | 11 [6.75; 18.2] | 0.003 |
| Duration of antiviral treatment (days) | 5.5 [4.2; 9.7] | 4 [3; 5] | 0.001 |
| Duration of corticotherapy(days) | 10 [7; 16.7] | 7 [1; 12] | 0.005 |
| Serum glucose (mg/dL) | 122.5 [109.5; 142] | 112 [100; 129] | 0.012 |
| HbA1c % | 5.9 [5.3; 6.6] | 5.8 [5.3; 6.6] | 0.816 |
| CRP (mg/L) | 92.3 [38.2; 140.4] | 39 [12;73.1] | <0.001 |
| Fibrinogen (mg/dL) | 635 [419.5; 708.5] | 447 [335.5; 562] | 0.001 |
| ESR(mm/h) | 59 [52; 69] | 45 [20; 55] | <0.001 |
| LDH (U/L) | 381 [308.5; 456.7] | 257 [216; 330] | <0.001 |
| AST (U/L) | 46 [38.5; 65] | 38 [30; 53.7] | 0.002 |
| ALT (U/L) | 43 [30.2; 54] | 38 [24; 54] | 0.331 |
| CK (U/L) | 107 [50.7; 232.7] | 104 [48; 148] | 0.271 |
| Serum lipase (U/L) | 552.5 [462.5; 761.2] | 124 [84; 195.5] | <0.001 |
| Serum urea (mg/dL) | 31.4 [28.6; 40.6] | 32.6 [26; 40.5] | 0.667 |
| Serum creatinine (mg/dL) | 0.8 [0.7; 1] | 0.8 [0.6; 0.9] | 0.206 |
| DB (mg/dL) | 0.3 [0.2; 0.5] | 0.4 [0.3; 0.5] | 0.220 |
| IB (mg/dL) | 0.4 [0.3; 0.4] | 0.5 [0.3; 0.6] | 0.673 |
| Serum ferritin(ng/mL) | 715 [493; 1163] | 633 [311.2; 1134.2] | 0.234 |
| IL1 (pg/mL) | 0.3 [0.1; 3.5] | 5.7 [0.5; 18.7] | 0.024 |
| IL6 (pg/mL) | 107.2 [45.2; 164.4] | 91.4 [36.2; 149.1] | 0.418 |
| TNFα (pg/mL) | 9.4 [3.3; 17.5] | 10.7 [4.8; 18.3] | 0.636 |
| RBC (×106/µL) | 4.5 [3.9; 4.8] | 4.4 [3.9; 4.9] | 0.859 |
| WBC (×103/µL) | 8.7 [6.9; 12.8] | 6.4 [5; 9.4] | 0.001 |
| Lymphocytes (×103/µL) | 0.9 [0.5; 1.1] | 0.9 [0.6; 1.4] | 0.341 |
| Neutrophils (×103/µL) | 7.2 [5.9; 11] | 4.5 [3.2; 7.3] | <0.001 |
| Platelets (×103/µL) | 232 [159.5; 332.5] | 188 [138; 232.5] | 0.028 |
| D-dimers (ng/mL) | 264 [193.5; 487] | 187.5 [144; 259] | 0.001 |
| Serum Na (mEq/L) | 138 [132.2; 159.5] | 137 [130; 142] | 0.123 |
| Serum K (mEq/L) | 4.5 [3.9; 5.2] | 4.2 [3.7; 4.6] | 0.021 |
| Number of pulmonary lobes involved | 5 [5; 5] | 5 [4; 5] | 0.008 |
| Consolidation (% from total lung volume) | 1 [0.7; 1.7] | 0.9 [0.7; 1.6] | 0.526 |
| Mixed lesions (% from total lung volume) | 2.9 [2.6; 4.9] | 2.4 [1.2; 3.8] | 0.005 |
| Interstitial lesions (% from total lung volume) | 42.1 [33.9; 54.6] | 30.3 [21.5; 43.4] | <0.001 |
| Normal pulmonary densities (% from total lung volume) | 48.7 [34.6; 56.9] | 61.4 [47.2; 69.5] | <0.001 |
| Total pulmonary lesions (% from total lung volume) | 47.4 [37.9; 63.4] | 34.4 [23.6; 49.1] | <0.001 |
| Parameter | Patients in Wave 1 | Patients in Wave 2 | Patients in Wave 3 | |
|---|---|---|---|---|
| Balthazar grade | 0 | 0 | 0 | 0 |
| 1 | 4 | 5 | 3 | |
| 2 | 4 | 3 | 5 | |
| 3 | 2 | 1 | 4 | |
| 4 | 0 | 0 | 1 | |
| Pancreaticnecrosisscore | 0% | 9 | 9 | 11 |
| ≤30% | 1 | 0 | 2 | |
| 30–50% | 0 | 0 | 0 | |
| 50% | 0 | 0 | 0 | |
| CTSI | 0–3 | 9 | 9 | 11 |
| 4–6 | 1 | 0 | 2 | |
| 7–10 | 0 | 0 | 0 | |
| Lipase level | normal | 0 | 0 | 0 |
| 1–3× | 7 | 8 | 10 | |
| >3× | 3 | 1 | 3 | |
| Clinical, Biological, and Imaging Characteristics | Spearman’s Rho | p-Value |
|---|---|---|
| Hospital stay | 0.163 | 0.003 |
| Duration of antiviral treatment (days) | 0.179 | 0.001 |
| Duration of corticotherapy | 0.148 | 0.005 |
| Serum glucose | 0.124 | 0.012 |
| CRP | 0.197 | <0.001 |
| Fibrinogen | 0.164 | 0.001 |
| ESR | 0.259 | <0.001 |
| LDH | 0.290 | <0.001 |
| AST | 0.154 | 0.002 |
| IL1 | −0.164 | 0.023 |
| WBC | 0.159 | 0.001 |
| Neutrophils | 0.193 | <0.001 |
| Platelets | 0.109 | 0.028 |
| D-dimers | 0.172 | 0.001 |
| Serum K | 0.116 | 0.020 |
| Number of pulmonary lobes involved | 0.161 | 0.001 |
| Mixed lesions (% from total lung volume) | 0.140 | 0.005 |
| Interstitial lesions (% from total lung volume) | 0.230 | <0.001 |
| Normal pulmonary densities (% from total lung volume) | −0.211 | <0.001 |
| Total pulmonary lesions (% from total lung volume) | 0.217 | <0.001 |
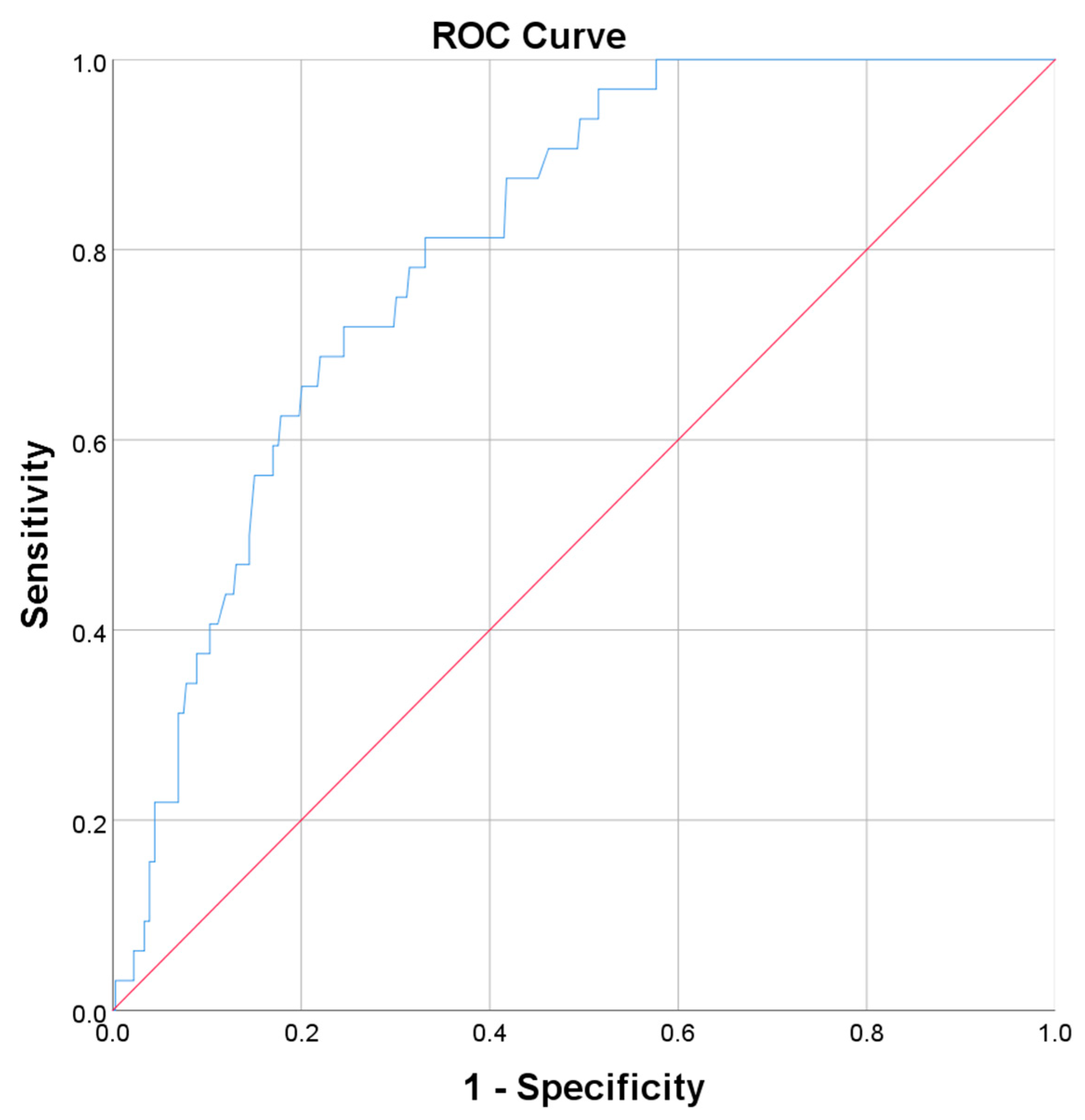
| Predictor | AUC | Std Error | p-Value | CI 95% | |
|---|---|---|---|---|---|
| Lower Bound | Upper Bound | ||||
| Hospital stay | 0.661 | 0.041 | 0.003 | 0.581 | 0.741 |
| Duration of antiviral treatment (days) | 0.677 | 0.051 | 0.001 | 0.576 | 0.777 |
| Duration of corticotherapy | 0.650 | 0.048 | 0.005 | 0.556 | 0.745 |
| Serum glucose | 0.633 | 0.046 | 0.012 | 0.542 | 0.724 |
| CRP | 0.702 | 0.048 | <0.001 | 0.609 | 0.795 |
| Fibrinogen | 0.675 | 0.057 | 0.001 | 0.563 | 0.787 |
| ESR | 0.777 | 0.033 | <0.001 | 0.711 | 0.842 |
| LDH | 0.806 | 0.032 | <0.001 | 0.743 | 0.868 |
| AST | 0.662 | 0.037 | 0.002 | 0.589 | 0.735 |
| IL1 | 0.695 | 0.071 | 0.024 | 0.556 | 0.834 |
| WBC | 0.670 | 0.048 | 0.001 | 0.575 | 0.764 |
| Neutrophils | 0.707 | 0.046 | <0.001 | 0.618 | 0.796 |
| Platelets | 0.617 | 0.055 | 0.028 | 0.509 | 0.725 |
| D-dimers | 0.684 | 0.057 | 0.001 | 0.573 | 0.795 |
| Serum K | 0.623 | 0.058 | 0.021 | 0.510 | 0.736 |
| Number of pulmonary lobes involved | 0.649 | 0.041 | 0.005 | 0.568 | 0.730 |
| Mixed lesions (% from total lung volume) | 0.652 | 0.040 | 0.005 | 0.572 | 0.727 |
| Interstitial lesions (% from total lung volume) | 0.746 | 0.036 | <0.001 | 0.675 | 0.818 |
| Normal pulmonary densities (% from total lung volume) | 0.726 | 0.035 | <0.001 | 0.657 | 0.795 |
| Total pulmonary lesions (% from total lung volume) | 0.733 | 0.036 | <0.001 | 0.663 | 0.802 |
| Variable | B | S.E. | Wald | p | OR | 95% CI for OR | |
|---|---|---|---|---|---|---|---|
| Lower | Upper | ||||||
| D-Dimers | 0.002 | 0.001 | 7.054 | 0.008 | 1.002 | 1.000 | 1.003 |
| Lung interstitial lesions | 0.045 | 0.017 | 7.355 | 0.007 | 1.046 | 1.013 | 1.081 |
| CRP | 0.011 | 0.003 | 13.083 | <0.001 | 1.011 | 1.005 | 1.018 |
| Constant | −5.552 | 0.820 | 45.808 | <0.001 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lazar, M.; Chitu, C.E.; Barbu, E.C. Pancreatic Injury in Severe SARS-CoV-2 Infection: A Retrospective Study Across Three Pandemic Waves. Life 2025, 15, 1439. https://doi.org/10.3390/life15091439
Lazar M, Chitu CE, Barbu EC. Pancreatic Injury in Severe SARS-CoV-2 Infection: A Retrospective Study Across Three Pandemic Waves. Life. 2025; 15(9):1439. https://doi.org/10.3390/life15091439
Chicago/Turabian StyleLazar, Mihai, Cristina Emilia Chitu, and Ecaterina Constanta Barbu. 2025. "Pancreatic Injury in Severe SARS-CoV-2 Infection: A Retrospective Study Across Three Pandemic Waves" Life 15, no. 9: 1439. https://doi.org/10.3390/life15091439
APA StyleLazar, M., Chitu, C. E., & Barbu, E. C. (2025). Pancreatic Injury in Severe SARS-CoV-2 Infection: A Retrospective Study Across Three Pandemic Waves. Life, 15(9), 1439. https://doi.org/10.3390/life15091439






