Investigating the Hemorheological, Metabolic, and Physical Performance Effect of a Core Muscle Strengthening Training Program
Abstract
1. Introduction
2. Materials and Methods
2.1. Volunteers
2.2. The Core Muscle Training Program
2.3. Body Composition, Spiroergometry Test, and Collection of Blood Samples
2.4. Laboratory Blood Tests
2.5. Statistical Analysis
3. Results
3.1. Bodyweight, Body Mass Index, and Body Composition Parameters
3.2. Spiroergometry Test
3.3. Blood Gas, pH, Acid–Base Parameters, Electrolytes, and Metabolites
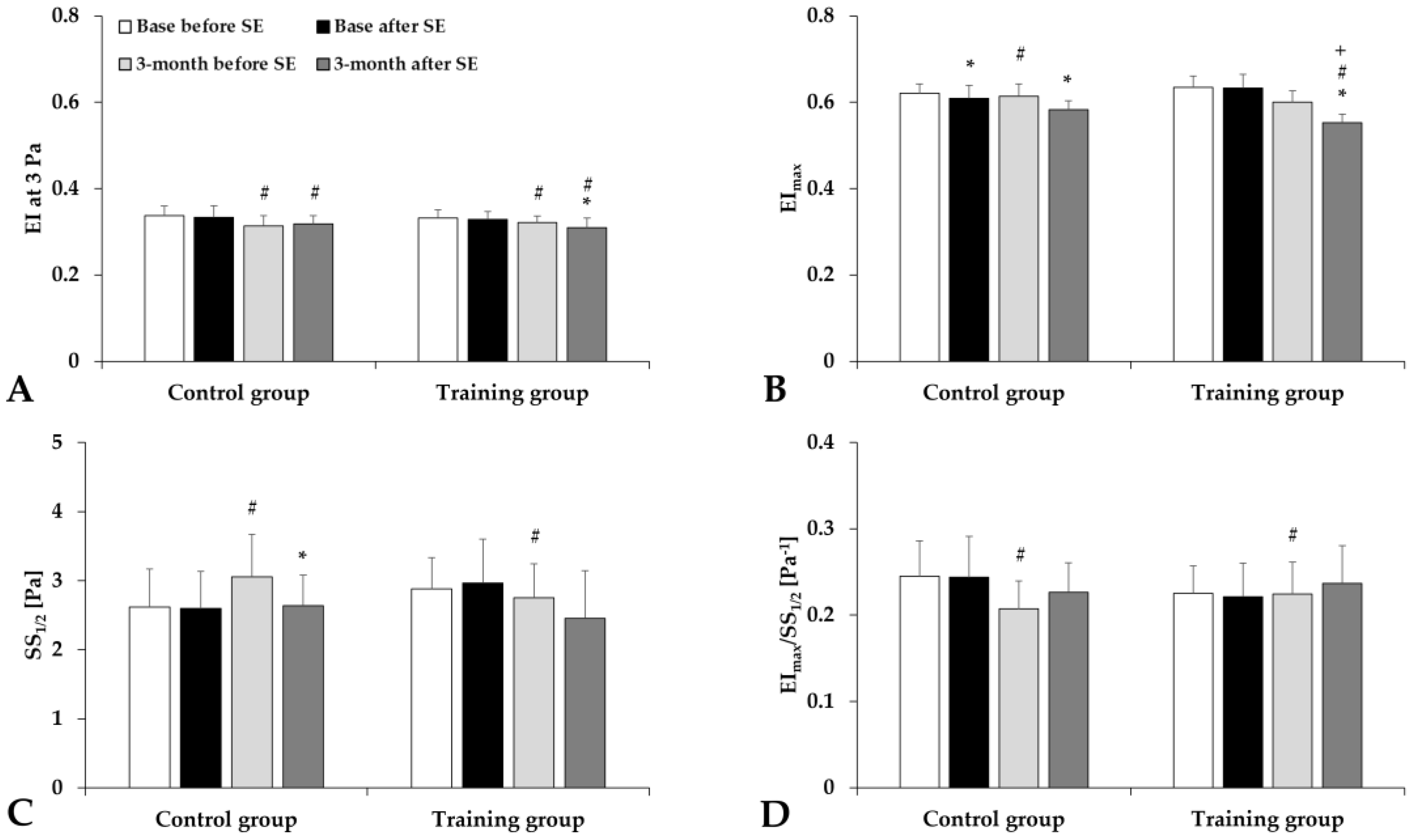
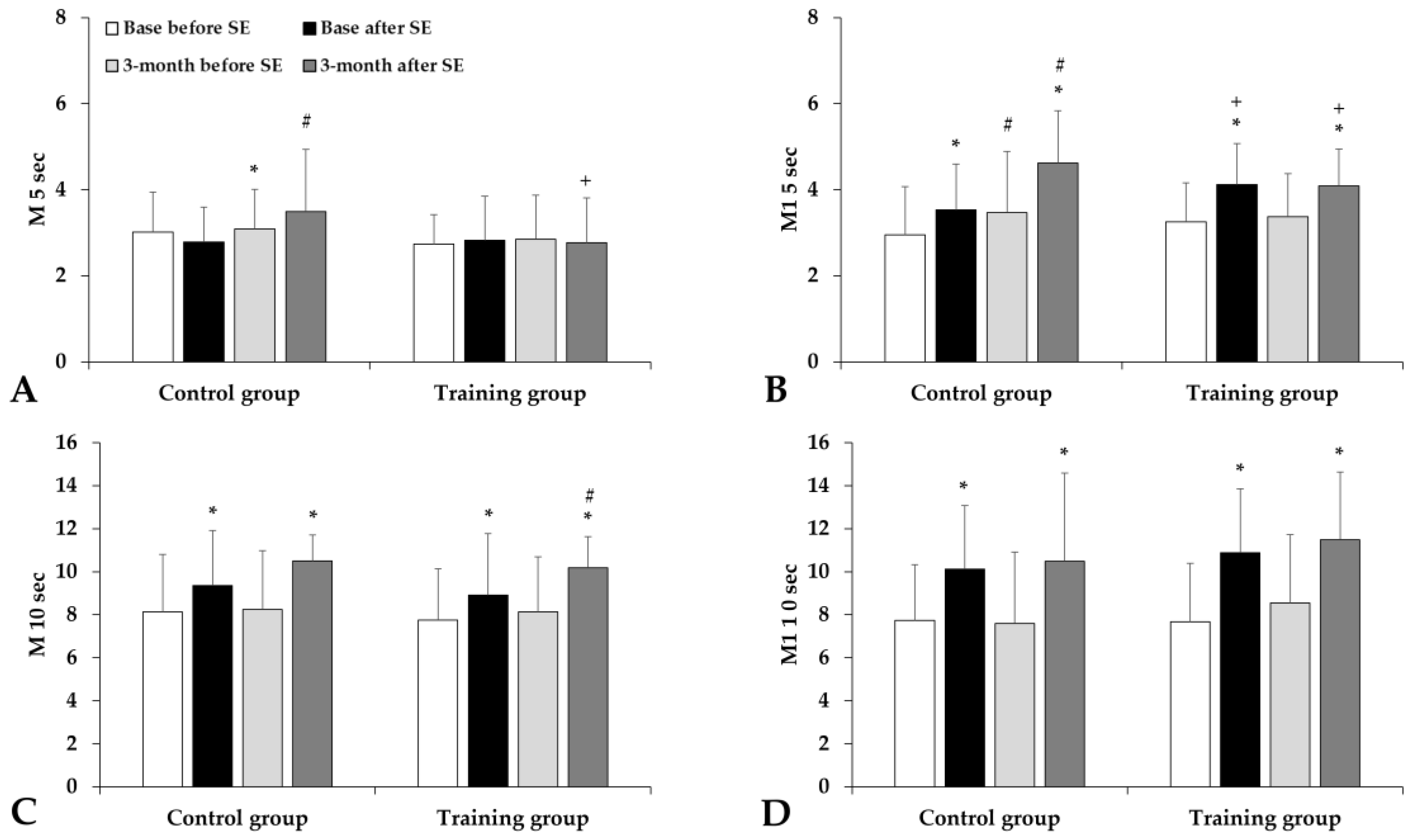
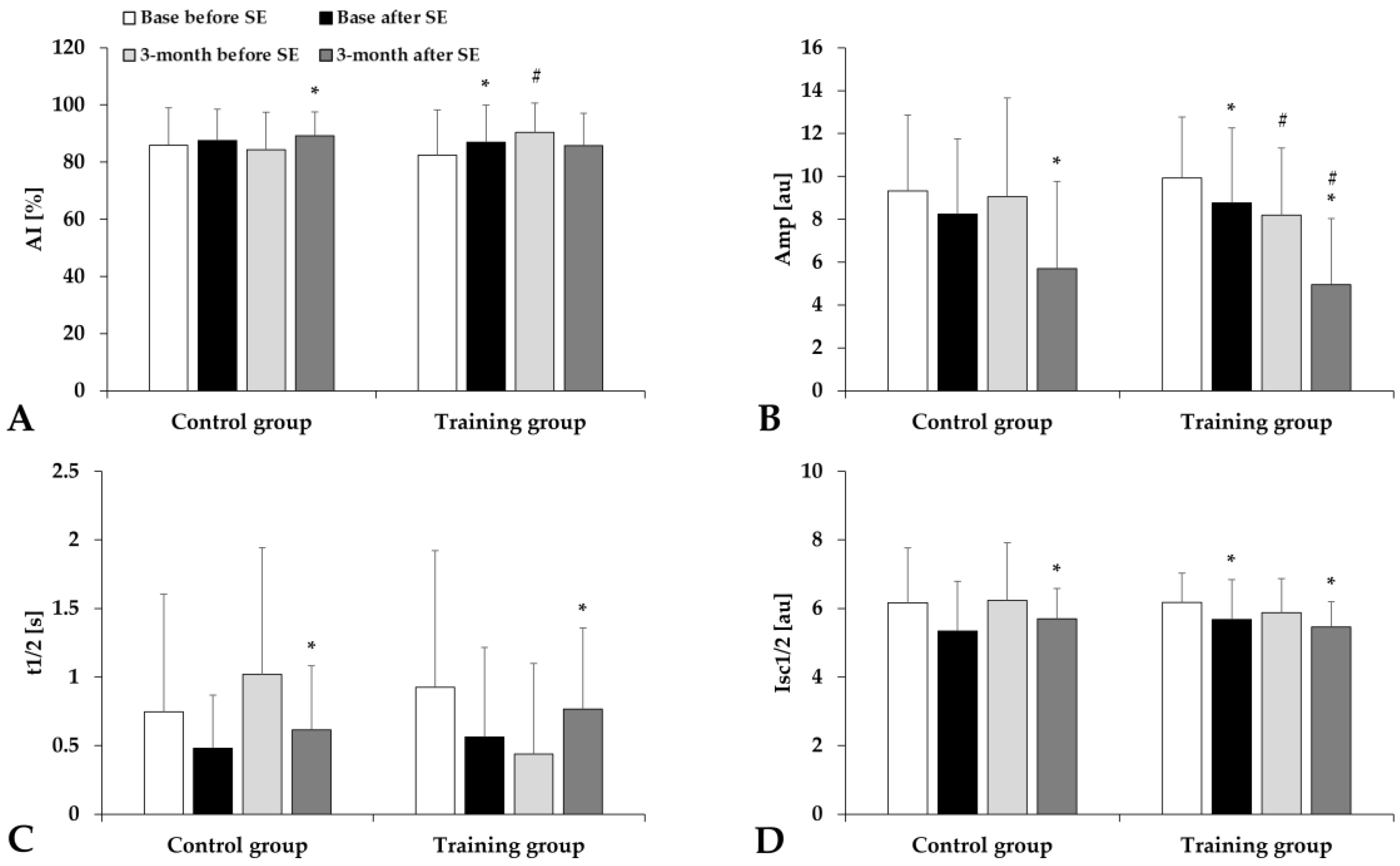
3.4. Hematological and Hemorheological Parameters
4. Discussion
5. Summary and Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bassett, D.R. Limiting Factors for Maximum Oxygen Uptake and Determinants of Endurance Performance. Med. Sci. Sports Exerc. 2000, 32, 70. [Google Scholar] [CrossRef]
- Sandor, B.; Nagy, A.; Toth, A.; Rabai, M.; Mezey, B.; Csatho, A.; Czuriga, I.; Toth, K.; Szabados, E. Effects of Moderate Aerobic Exercise Training on Hemorheological and Laboratory Parameters in Ischemic Heart Disease Patients. PLoS ONE 2014, 9, e110751. [Google Scholar] [CrossRef] [PubMed]
- Nader, E.; Guillot, N.; Lavorel, L.; Hancco, I.; Fort, R.; Stauffer, E.; Renoux, C.; Joly, P.; Germain, M.; Connes, P. Eryptosis and Hemorheological Responses to Maximal Exercise in Athletes: Comparison between Running and Cycling. Scand. Med. Sci. Sports 2018, 28, 1532–1540. [Google Scholar] [CrossRef]
- Nader, E.; Skinner, S.; Romana, M.; Fort, R.; Lemonne, N.; Guillot, N.; Gauthier, A.; Antoine-Jonville, S.; Renoux, C.; Hardy-Dessources, M.-D.; et al. Blood Rheology: Key Parameters, Impact on Blood Flow, Role in Sickle Cell Disease and Effects of Exercise. Front. Physiol. 2019, 10, 1329. [Google Scholar] [CrossRef] [PubMed]
- El-Sayed, M.S. Effects of Exercise and Training on Blood Rheology. Sports Med. 1998, 26, 281–292. [Google Scholar] [CrossRef] [PubMed]
- Cokelet, G.R.; Meiselman, H.J. Macro- and micro-rheological properties of blood. In Handbook of Hemorheology and Hemodynamics; Baskurt, O.K., Hardeman, M.R., Rampling, M.W., Meiselman, H.J., Eds.; IOS Press: Amsterdam, The Netherlands, 2007; pp. 45–71. [Google Scholar]
- Baskurt, O.K. Mechanisms of blood rheology alterations. In Handbook of Hemorheology and Hemodynamics; Baskurt, O.K., Hardeman, M.R., Rampling, M.W., Meiselman, H.J., Eds.; IOS Press: Amsterdam, The Netherlands, 2007; pp. 170–190. [Google Scholar]
- Ernst, E. Influence of Regular Physical Activity on Blood Rheology. Eur. Heart J. 1987, 8, 59–62. [Google Scholar] [CrossRef]
- Connes, P.; Caillaud, C.; Py, G.; Mercier, J.; Hue, O.; Brun, J.-F. Maximal Exercise and Lactate Do Not Change Red Blood Cell Aggregation in Well Trained Athletes. Clin. Hemorheol. Microcirc. 2007, 36, 319–326. [Google Scholar]
- Brun, J.-F.; Varlet-Marie, E.; Connes, P.; Aloulou, I. Hemorheological Alterations Related to Training and Overtraining. Biorheology 2010, 47, 95–115. [Google Scholar] [CrossRef]
- Romain, A.-J.; Brun, J.-F.; Varlet-Marie, E.; Raynaud de Mauverger, E. Effects of Exercise Training on Blood Rheology: A Meta-Analysis. Clin. Hemorheol. Microcirc. 2011, 49, 199–205. [Google Scholar] [CrossRef]
- Brun, J.-F.; Varlet-Marie, E.; Romain, A.-J.; Guiraudou, M.; Raynaud de Mauverger, E. Exercise Hemorheology: Moving from Old Simplistic Paradigms to a More Complex Picture. Clin. Hemorheol. Microcirc. 2013, 55, 15–27. [Google Scholar] [CrossRef]
- Connes, P.; Simmonds, M.J.; Brun, J.-F.; Baskurt, O.K. Exercise Hemorheology: Classical Data, Recent Findings and Unresolved Issues. Clin. Hemorheol. Microcirc. 2013, 53, 187–199. [Google Scholar] [CrossRef]
- Bizjak, D.A.; Tomschi, F.; Bales, G.; Nader, E.; Romana, M.; Connes, P.; Bloch, W.; Grau, M. Does Endurance Training Improve Red Blood Cell Aging and Hemorheology in Moderate-Trained Healthy Individuals? J. Sport Health Sci. 2020, 9, 595–603. [Google Scholar] [CrossRef]
- Szanto, S.; Mody, T.; Gyurcsik, Z.; Babjak, L.B.; Somogyi, V.; Barath, B.; Varga, A.; Matrai, A.A.; Nemeth, N. Alterations of Selected Hemorheological and Metabolic Parameters Induced by Physical Activity in Untrained Men and Sportsmen. Metabolites 2021, 11, 870. [Google Scholar] [CrossRef]
- Tomschi, F.; Bloch, W.; Grau, M. Impact of Type of Sport, Gender and Age on Red Blood Cell Deformability of Elite Athletes. Int. J. Sports Med. 2018, 39, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Saldanha, C. Fibrinogen Interaction with the Red Blood Cell Membrane. Clin. Hemorheol. Microcirc. 2013, 53, 39–44. [Google Scholar] [CrossRef] [PubMed]
- Kyröläinen, H.; Pihlainen, K.; Vaara, J.P.; Ojanen, T.; Santtila, M. Optimising Training Adaptations and Performance in Military Environment. J. Sci. Med. Sport 2018, 21, 1131–1138. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.; Doma, K.; Heilbronn, B.; Leicht, A. Effect of Exercise Training Programs on Physical Fitness Domains in Military Personnel: A Systematic Review and Meta-Analysis. Mil. Med. 2022, 187, 1065–1073. [Google Scholar] [CrossRef]
- Haddock, C.K.; Poston, W.S.C.; Heinrich, K.M.; Jahnke, S.A.; Jitnarin, N. The Benefits of High-Intensity Functional Training Fitness Programs for Military Personnel. Mil. Med. 2016, 181, e1508–e1514. [Google Scholar] [CrossRef]
- Burley, S.D.; Drain, J.R.; Sampson, J.A.; Nindl, B.C.; Groeller, H. Effect of a Novel Low Volume, High Intensity Concurrent Training Regimen on Recruit Fitness and Resilience. J. Sci. Med. Sport 2020, 23, 979–984. [Google Scholar] [CrossRef]
- Wang, X.; Song, W.; Ruan, Y.; Li, B.; Lü, C.; Huang, N.; Fang, F.; Gu, W. Core Muscle Functional Strength Training for Reducing the Risk of Low Back Pain in Military Recruits: An Open-Label Randomized Controlled Trial. J. Integr. Med. 2022, 20, 145–152. [Google Scholar] [CrossRef]
- Ferri-Caruana, A.; Prades-Insa, B.; Serra-AÑÓ, P. Effects of Pelvic and Core Strength Training on Biomechanical Risk Factors for Anterior Cruciate Ligament Injuries. J. Sports Med. Phys. Fit. 2020, 60, 1128–1136. [Google Scholar] [CrossRef]
- Oja, P.; Memon, A.R.; Titze, S.; Jurakic, D.; Chen, S.-T.; Shrestha, N.; Em, S.; Matolic, T.; Vasankari, T.; Heinonen, A.; et al. Health Benefits of Different Sports: A Systematic Review and Meta-Analysis of Longitudinal and Intervention Studies Including 2.6 Million Adult Participants. Sports Med.-Open 2024, 10, 1–17. [Google Scholar] [CrossRef]
- Mayer-Berger, W. Endurance training in cardiovascular patients—Practical aspects. Dtsch. Z. Sportmed. 2018, 69, 87–92. [Google Scholar] [CrossRef]
- Pinto, R.; Melo, X.; Angarten, V.; Pires, M.L.; Borges, M.; Santos, V.; Abreu, A.; Santa-Clara, H. The effects of 12-months supervised periodized training on health-related physical fitness in coronary artery disease: A randomized controlled trial. J. Sports Sci. 2021, 39, 1893–1902. [Google Scholar] [CrossRef]
- Cakir-Atabek, H.; Atsak, P.; Gunduz, N.; Bor-Kucukatay, M. Effects of resistance training intensity on deformability and aggregation of red blood cells. Clin. Hemorheol. Microcirc. 2009, 41, 251–261. [Google Scholar] [CrossRef] [PubMed]
- Schilling, J.F.; Murphy, J.C.; Bonney, J.R.; Thich, J.L. Effect of Core Strength and Endurance Training on Performance in College Students: Randomized Pilot Study. J. Bodyw. Mov. Ther. 2013, 17, 278–290. [Google Scholar] [CrossRef]
- Akuthota, V.; Ferreiro, A.; Moore, T.; Fredericson, M. Core stability exercise principles. Curr. Sports Med. Rep. 2008, 7, 39–44. [Google Scholar] [CrossRef] [PubMed]
- Barroso, R.; Tricoli, V.; Santos Gil, S.D.; Ugrinowitsch, C.; Roschel, H. Maximal strength, number of repetitions, and total volume are differently affected by static-, ballistic-, and proprioceptive neuromuscular facilitation stretching. J. Strength Cond. Res. 2012, 26, 2432–2437. [Google Scholar] [CrossRef]
- Moghadam, N.; Ghaffari, M.S.; Noormohammadpour, P.; Rostami, M.; Zarei, M.; Moosavi, M.; Kordi, R. Comparison of the recruitment of transverse abdominis through drawing-in and bracing in different core stability training positions. J. Exerc. Rehabil. 2019, 15, 819–825. [Google Scholar] [CrossRef] [PubMed]
- Silva, S.C.; Monteiro, W.D.; Cunha, F.A.; Farinatti, P. Influence of Different Treadmill Inclinations on Vo2max and Ventilatory Thresholds During Maximal Ramp Protocols. J. Strength Cond. Res. 2021, 35, 233–239. [Google Scholar] [CrossRef]
- Figueiredo, P.S.; Looney, D.P.; Pryor, J.L.; Doughty, E.M.; McClung, H.L.; Vangala, S.V.; Santee, W.R.; Beidleman, B.A.; Potter, A.W. Verification of Maximal Oxygen Uptake in Active Military Personnel During Treadmill Running. J. Strength Cond. Res. 2022, 36, 1053–1058. [Google Scholar] [CrossRef]
- Matrai, A.; Whittington, R.B.; Ernst, E. A Simple Method of Estimating Whole Blood Viscosity at Standardized Hematocrit. Clin. Hemorheol. Microcirc. 1987, 7, 261–265. [Google Scholar] [CrossRef]
- Hardeman, M.R.; Goedhart, P.T.; Shin, S. Methods in hemorheology. In Handbook of Hemorheology and Hemodynamics; Baskurt, O.K., Hardeman, M.R., Rampling, M.W., Meiselman, H.J., Eds.; IOS Press: Amsterdam, The Netherlands, 2007; pp. 242–266. [Google Scholar]
- Baskurt, O.K.; Boynard, M.; Cokelet, G.C.; Connes, P.; Cooke, B.M.; Forconi, S.; Liao, F.; Hardeman, M.R.; Jung, F.; Meiselman, H.J.; et al. International Expert Panel for Standardization of Hemorheological Methods. New Guidelines for Hemorheological Laboratory Techniques. Clin. Hemorheol. Microcirc. 2009, 42, 75–97. [Google Scholar] [CrossRef] [PubMed]
- Baskurt, O.K.; Hardeman, M.R.; Uyuklu, M.; Ulker, P.; Cengiz, M.; Nemeth, N.; Shin, S.; Alexy, T.; Meiselman, H.J. Parameterization of Red Blood Cell Elongation Index–Shear Stress Curves Obtained by Ektacytometry. Scand. J. Clin. Lab. Investig. 2009, 69, 777–788. [Google Scholar] [CrossRef] [PubMed]
- Simmonds, M.J.; Connes, P.; Sabapathy, S. Exercise-Induced Blood Lactate Increase Does Not Change Red Blood Cell Deformability in Cyclists. PLoS ONE 2013, 8, e71219. [Google Scholar] [CrossRef]
- Smith, J.A.; Martin, D.T.; Telford, R.D.; Ballas, S.K. Greater Erythrocyte Deformability in World-Class Endurance Athletes. Am. J. Physiol-Heart Circ. Physiol. 1999, 276, H2188–H2193. [Google Scholar] [CrossRef]
- Mohanty, J.G.; Nagababu, E.; Rifkind, J.M. Red Blood Cell Oxidative Stress Impairs Oxygen Delivery and Induces Red Blood Cell Aging. Front. Physiol. 2014, 5, 84. [Google Scholar] [CrossRef]
- Fisher-Wellman, K.; Bloomer, R.J. Acute Exercise and Oxidative Stress: A 30 Year History. Dyn. Med. 2009, 8, 1. [Google Scholar] [CrossRef]
- Sawka, M.N.; Cheuvront, S.N.; Carter, R. Human Water Needs. Nutr. Rev. 2005, 63, S30–S39. [Google Scholar] [CrossRef]
- Latunde-Dada, G.O. Iron Metabolism in Athletes—Achieving a Gold Standard. Eur. J. Haematol. 2013, 90, 10–15. [Google Scholar] [CrossRef]
- Talebi, S.; Mohammadi, H.; Zeraattalab-Motlagh, S.; Arab, A.; Keshavarz Mohammadian, M.; Ghoreishy, S.M.; Abbaspour Tehrani Fard, M.; Amiri Khosroshahi, R.; Djafarian, K. Nutritional Interventions for Exercise-Induced Muscle Damage: An Umbrella Review of Systematic Reviews and Meta-Analyses of Randomized Trials. Nutr. Rev. 2024, 82, 639–653. [Google Scholar] [CrossRef]

| Variables | Control Group | Training Group | ||
|---|---|---|---|---|
| Base | End of 3-Month Period | Base | End of 3-Month Period | |
| Bodyweight [kg] | 83.31 ± 13.15 | 84.01 ± 13.22 | 83.78 ± 12.02 | 83.23 ± 10.90 |
| BMI [kg/m2] | 25.48 ± 2.93 | 25.73 ± 2.71 | 25.59 ± 2.82 | 25.41 ± 2.18 |
| Percent body fat [%] | 16.37 ± 5.89 | 15.86 ± 5.13 | 16.06 ± 6.34 | 14.36 ± 5.43 |
| Skeletal muscle mass [kg] | 39.82 ± 5.53 | 40.49 ± 6.08 | 40.35 ± 6.57 | 40.89 ± 6.13 |
| Variables | Control Group | Training Group | ||
|---|---|---|---|---|
| Base | End of 3-Month Period | Base | End of 3-Month Period | |
| RER | 1.11 ± 0.07 | 1.14 ± 0.09 | 1.13 ± 0.06 | 1.17 ± 0.07 |
| Lactatemax [mmol/L] | 11.30 ± 2.22 | 10.61 ± 2.49 | 12.60 ± 1.95 | 11.74 ± 2.46 |
| Lactate5’ [mmol/L] | 10.89 ± 2.05 | 10.23 ± 2.34 | 12.02 ± 2.11 | 10.25 ± 2.00 |
| Lactatemax/Lactate5’ | 1.06 ± 0.22 | 1.05 ± 0.16 | 1.06 ± 0.09 | 1.17 ± 0.28 |
| Lactate decrease in 5 min (%) | 2 ± 19% | 2 ± 18% | 4 ± 9% | 11 ± 14% |
| R2 of RER and Lactate5’ | 0.4707 | 0.2199 | 0.2716 | 0.0503 |
| Variables | Group | Base | End of 3-Month Period | ||||
|---|---|---|---|---|---|---|---|
| Before Spiroergometry | After Spiroergometry | After/Before Ratio | Before Spiroergometry | After Spiroergometry | After/Before Ratio | ||
| pO2 [mmHg] | Control | 30.62 ± 10.34 | 68.97 ± 26.29 * | 2.43 ± 1.12 | 31.15 ± 8.24 | 54.85 ± 17.95 * | 1.91 ± 0.80 |
| Training | 28.78 ± 7.73 | 70.29 ± 23.41 * | 2.56 ± 1.03 | 32.56 ± 5.43 | 52.51 ± 17.42 * | 1.66 ± 0.64 # | |
| pCO2 [mmHg] | Control | 53.92 ± 5.36 | 43.60 ± 7.50 * | 0.81 ± 0.14 | 52.01 ± 4.37 | 48.39 ± 7.21 # | 0.93 ± 0.19 # |
| Training | 54.70 ± 4.01 | 42.37 ± 7.54 * + | 0.78 ± 0.13 | 55.90 ± 4.58 # + | 49.76 ± 6.66 * # | 0.89 ± 0.11 # | |
| pH | Control | 7.34 ± 0.03 | 7.23 ± 0.06 * | 0.98 ± 0.01 | 7.34 ± 0.04 | 7.21 ± 0.05 * | 0.98 ± 0.01 |
| Training | 7.34 ± 0.02 | 7.22 ± 0.07 * | 0.98 ± 0.01 | 7.33 ± 0.02 | 7.22 ± 0.06 * | 0.99 ± 0.01 | |
| Lactate [mmol/L] | Control | 1.41 ± 0.50 | 12.57 ± 1.88 * | 9.82 ± 3.24 | 1.74 ± 1.83 | 13.38 ± 2.99 * | 10.40 ± 5.19 |
| Training | 1.35 ± 0.29 | 13.87 ± 2.42 * | 10.65 ± 2.97 | 1.76 ± 0.52 | 13.43 ± 3.43 * | 8.48 ± 3.65 | |
| Creatinine [µmol/L] | Control | 84.75 ± 16.75 | 94.42 ± 12.02 * | 1.08 ± 0.30 | 107.67 ± 19.28 # | 121.25 ± 19.01 * # | 1.10 ± 0.12 |
| Training | 93.84 ± 21.67 | 112.47 ± 23.3 * + | 1.26 ± 0.46 | 104.59 ± 14.49 # | 116.47 ± 12.76 * | 1.12 ± 0.10 | |
| Glucose [mmol/L] | Control | 5.24 ± 0.58 | 6.57 ± 1.26 * | 1.26 ± 0.23 | 4.94 ± 0.49 # | 5.96 ± 1.37 * | 1.20 ± 0.25 |
| Training | 5.00 ± 0.55 | 6.48 ± 1.24 * | 1.32 ± 0.31 | 4.44 ± 0.56 # + | 5.55 ± 0.95 * # | 1.29 ± 0.38 | |
| Variables | Group | Base | End of 3-Month Period | ||||
|---|---|---|---|---|---|---|---|
| Before Spiroergometry | After Spiroergometry | After/Before Ratio | Before Spiroergometry | After Spiroergometry | After/Before Ratio | ||
| WBC [×109/L] | Control | 6.29 ± 1.17 | 12.67 ± 2.67 * | 2.06 ± 0.47 | 6.73 ± 1.15 | 11.37 ± 1.94 * | 1.71 ± 0.24 # |
| Training | 5.82 ± 1.26 | 11.11 ± 3.22 * | 1.92 ± 0.43 | 5.51 ± 0.99 | 10.31 ± 2.20 * | 1.91 ± 0.51 | |
| RBC [×1012/L] | Control | 5.05 ± 0.28 | 5.29 ± 0.37 | 1.05 ± 0.05 | 5.09 ± 0.36 | 5.32 ± 0.31 | 1.06 ± 0.04 |
| Training | 5.20 ± 0.30 | 5.36 ± 0.32 | 1.03 ± 0.02 | 5.15 ± 0.27 | 5.35 ± 0.26 | 1.04 ± 0.04 | |
| Hgb [g/L] | Control | 15.27 ± 0.80 | 16.05 ± 1.02 * | 1.05 ± 0.04 | 15.28 ± 1.00 | 16.09 ± 0.90 * | 1.06 ± 0.03 |
| Training | 15.82 ± 0.99 | 16.41 ± 1.04 * | 1.04 ± 0.02 | 15.53 ± 1.06 | 16.21 ± 0.89 * | 1.05 ± 0.05 | |
| Hct [%] | Control | 44.22 ± 1.82 | 46.96 ± 2.50 * | 1.06 ± 0.05 | 44.08 ± 2.60 | 47.34 ± 1.83 * | 1.08 ± 0.03 |
| Training | 45.45 ± 2.15 | 47.57 ± 2.61 * | 1.05 ± 0.02 | 45.27 ± 2.47 | 48.03 ± 2.25 * | 1.06 ± 0.04 + | |
| MCV [fL] | Control | 87.71 ± 3.33 | 88.96 ± 3.61 | 1.01 ± 0.01 | 86.66 ± 3.09 | 89.06 ± 3.49 | 1.03 ± 0.03 |
| Training | 87.51 ± 2.74 | 88.80 ± 2.93 | 1.01 ± 0.01 | 87.99 ± 2.80 | 89.84 ± 2.77 | 1.02 ± 0.08 + | |
| MCH [pg] | Control | 30.28 ± 1.20 | 30.39 ± 1.22 | 1.00 ± 0.01 | 30.04 ± 1.31 | 30.27 ± 1.38 | 1.01 ± 0.02 |
| Training | 30.46 ± 1.19 | 30.63 ± 1.19 | 1.01 ± 0.01 | 30.18 ± 1.22 | 30.34 ± 1.23 | 1.01 ± 0.02 | |
| MCHC [g/L] | Control | 34.52 ± 0.79 | 34.17 ± 0.95 | 0.99 ± 0.01 | 34.67 ± 0.89 | 33.98 ± 1.00 | 0.98 ± 0.02 |
| Training | 34.80 ± 0.92 | 34.50 ± 0.90 | 0.99 ± 0.01 | 34.29 ± 0.79 | 33.76 ± 0.87 | 0.98 ± 0.02 | |
| Plt [×109/L] | Control | 254.80 ± 47.62 | 318.18 ± 42.46 * | 1.29 ± 0.34 | 258.17 ± 48.91 | 322.79 ± 42.55 * | 1.26 ± 0.18 |
| Training | 217.47 ± 39.69 | 279.08 ± 66.84 * | 1.27 ± 0.12 | 219.85 ± 40.28 | 269.35 ± 50.35 * | 1.23 ± 0.13 | |
| Variables | Group | Base | End of 3-Month Period | ||||
|---|---|---|---|---|---|---|---|
| Before Spiroergometry | After Spiroergometry | After/Before Ratio | Before Spiroergometry | After Spiroergometry | After/Before Ratio | ||
| WBV [mPas] | Control | 5.03 ± 0.68 | 5.10 ± 0.56 | 1.02 ± 0.14 | 4.57 ± 0.52 | 5.07 ± 0.46 * | 1.11 ± 0.11 |
| Training | 4.70 ± 0.68 | 5.50 ± 0.64 * | 1.18 ± 0.15 | 5.04 ± 1.01 | 4.98 ± 0.61 # | 1.02 ± 0.20 | |
| PV [mPas] | Control | 1.45 ± 0.26 | 1.45 ± 0.34 | 1.02 ± 0.27 | 1.36 ± 0.27 | 1.63 ± 0.45 * | 1.21 ± 0.31 |
| Training | 1.45 ± 0.40 | 1.63 ± 0.27 | 1.10 ± 0.38 | 1.49 ± 0.48 | 1.66 ± 0.40 | 1.19 ± 0.41 | |
| WBV 40% [mPas] | Control | 4.41 ± 0.55 | 4.21 ± 0.46 | 0.97 ± 0.14 | 4.11 ± 0.55 | 4.21 ± 0.33 | 1.02 ± 0.09 |
| Training | 4.07 ± 0.48 | 4.55 ± 0.43 * | 1.07 ± 0.29 | 4.37 ± 0.84 | 4.11 ± 0.30 # | 0.97 ± 0.19 | |
| Hct/WBV | Control | 9.04 ± 1.02 | 9.30 ± 0.88 | 1.04 ± 0.18 | 9.76 ± 1.25 | 9.40 ± 0.78 | 0.99 ± 0.10 |
| Training | 9.82 ± 1.21 | 8.73 ± 0.88 * | 0.90 ± 0.12 | 9.25 ± 1.56 | 9.73 ± 0.79 # | 1.09 ± 0.29 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mody, T.; Gyurcsik, Z.N.; Bakos, C.A.; Horvath, B.; Bedocs-Barath, B.; Varga, A.; Matrai, A.A.; Nemeth, N.; Szanto, S. Investigating the Hemorheological, Metabolic, and Physical Performance Effect of a Core Muscle Strengthening Training Program. Life 2025, 15, 1438. https://doi.org/10.3390/life15091438
Mody T, Gyurcsik ZN, Bakos CA, Horvath B, Bedocs-Barath B, Varga A, Matrai AA, Nemeth N, Szanto S. Investigating the Hemorheological, Metabolic, and Physical Performance Effect of a Core Muscle Strengthening Training Program. Life. 2025; 15(9):1438. https://doi.org/10.3390/life15091438
Chicago/Turabian StyleMody, Tobias, Zsuzsanna Nemethne Gyurcsik, Csaba Attila Bakos, Bela Horvath, Barbara Bedocs-Barath, Adam Varga, Adam Attila Matrai, Norbert Nemeth, and Sandor Szanto. 2025. "Investigating the Hemorheological, Metabolic, and Physical Performance Effect of a Core Muscle Strengthening Training Program" Life 15, no. 9: 1438. https://doi.org/10.3390/life15091438
APA StyleMody, T., Gyurcsik, Z. N., Bakos, C. A., Horvath, B., Bedocs-Barath, B., Varga, A., Matrai, A. A., Nemeth, N., & Szanto, S. (2025). Investigating the Hemorheological, Metabolic, and Physical Performance Effect of a Core Muscle Strengthening Training Program. Life, 15(9), 1438. https://doi.org/10.3390/life15091438








